Reaction Behaviors of Associated Minerals in Molten Salt Smelting of Stibnite and Kilogram-Class Trials
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Methods and Procedures
2.2.1. Laboratory Experiments
2.2.2. Kilogram-ClassPilot Tests
3. Results and Discussion
3.1. Thermogravimetric Analysis
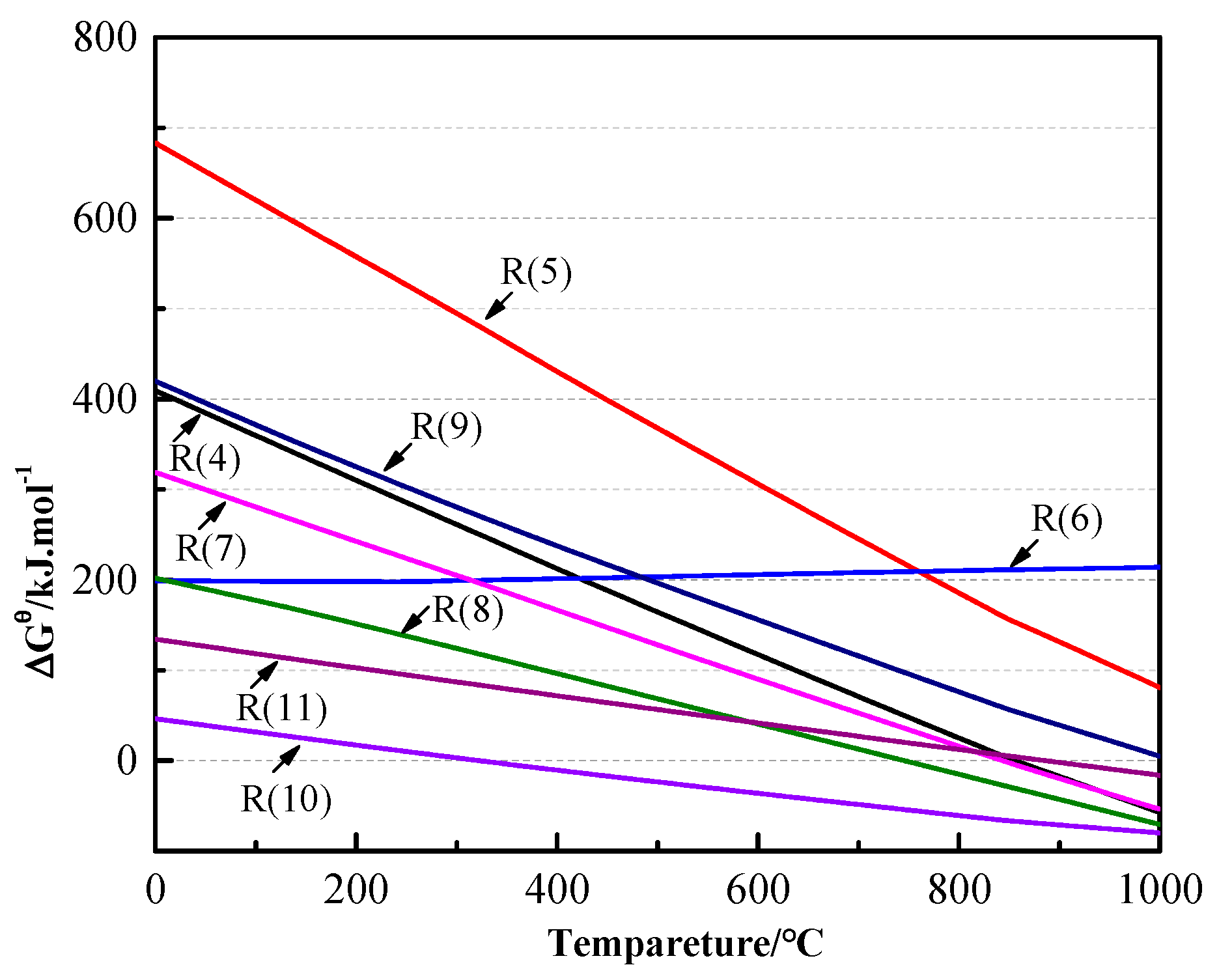
3.2. Thermodynamic Analysis
3.3. Process Analysis
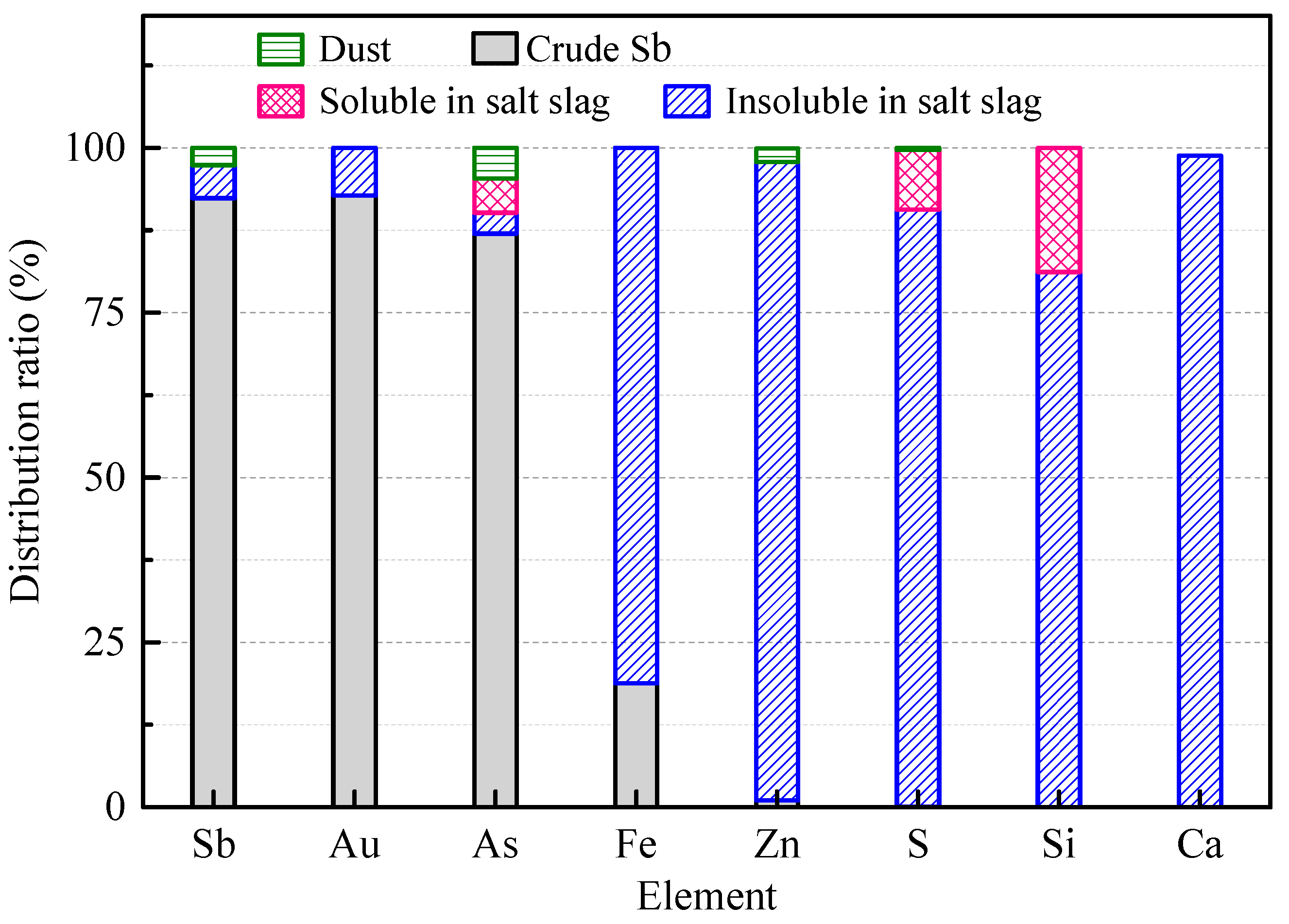
3.4. Kilogram-Class Pilot Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gierlotka, W. Thermodynamic description of the binary Au-Sb and ternary Au-In-Sb systems. J. Alloys Compd. 2013, 579, 533–539. [Google Scholar] [CrossRef]
- Anderson, C.G. The metallurgy of antimony. Chem. Erde-Geochem. 2012, 72, 3–8. [Google Scholar] [CrossRef]
- Yang, J.G.; Tang, C.B.; Chen, Y.M.; Tang, M.T. Separation of antimony from a stibnite concentrate through a low-temperature smelting process to eliminate SO2 emission. Metall. Mater. Trans. B 2011, 42, 30–36. [Google Scholar] [CrossRef]
- Habashi, F. Handbook of Extractive Metallurgy, Volume II, 1st ed.; WILEY-VCH: Weinheim, Germany, 1997. [Google Scholar]
- Awe, S.A.; Sundkvist, J.E.; Bolin, N.J.; Sandstrom, A. Process flowsheet development for recovering antimony from Sb-bearing copper concentrates. Miner. Eng. 2013, 49, 45–53. [Google Scholar] [CrossRef]
- Awe, S.A.; Sandstrom, A. Selective leaching of arsenic and antimony from a tetrahedrite rich complex sulphide concentrate using alkaline sulphide solution. Miner. Eng. 2010, 23, 1227–1236. [Google Scholar] [CrossRef]
- Lie, X.Q.; Zhong, Q.Y.; Lin, S.Y.; Chu, S.Z. Antimony white production from antimony sulfide ore using acidic solution of antimony pentachloride as lixiviant. J. Cent. South. Inst. Min. Metall. 1990, 21, 615–621. [Google Scholar]
- Yang, J.G.; Wu, Y.T. A hydrometallurgical process for the separation and recovery of antimony. Hydrometallurgy 2014, 143, 68–74. [Google Scholar] [CrossRef]
- Mahlangu, T.; Gudyanga, F.P.; Simbi, D.J. Reductive leaching of stibnite (Sb2S3)flotation concentrates using metallic iron in a hydrochloric acid medium II: Kinetics. Hydrometallurgy 2007, 88, 132–142. [Google Scholar] [CrossRef]
- Xie, Z.F.; Li, B.; Liu, W.L.; Li, H.; Jiang, C.J.; Huang, H.F. A new process for preparation of qualified antimony white from jamesonite. Min. Metall. Eng. 2015, 35, 80–84. [Google Scholar]
- Wang, Q.M.; Guo, X.Y.; Tian, Q.H.; Jiang, T.; Chen, M.; Zhao, B.J. Effects of Matte grade on the distribution of minor elements (Pb, Zn, As, Sb, and Bi) in the bottom blown copper smelting process. Metals 2017, 7, 502. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.M.; Guo, X.Y.; Tian, Q.H.; Chen, M.; Zhao, B.J. Reaction mechanism and distribution behavior of arsenic in the bottom blown copper smelting process. Metals 2017, 7, 302. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Luo, H.L.; Qing, W.Q.; Zheng, Y.X.; Yang, K.; Han, J.W. Investigation into oxygen-enriched bottom-blown stibnite and direct reduction. Metall. Mater. Trans. B 2014, 45, 1281–1290. [Google Scholar] [CrossRef]
- Padilla, R.; Chambi, L.C.; Ruiz, M.C. Antimony production by carbothermic reduction of stibnite in presence of lime. J. Min. Metall. Sect. B-Metall. 2014, 50, 5–13. [Google Scholar] [CrossRef]
- Padilla, R.; Aracena, A.; Ruiz, M.C. Kinetics of stibnite (Sb2S3) oxidation at roasting temperatures. J. Min. Metall. Sect. B-Metall. 2014, 50, 127–132. [Google Scholar] [CrossRef] [Green Version]
- Smirnov, M.P. Direct smelting of lead at low temperature. Nonferr. Met. 1990, 46, 34–36. [Google Scholar]
- Efim, V.M. Low temperature smelting of lead metallic scrap. Erzmetall 2000, 53, 85–89. [Google Scholar]
- Kokoro, I.; Shu, Y.; Masafumi, M. Phase relation and thermodynamic properties of NaCl-Na2CO3 system as a basic system for secondary fly ash incineration processes of municipal wastes. Mater. Trans. 2001, 42, 2480–2486. [Google Scholar]
- Olivarea, R.I.; Chen, C.L.; Wright, S. Thermal stability of molten lithium-sodium-potassium carbonate and influence of additives on the melting point. J. Sol. Energy Eng. 2012, 134, 041002. [Google Scholar] [CrossRef]
- Wang, T.; Mantha, D.; Reddy, R.G. Thermal stability of the eutectic composition in LiNO3-NaNO3-KNO3 ternary system used for thermal energy storage. Sol. Energy Mater. Sol. Cells 2012, 100, 162–168. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Wu, Z.G. Thermal property characterization of a low melting-temperature ternary nitrate salt mixture for thermal energy storage systems. Sol. Energy Mater. Sol. Cells 2011, 96, 3341–3346. [Google Scholar] [CrossRef]
- Ye, L.G.; Tang, C.B.; Chen, Y.M.; Yang, S.H.; Yang, J.G.; Zhang, W.H. One-step extraction of antimony from low-grade stibnite in Sodium Carbonate e Sodium Chloride binary molten salt. J. Clean. Prod. 2015, 93, 134–139. [Google Scholar] [CrossRef]
- Chen, Y.M.; Ye, L.G.; Tang, C.B.; Yang, S.H.; Tang, M.T.; Zhang, W.H. Solubility of Sb in binary Na2CO3-NaCl molten salt. Trans. Nonferr. Met. Soc. China 2015, 25, 3146–3151. [Google Scholar] [CrossRef]
- Jun, Y.; Katsunari, O.; Koichi, A. Thermodynamic assessment of the KCl-K2CO3-NaCl-Na2CO3 system. Calphad 2007, 31, 155–163. [Google Scholar]
- Lu, W.Y.; Zeng, C.L. Preparation of cohesive graphite films by electroreduction of CO32− in molten Na2CO3-NaCl. Surf. Coat. Technol. 2012, 206, 4287–4292. [Google Scholar]
- Jun, Y.; Daisuke, M.; Koichi, A.; Youji, Y.; Hiroshi, Y. Strength of salt core composed of alkali carbonate and alkali chloride mixtures made by casting technique. Mater. Trans. 2007, 48, 1034–1041. [Google Scholar]
- Kim, J.W.; Lee, H.E. Thermal and carbothermic decomposition of Na2CO3 and Li2CO3. Metall. Mater. Trans. B 2001, 32, 17–24. [Google Scholar] [CrossRef]
- Lewis, A.E.; Beautment, C. Prioritising objectives for waste reprocessing: A case study in secondary lead refining. Waste Manag. 2002, 22, 277–285. [Google Scholar] [CrossRef]
- Ye, L.G.; Tang, C.B.; Chen, Y.M.; Yang, S.H.; Tang, M.T. The thermal physical properties and stability of the eutectic composition in a Na2CO3-NaCl binary system. Thermochim. Acta 2014, 596, 14–20. [Google Scholar] [CrossRef]
- Brain, I.; Knacke, O.; Kubaschewski, O. Thermochemical Properties of Inorganic Substance, 1st ed.; Springer: New York, NY, USA, 1972. [Google Scholar]
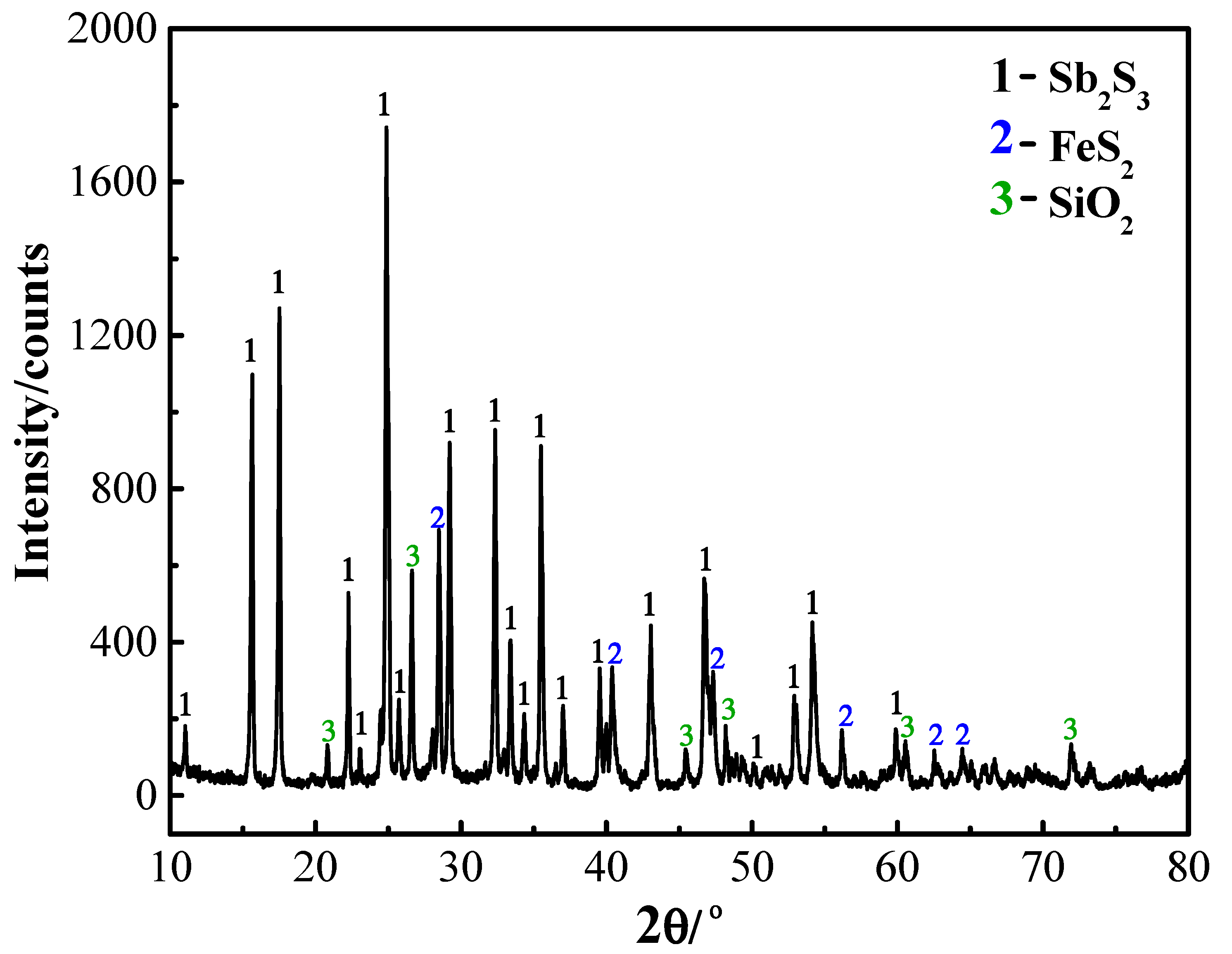
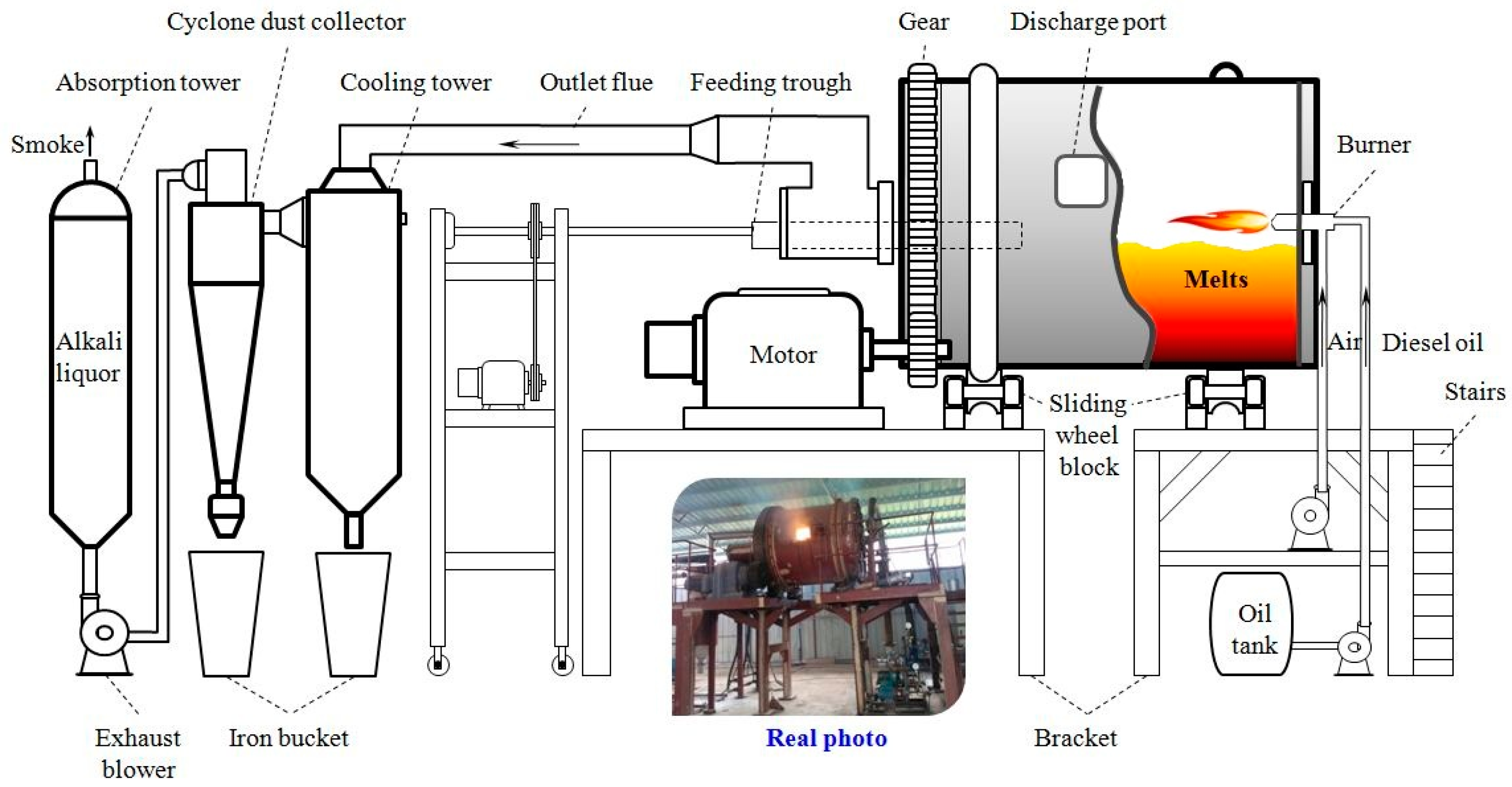


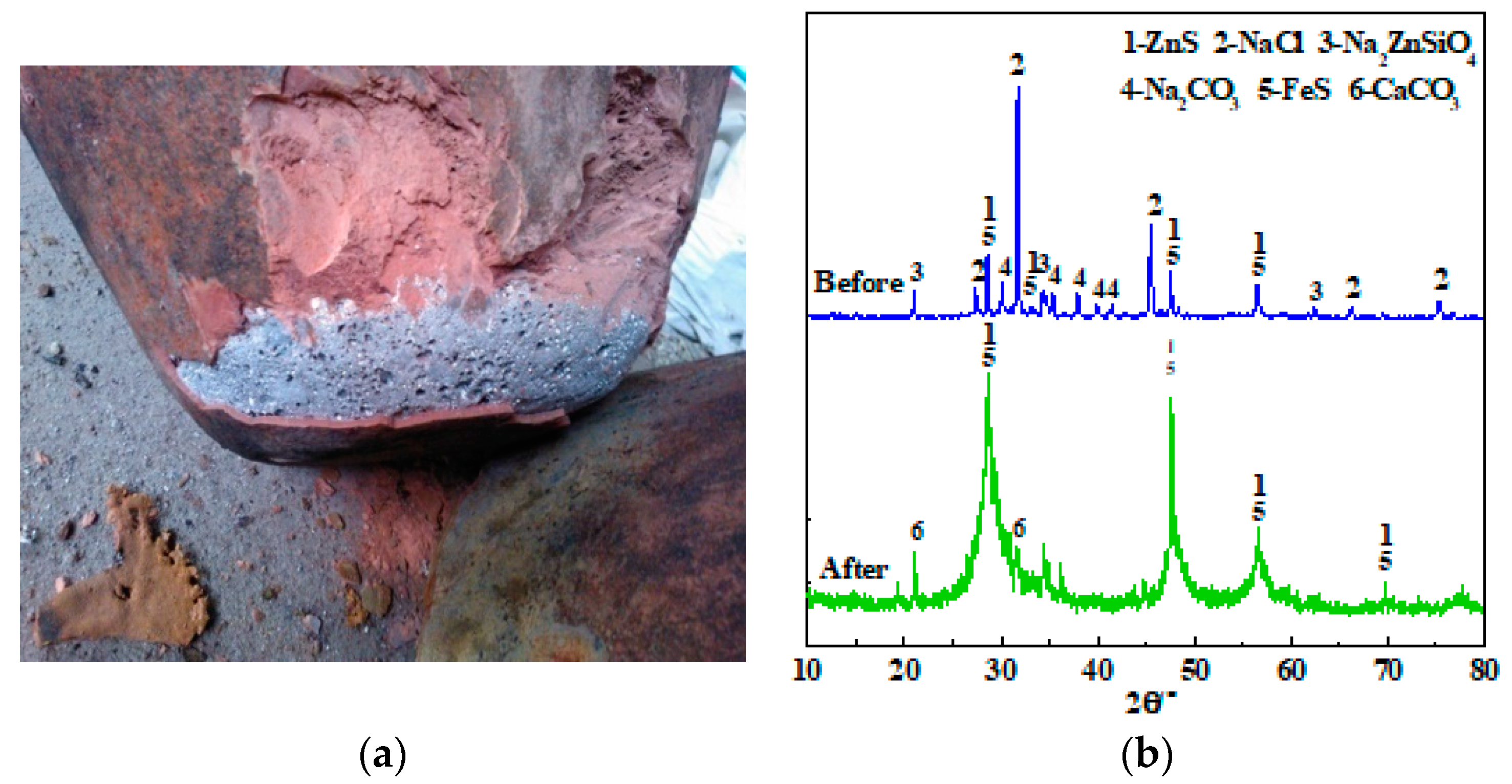
| Elements | Sb | S | Fe | Pb | Cu | As | Bi | Au/(g/t) | SiO2 | CaO |
|---|---|---|---|---|---|---|---|---|---|---|
| Content (wt%) | 48.08 | 25.13 | 5.14 | 0.28 | 0.039 | 0.50 | 0.0037 | 101.05 | 12.14 | 0.90 |
| Composition | C | Volatile | Ash | Other |
|---|---|---|---|---|
| Content (wt%) | 70.00 | 12.00 | 10.00 | 8.00 |
| Reaction | Number |
|---|---|
| 2PbS + 2Na2CO3 + C = 2Pb + 2Na2S + 3CO2(g) | (4) |
| 4PbS + 4Na2CO3 = 4Pb + 3Na2S + Na2SO4 + 4CO2(g) | (5) |
| PbS + Na2SO4 = PbSO4 + Na2S | (6) |
| PbSO4 + PbS = 2Pb + 2SO2(g) | (7) |
| 2FeS2 = 2FeS + S2(g) | (8) |
| 2FeS + 2Na2CO3 + C = 2Na2S + 2Fe + 3CO2(g) | (9) |
| SiO2 + Na2CO3 = Na2SiO3 + CO2(g) | (10) |
| CaCO3 = CaO + CO2(g) | (11) |
| Test No. | Conditions | Metal | Molten Salt Slag | Smoke | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na2CO3 (kg) | NaCl (kg) | ZnO (times) | Carbon (kg) | Temperature (°C) | Time (h) | Sb (pct) | Au (g/t) | DRR of Sb (%) | DRR of Au (%) | Sb (pct) | Au (g/t) | SFR (%) | VR of Sb (%) | |
| 1 | 145.00 | 5.00 | 1.00 | 7.50 | 920 | 5 | 89.00 | 143 | 10.23 | 7.83 | 11.48 | 24 | 81.03 | 3.83 |
| 2 | 120.00 | 30.00 | 1.00 | 7.50 | 920 | 8 | 93.76 | 166 | 58.20 | 49.04 | 6.23 | 17 | 94.99 | 4.12 |
| 3 | 85.50 | 64.50 | 1.00 | 7.50 | 920 | 8 | 94.24 | 202 | 76.54 | 78.16 | 3.40 | 7 | 96.20 | 4.03 |
| 4 | 70.00 | 80.00 | 1.00 | 7.50 | 920 | 8 | 93.11 | 212 | 70.38 | 76.22 | 4.40 | 8 | 96.06 | 3.95 |
| 5 | 85.50 | 64.50 | 1.20 | 7.50 | 920 | 8 | 84.36 | 195 | 68.27 | 75.19 | 4.19 | 8 | 98.75 | 4.69 |
| 6 | 85.50 | 64.50 | 1.00 | 10.00 | 920 | 8 | 92.09 | 201 | 77.19 | 80.24 | 3.24 | 8 | 98.35 | 4.55 |
| 7 | 85.50 | 64.50 | 1.00 | 10.00 | 970 | 8 | 94.14 | 207 | 79.44 | 83.15 | 2.69 | 6 | 97.28 | 5.18 |
| 8 | 85.50 | 64.50 | 1.00 | 10.00 | 970 | 12 | 92.58 | 207 | 80.03 | 85.17 | 2.38 | 5 | 98.03 | 6.40 |
| 9 | 114.00 | 86.00 | 1.00 | 10.00 | 970 | 8 | 95.13 | 205 | 87.06 | 89.30 | 1.24 | 4 | 98.72 | 4.76 |
| 10 | 142.50 | 107.50 | 1.00 | 10.00 | 920 | 8 | 96.42 | 200 | 93.22 | 92.06 | 0.46 | 2 | 99.30 | 4.02 |
| 11 | 142.50 | 107.50 | 1.00 | 10.00 | 870 | 10 | 95.58 | 202 | 92.37 | 92.77 | 0.48 | 2 | 99.49 | 2.65 |
| Product | Composition (Solid: %; Solution: mg/L) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sb | Fe | S | Zn | As | Au | Na | Si | Ca | Al | |
| Crude Sb | 95.58 | 2.08 | 0.05 | 0.95 | 0.89 | 202 | - | - | - | - |
| Residue | 2.34 | 13.14 | 25.44 | 38.01 | 0.003 | 6 | 3.17 | 6.19 | 0.14 | 2.17 |
| Lixivium | 48.89 | - | 1338.00 | 106.00 | 2.50 | - | >3000 | 613.00 | - | 21.39 |
| Dust | 33.52 | 0.75 | 3.15 | 6.88 | 0.13 | - | 20.47 | 1.20 | 1.06 | 0.79 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, Z.; Ye, L.; Tang, C.; Xin, Y. Reaction Behaviors of Associated Minerals in Molten Salt Smelting of Stibnite and Kilogram-Class Trials. Metals 2020, 10, 43. https://doi.org/10.3390/met10010043
Ouyang Z, Ye L, Tang C, Xin Y. Reaction Behaviors of Associated Minerals in Molten Salt Smelting of Stibnite and Kilogram-Class Trials. Metals. 2020; 10(1):43. https://doi.org/10.3390/met10010043
Chicago/Turabian StyleOuyang, Zhen, Longgang Ye, Chaobo Tang, and Yuntao Xin. 2020. "Reaction Behaviors of Associated Minerals in Molten Salt Smelting of Stibnite and Kilogram-Class Trials" Metals 10, no. 1: 43. https://doi.org/10.3390/met10010043
APA StyleOuyang, Z., Ye, L., Tang, C., & Xin, Y. (2020). Reaction Behaviors of Associated Minerals in Molten Salt Smelting of Stibnite and Kilogram-Class Trials. Metals, 10(1), 43. https://doi.org/10.3390/met10010043




