Experimental and Numerical Investigation of Reaction Behavior of Carbon Composite Briquette in Blast Furnace
Abstract
1. Introduction
2. Experimental
2.1. Raw Materials
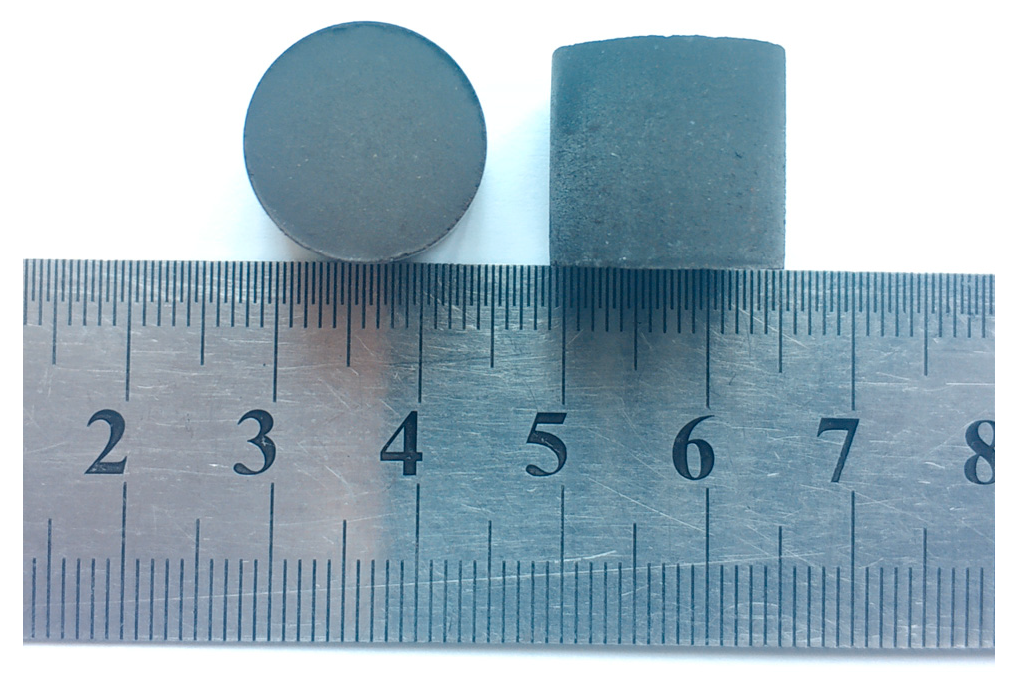
2.2. CCB Preparation
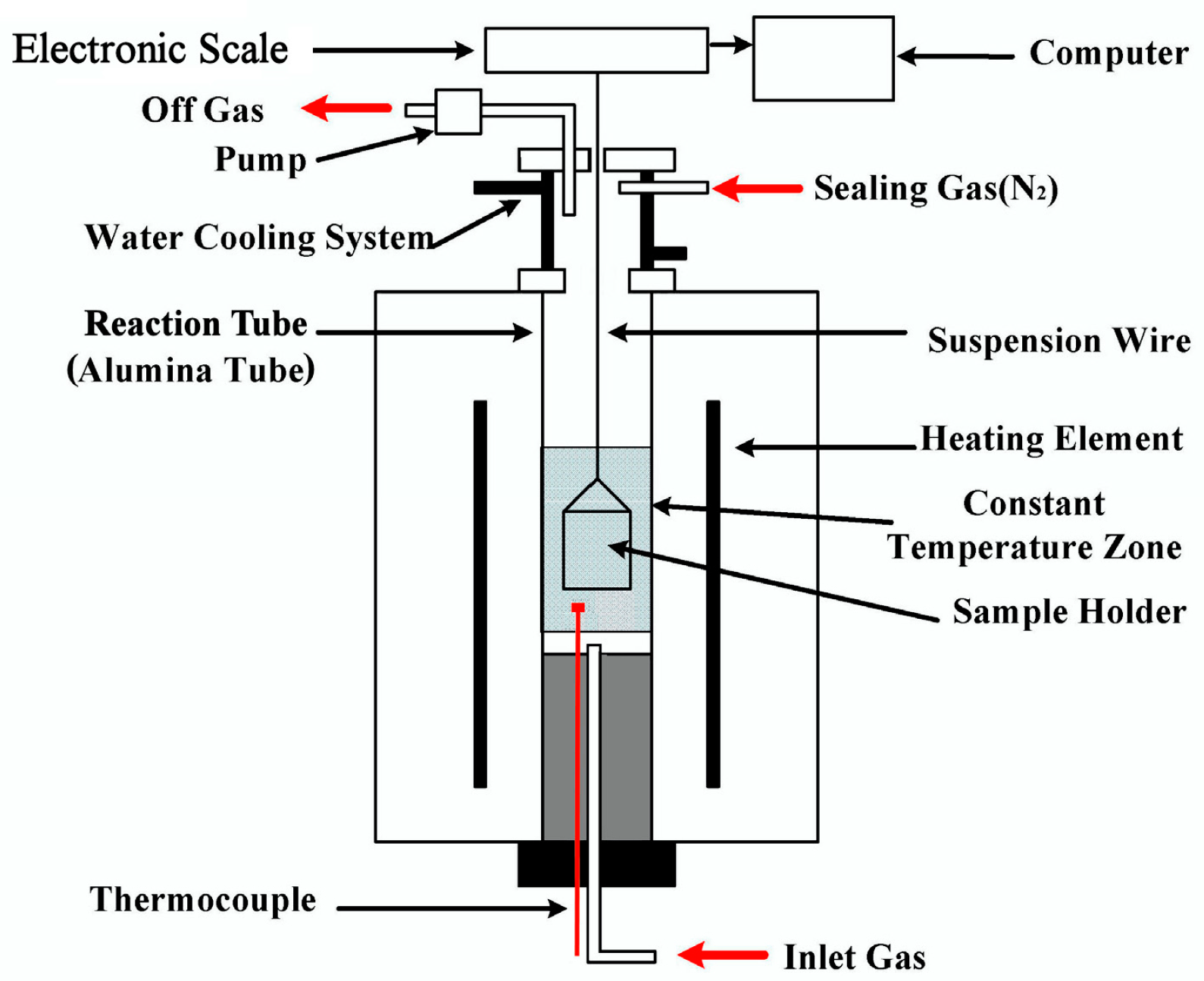
2.3. CCB Reaction Tests
2.4. Analysis and Characterization
3. Development of a CCB Model in BF Atmosphere
3.1. Theory
3.2. Solution Method
4. Results and Discussion
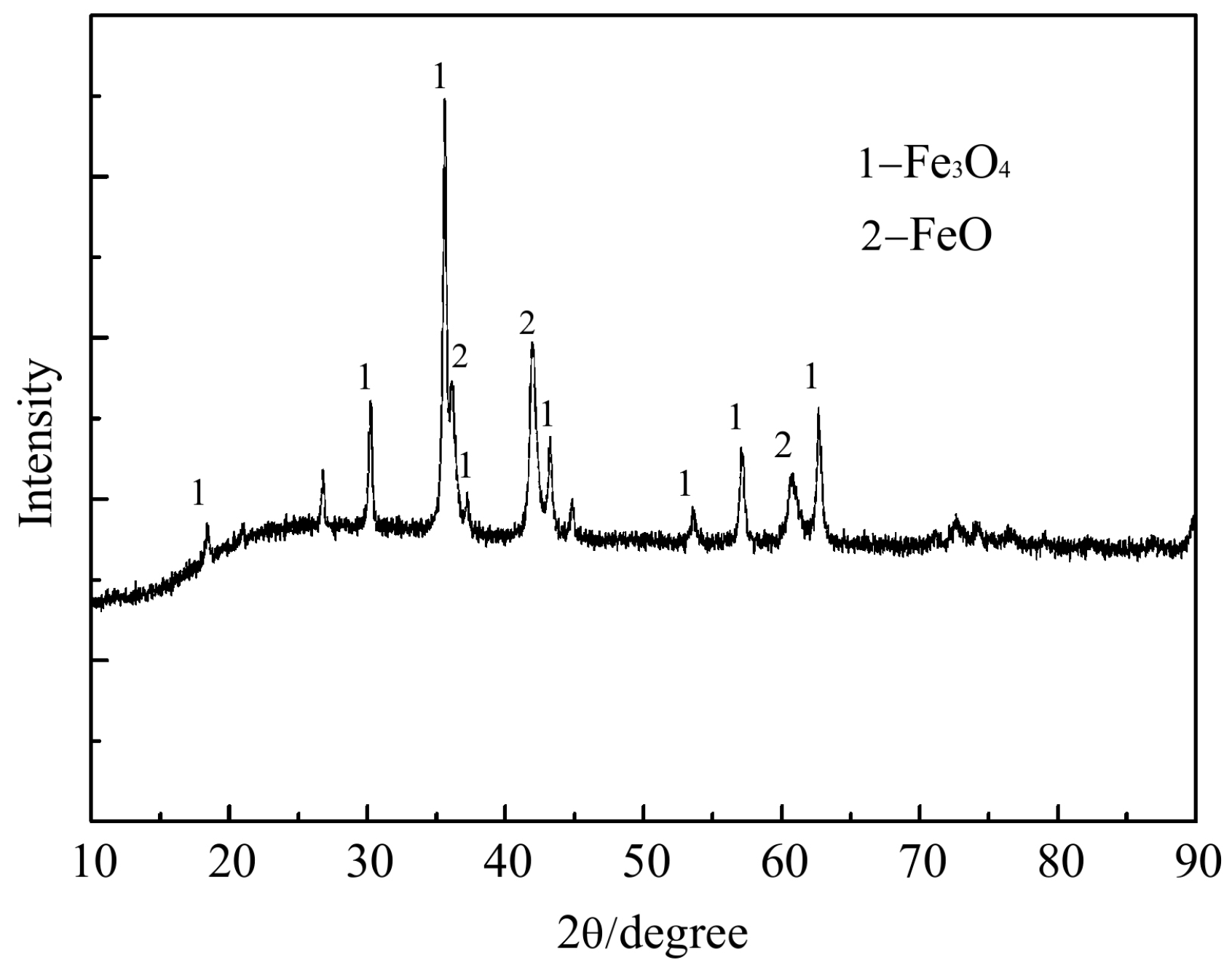
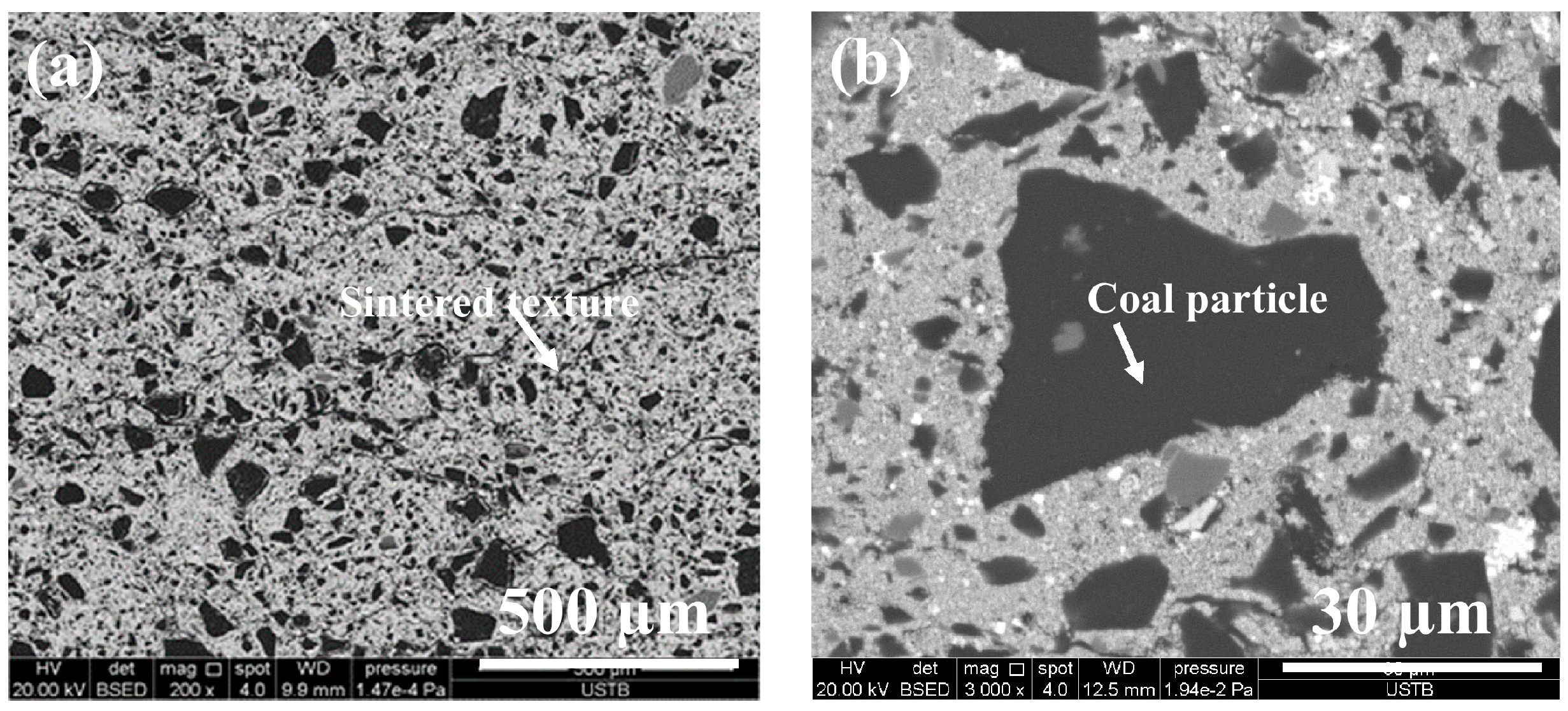
4.1. Characterization of CCB
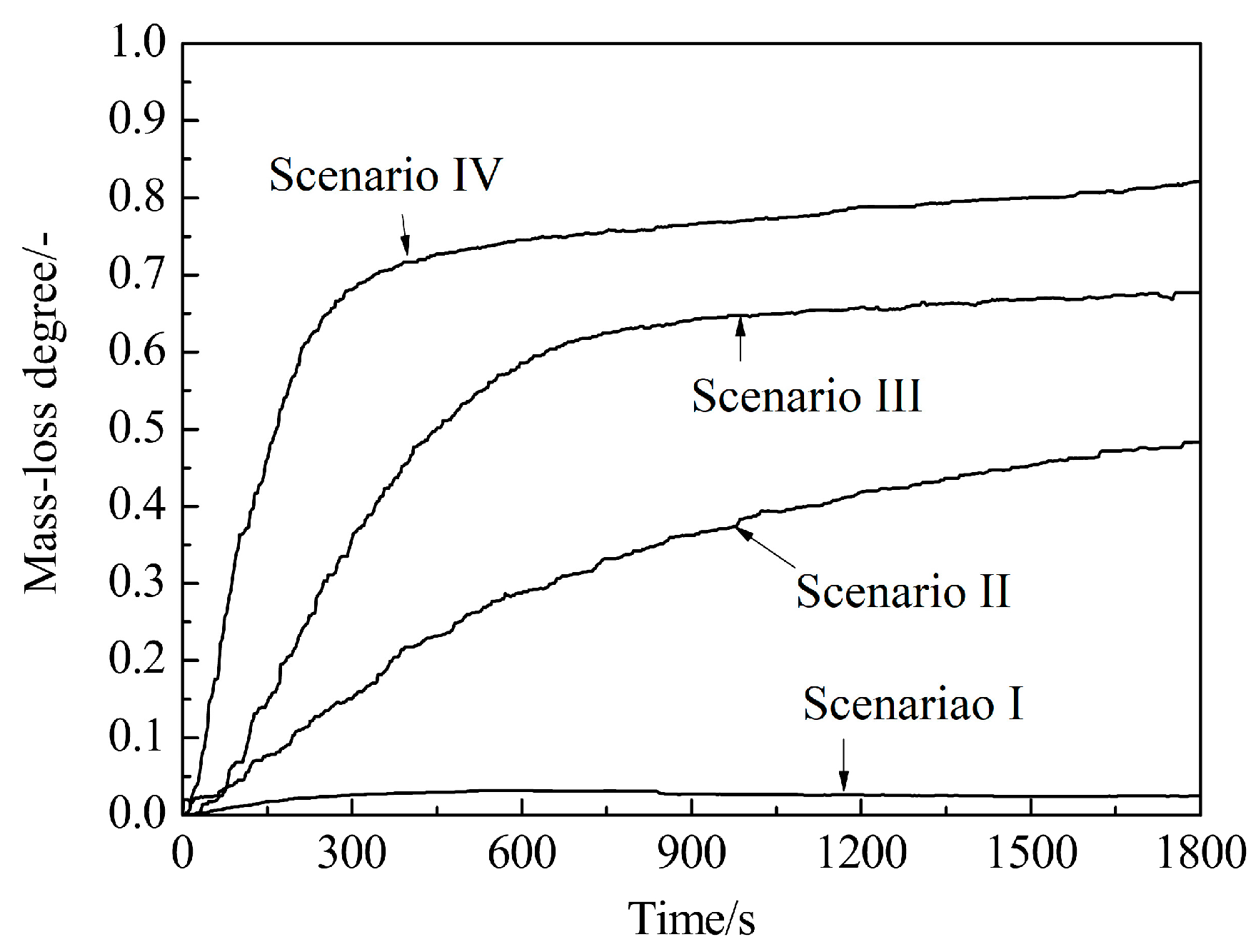
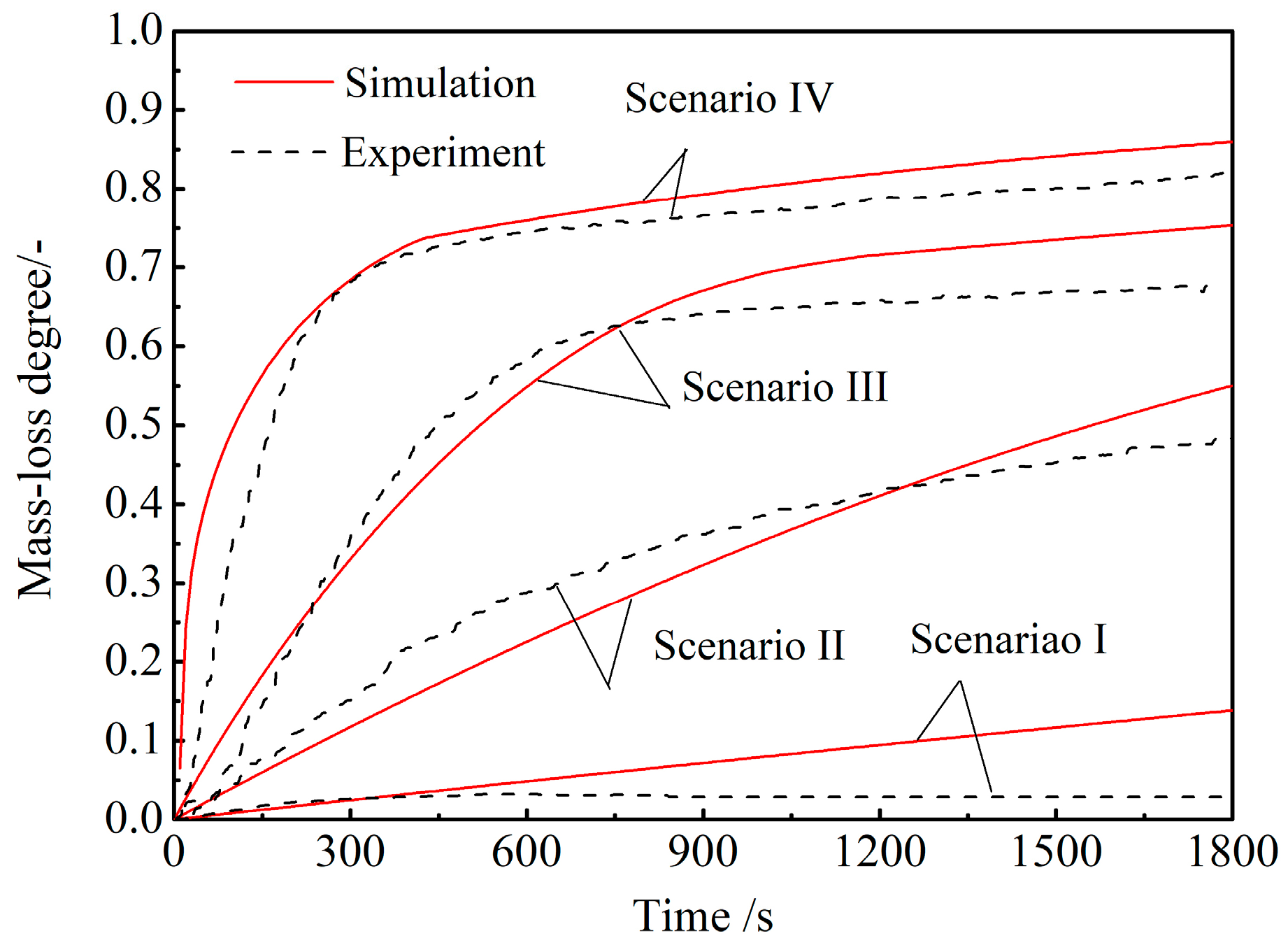
4.2. CCB Model Validation under Simulated BF Conditions
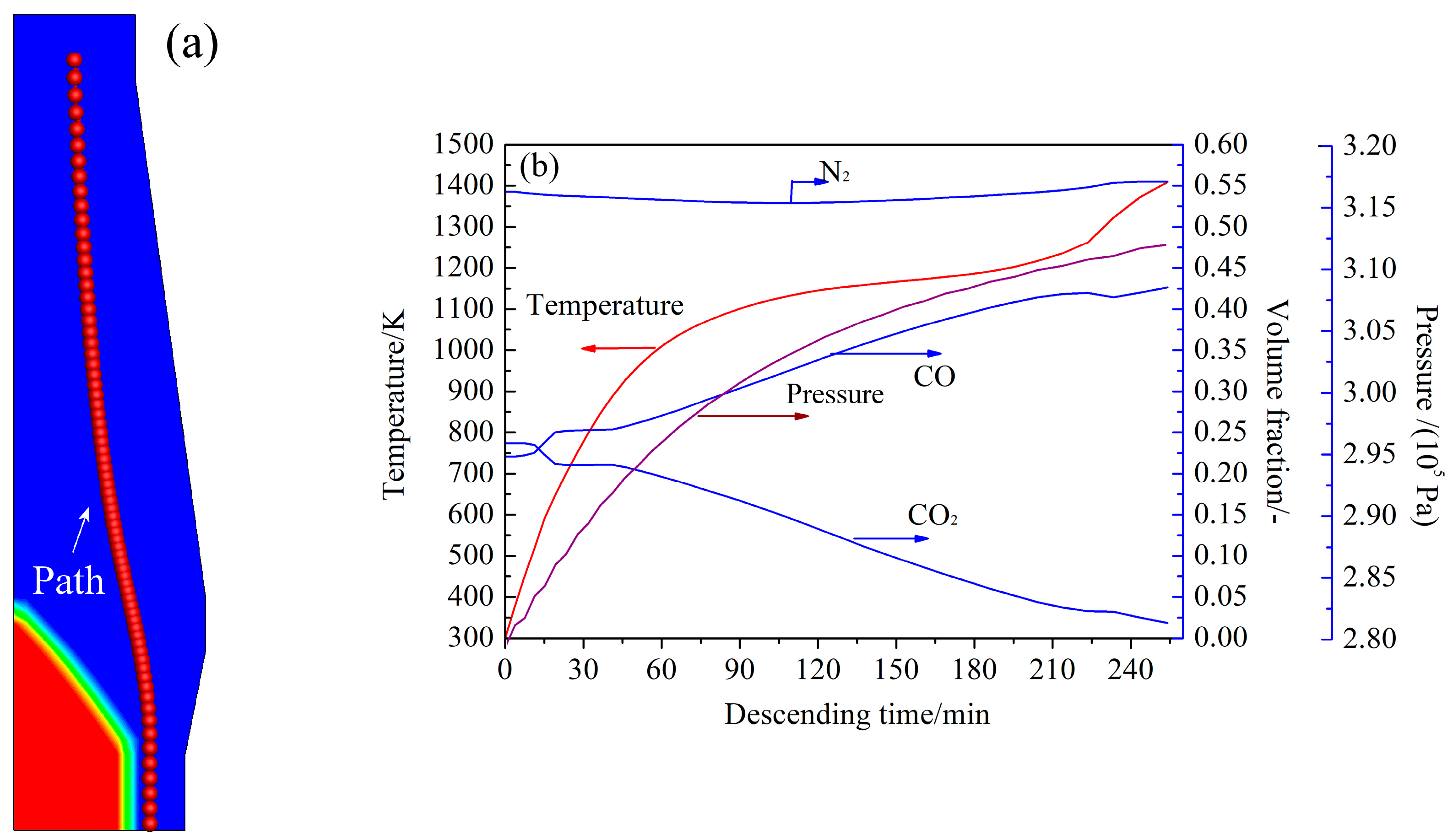
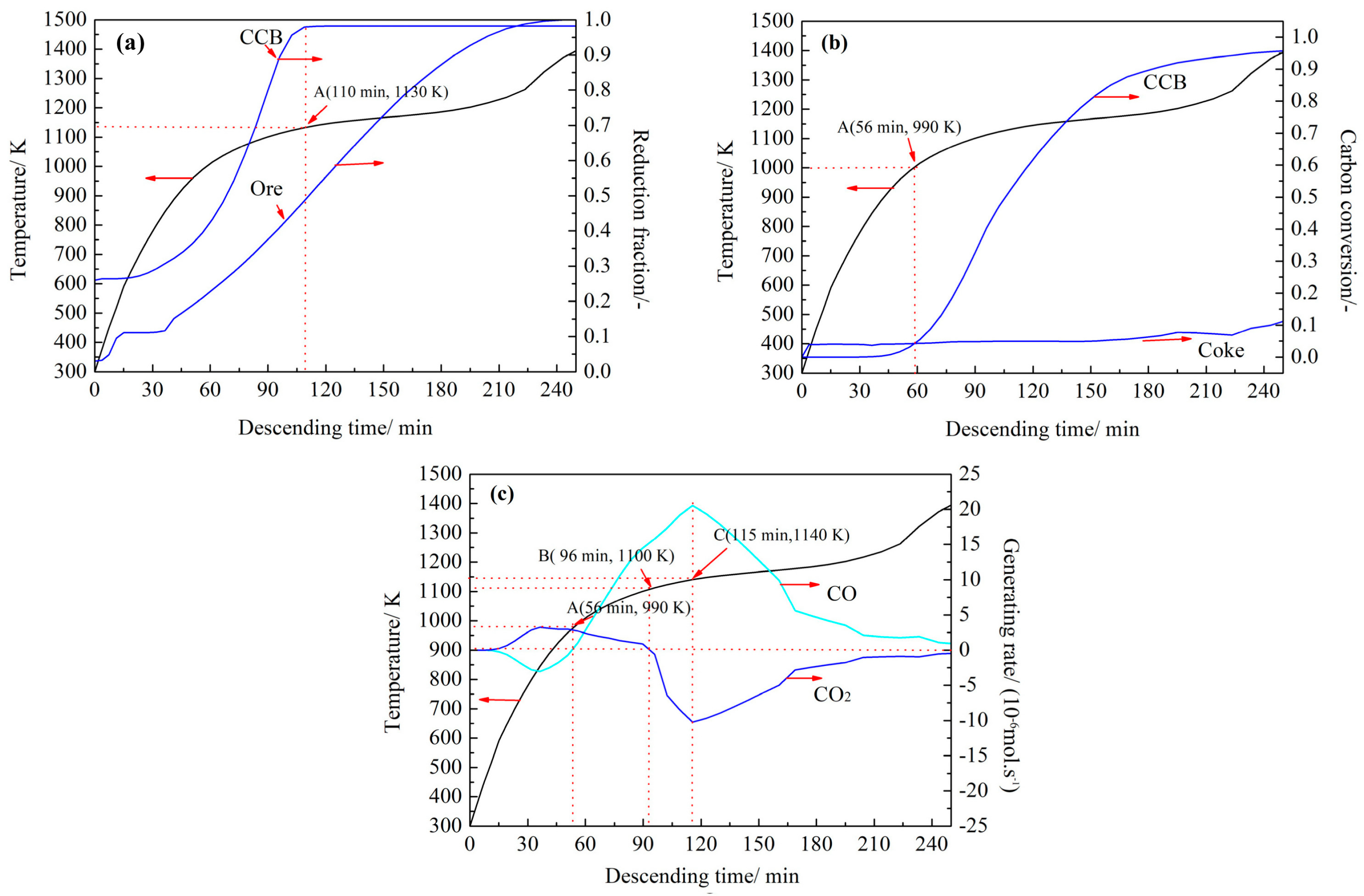
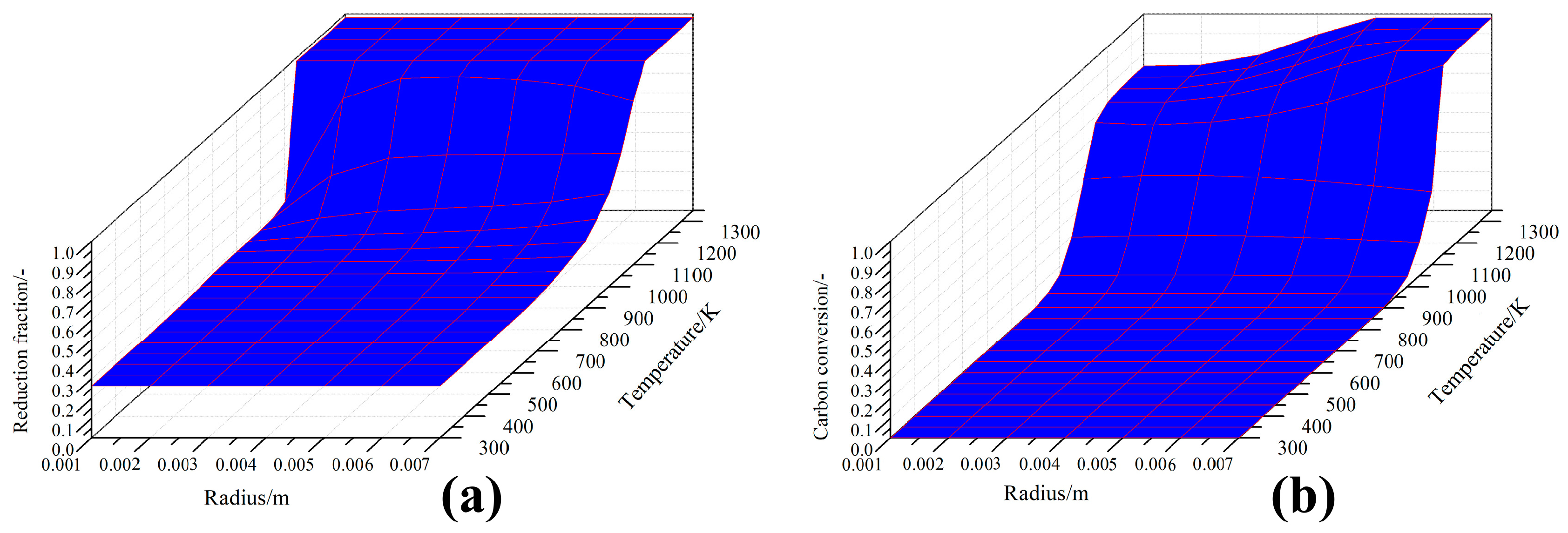
4.3. Simulation of CCB Reaction Behavior in Actual BF
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Table of Symbols | |
| ags | gas–ore interface area, (m2·m−3) |
| d | CCB diameter, 0.015 (m) |
| DCO-N2,DCO2-N2, Deff | gas diffusivity, effective gas diffusivity, (m2·s−1) |
| fi | reaction fraction, (-) |
| ki | reaction rate constant of Reaction i, (m·s−1, s−1·atm−1) |
| Ki | equilibrium constant of Reaction i, (-) |
| M | molar weight, (kg·mol−1) |
| m | mass, (kg) |
| P | pressure, (Pa) |
| r | radial direction, (m) |
| Re | Reynolds number, (-) |
| R | constant, 8.314 (J·mol-1·K-1) |
| Ri | chemical reaction rate of Reaction i, (mol·m−3⋅s−1) |
| Sc | Schemit number, (-) |
| T | temperature, (K) |
| t | time, (s) |
| u | velocity, (m·s−1) |
| µ | viscosity, (kg·m−1·s−1) |
| α | porosity, (-) |
| ρ | density, (kg·m−3) |
| Subscripts | |
| 0 | initial |
| g | gas |
| e | environment |
| Species or element name | variable of assigned species or element |
References
- Geerdes, M.; Chaigneau, R.; Kurunov, I. Modern Blast Furnace Ironmaking: An Introduction; IOS Press: Amsterdam, The Netherlands, 2015; pp. 10–12. ISBN 978-1-61499-498-5. [Google Scholar]
- Song, J.; Jiang, Z.; Bao, C.; Xu, A. Comparison of energy consumption and CO2 emission for three steel production routes—Integrated steel plant equipped with blast furnace, oxygen blast furnace or COREX. Metals 2019, 9, 364. [Google Scholar] [CrossRef]
- Tan, X.; Li, H.; Guo, J.; Gu, B.; Zeng, Y. Energy-saving and emission-reduction technology selection and CO2 emission reduction potential of China’s iron and steel industry under energy substitution policy. J. Clean. Prod. 2019, 222, 823–834. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Viswanathan, N.; Bjorkman, B. Composite pellets–a potential raw material for iron-making. Steel Res. Int. 2014, 85, 293–306. [Google Scholar] [CrossRef]
- Kasai, A.; Toyota, H.; Nozawa, K.; Kitayama, S. Reduction of reducing agent rate in blast furnace operation by carbon composite iron ore hot briquette. ISIJ Int. 2011, 51, 1333–1335. [Google Scholar] [CrossRef]
- Zhao, W.; Chu, M.S.; Wang, H.T.; Liu, Z.G.; Tang, Y.T. Novel blast furnace operation process involving charging with low-titanium vanadium–titanium magnetite carbon composite hot briquette. Int. J. Miner. Metall. Mater. 2016, 23, 501–510. [Google Scholar] [CrossRef]
- El-Hussiny, N.A.; Shalabi, M.E.H. A self-reduced intermediate product from iron and steel plants waste materials using a briquetting process. Powder Technol. 2011, 205, 217–223. [Google Scholar] [CrossRef]
- Flores, B.D.; Guerrero, A.; Flores, I.V.; Borrego, A.G.; Díez, M.A.; Osório, E.; Vilela, A.C.F. On the reduction behavior, structural and mechanical features of iron ore-carbon briquettes. Fuel Process. Technol. 2017, 155, 238–245. [Google Scholar] [CrossRef]
- Watanabe, K.; Ueda, S.; Inoue, R.; Ariyama, T. Enhancement of reactivity of carbon iron ore composite using redox reaction of iron oxide powder. ISIJ Int. 2010, 50, 524–530. [Google Scholar] [CrossRef]
- Kasai, A.; Matsui, Y. Lowering of thermal reserve zone temperature in blast furnace by adjoining carbonaceous material and iron ore. ISIJ Int. 2004, 44, 2073–2078. [Google Scholar] [CrossRef]
- Singh, M.; Björkman, B. Testing of cement-bonded briquettes under laboratory and blast furnace conditions Part 1–Effect of processing parameters. Ironmak. Steelmak. 2007, 34, 30–40. [Google Scholar] [CrossRef]
- Kowitwarangkul, P.; Babich, A.; Senk, D. Reduction behavior of self-reducing pellet (SRP) for low height blast furnace. Steel Res. Int. 2014, 85, 1501–1509. [Google Scholar] [CrossRef]
- Chu, M.; Liu, Z.; Wang, Z.; Yagi, J.I. Fundamental study on carbon composite iron ore hot briquette used as blast furnace burden. Steel Res. Int. 2011, 82, 521–528. [Google Scholar] [CrossRef]
- Matsui, Y.; Sawayama, M.; Kasai, A.; Yamagata, Y.; Noma, F. Reduction behavior of carbon composite iron ore hot briquette in shaft furnace and scope on blast furnace performance reinforcement. ISIJ Int. 2003, 43, 1904–1912. [Google Scholar] [CrossRef]
- Tang, H.; Liu, S.; Rong, T. Preparation of high-carbon metallic briquette for blast furnace application. ISIJ Int. 2019, 59, 22–30. [Google Scholar] [CrossRef]
- Narita, C.Y.B.; Mourao, M.; Takano, C. Development of composite briquettes of iron ore and coal hardened by heat treatment. Ironmak. Steelmak. 2015, 42, 548–552. [Google Scholar] [CrossRef]
- Yokoyama, H.; Higuchi, K.; Ito, T.; Oshio, A. Decrease in carbon consumption of a commercial blast furnace by using carbon composite iron ore. ISIJ Int. 2012, 52, 2000–2006. [Google Scholar] [CrossRef]
- Kemppainen, A.; Iljana, M.; Heikkinen, E.P.; Paananen, T.; Mattila, O.; Fabritius, T. Reduction behavior of cold-bonded briquettes under simulated blast furnace conditions. ISIJ Int. 2014, 54, 1539–1545. [Google Scholar] [CrossRef]
- Mochizuki, Y.; Tsubouchi, N.; Akiyama, T. Investigation of strength and reduction reactivity during heat treatment in simulated-experimental blast furnace of carbon-containing pellet prepared by vapor deposition of tar to cold-bonded pellet with large particle size. Fuel Process. Technol. 2018, 176, 21–32. [Google Scholar] [CrossRef]
- Man, Y.; Feng, J.X.; Li, F.J.; Ge, Q.; Chen, Y.M.; Zhou, J.Z. Influence of temperature and time on reduction behavior in iron ore–coal composite pellets. Powder Technol. 2014, 256, 361–366. [Google Scholar] [CrossRef]
- Liu, C.; Ding, X.; Dai, J.; Tang, Z.; Dong, Y. Comprehensive CFD modeling of reduction behavior inside coal-based composite pellets. Ironmak. Steelmak. 2019, 1–10. [Google Scholar] [CrossRef]
- Sun, K.; Lu, W.K. Mathematical modeling of the kinetics of carbothermic reduction of iron oxides in ore-coal composite pellets. Metall. Mater. Trans. B 2009, 40, 91–103. [Google Scholar] [CrossRef]
- Shi, J.; Donskoi, E.; McElwain, D.L.S.; Wibberley, L.J. Modelling the reduction of an iron ore-coal composite pellet with conduction and convection in an axisymmetric temperature field. Math. Comput. Model. 2005, 42, 45–60. [Google Scholar] [CrossRef]
- Donskoi, E.; McElwain, D.L.S. Mathematical modeling of no-isothermal reduction in highly swelling iron ore-coal char composite pellet. Ironmak. Steelmak. 2001, 28, 384–389. [Google Scholar] [CrossRef]
- Tang, H.; Yun, Z.; Fu, X.; Du, S. Modeling and experimental study of ore-carbon briquette reduction under CO–CO2 atmosphere. Metals 2018, 8, 205. [Google Scholar] [CrossRef]
- Chu, M.; Nogami, H.; Yagi, J.I. Numerical analysis on charging carbon composite agglomerates into blast furnace. ISIJ Int. 2004, 44, 510–517. [Google Scholar] [CrossRef]
- Ueda, S.; Yanagiya, K.; Watanabe, K.; Murakami, T.; Inoue, R.; Ariyama, T. Reaction model and reduction behavior of carbon iron ore composite in blast furnace. ISIJ Int. 2009, 49, 827–836. [Google Scholar] [CrossRef]
- Tien, R.H.; Turkdogan, E.T. Gaseous reduction of iron oxides: Part IV. Mathematical analysis of partial internal reduction-diffusion control. Metall. Trans. 1972, 3, 2039–2048. [Google Scholar] [CrossRef]
- Natsui, S.; Kikuchi, T.; Suzuki, R.O. Numerical analysis of carbon monoxide–hydrogen gas reduction of iron ore in a packed bed by an Euler–Lagrange approach. Metall. Mater. Trans. B 2014, 45, 2395–2413. [Google Scholar] [CrossRef]
- Leffler, A.J. Determination of effective diffusivities of catalysts by gas chromatography. J. Catal. 1966, 5, 22–26. [Google Scholar] [CrossRef]
- Ge, Q. Kinetics of Gas-Solid Reactions, 1st ed.; Nuclear Energy Press: Beijing, China, 1991; pp. 9–12. ISBN 7502204008. [Google Scholar]
- Guo, K.L.; Kong, X.; Chen, S. Numerical Heat Transfer, 1st ed.; China Science & Technology University Press: Hefei, China, 1988; pp. 42–48. ISBN 7-312-00038. [Google Scholar]
- Turkdogan, E.T. Blast furnace reactions. Metall. Mater. Trans. B 1978, 9, 163–179. [Google Scholar] [CrossRef]
- Tang, H.; Rong, T.; Fan, K. Numerical investigation of applying high-carbon metallic briquette in blast furnace ironmaking. ISIJ Int. 2019, 59, 810–819. [Google Scholar] [CrossRef]

| Proximate Analysis (* ad, wt.%) | Element Analysis (* daf, wt.%) | Ash Analysis (wt.%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Volatile | * FC | Ash | Moisture | C | H | O | N | S | SiO2 | Al2O3 | CaO | Fe2O3 | Other |
| 17.87 | 71.71 | 9.25 | 1.17 | 89.44 | 3.06 | 5.92 | 1.18 | 0.40 | 46.7 | 35.0 | 7.59 | 4.8 | 1.54 |
| No. | Reaction | Reaction Rate (mol·m−3·s) | Ref. |
|---|---|---|---|
| 1 | (i = 1,2,3), , , , , | [27,28,29] | |
| 2 | |||
| 3 | |||
| 4 | [15,25] |
| Carbon | Magnetite | Wüstite | Metallic Iron | Gangue |
|---|---|---|---|---|
| 20.30 | 29.70 | 39.70 | 1.57 | 8.73 |
| Scenario | Temperature (K) | CO2 (vol.%) | CO (vol.%) | N2 (vol.%) |
|---|---|---|---|---|
| I | 1073 | 20 | 30 | 50 |
| II | 1173 | 15 | 35 | 50 |
| III | 1273 | 10 | 40 | 50 |
| IV | 1373 | 10 | 40 | 50 |
| Item | Reduction Fraction | Carbon Conversion | ||||||
|---|---|---|---|---|---|---|---|---|
| Scenario | I | II | III | VI | I | II | III | IV |
| Measured | 0.30 | 0.53 | 0.84 | 0.90 | 0.05 | 0.18 | 0.53 | 0.65 |
| Model-predicted | 0.39 | 0.88 | 0.98 | 0.98 | 0.10 | 0.30 | 0.56 | 0.70 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, H.; Sun, Y.; Rong, T. Experimental and Numerical Investigation of Reaction Behavior of Carbon Composite Briquette in Blast Furnace. Metals 2020, 10, 49. https://doi.org/10.3390/met10010049
Tang H, Sun Y, Rong T. Experimental and Numerical Investigation of Reaction Behavior of Carbon Composite Briquette in Blast Furnace. Metals. 2020; 10(1):49. https://doi.org/10.3390/met10010049
Chicago/Turabian StyleTang, Huiqing, Yanjun Sun, and Tao Rong. 2020. "Experimental and Numerical Investigation of Reaction Behavior of Carbon Composite Briquette in Blast Furnace" Metals 10, no. 1: 49. https://doi.org/10.3390/met10010049
APA StyleTang, H., Sun, Y., & Rong, T. (2020). Experimental and Numerical Investigation of Reaction Behavior of Carbon Composite Briquette in Blast Furnace. Metals, 10(1), 49. https://doi.org/10.3390/met10010049





