Abstract
The molten salt metallurgy of Sb, which involves the smelting of stibnite in a binary NaCl-Na2CO3 salt with sulfur-fixing and the addition of a reductant, has been proposed as a clean method for Sb extraction. However, the reacting behaviors of the minerals associated with stibnite (Sb2S3) during the smelting are still unclear, and industrial tests have not been conducted. This study investigated the behaviors of PbS, FeS2, SiO2, and CaCO3, which are the main minerals associated with stibnite, during reducing smelting by using the NaCl-Na2CO3 molten salt. The results showed that PbS could react with Na2CO3 to generate metallic Pb at 950 °C. FeS2 and SiO2 formed stable NaFeS2 and Na2SiO3 with the molten salt at a high temperature, respectively. CaCO3 formed an unstable intermediate product of Na2Ca(CO3)2 at 675 °C and decomposed with increasing temperature. Kilogram-class trials were also performed using 50 kg of concentrate and more than 300 kg of mixture material, and the results showed that the direct recovery rate of Sb and Au reached maximum values of 93.22% and 92.06% at temperature 920 °C in eutectic Na2CO3-NaCl molten salt, respectively, while the total sulfur-fixing ratio reached 99.49%. Thus, the associated minerals consumed the molten salt, and the feasibility of molten salt smelting was verified by this kilogram-class pilot experiment.
1. Introduction
Sb is mainly used for manufacturing alloys, semiconductor optoelectronic devices, and Sb compounds [1,2]. More than 80% of the world’s Sb is produced in China [3]. Stibnite (Sb2S3)and jamesonite (Pb4FeSb6S14) are the two main raw ores used for Sb smelting [3,4], and a pyrometallurgical process is the primary method used to extract Sb. Smelting under high temperatures (1100–1350 °C) has many shortcomings, including the consumption of large amounts of energy and materials, the consumption of refractory materials, and environmental pollution from the volatilization of Pb and As, as well as a low concentration of SO2, it cannot be used to make acid and required plenty of alkaline solution for absorption. At present, the research on green Sb extraction includes hydrometallurgical and pyrometallurgical processes. In the field of hydrometallurgy, NaOH and Na2S [5,6] have been investigated as leaching agents to leach Sb sulfide ore, after which the Sb metal can be obtained by electrolysis. This method has been attributed to alkaline leaching. In addition, acidic agents, including SbCl5-HCl and FeCl3-HCl [7,8,9,10], have been used to leach Sb sulfide ore, after which Sb was produced using diaphragm electrowinning. A low current efficiency and serious equipment corrosion were the main barriers in this hydrometallurgical process. At present, only a few plants have adopted it to produce Sb white.
In the field of pyrometallurgy, intensified smelting, using oxygen enrichment as an oxidant, was proposed to improve the traditional Sb extraction process [11,12,13]. These methods can improve the SO2 concentration in the flue gas and allow it to meet the acid production requirement. But the problems of flue bonding and the dispersion of valuable metals remain unsolved. Currently, many Sb smelteries are small, the acid-making process is a large investment for them. Therefore, sulfur-fixing roasting and smelting based on the difference between the sulphophile affinity of Sb and those of other metals were investigated. Padilla [14,15] researched the carbon reduction of stibnite to produce Sb oxide and metallic Sb using CaO as a sulfur-fixing agent. This method solved the problem of SO2 flue gas pollution in the traditional process. While the separation of the calcium sulfide and Sb produced by the reaction is difficult, and calcium sulfide is not easy to recover and utilize. Thus, sulfur-fixing had the advantage of free SO2 emission, and the low-temperature and short-process extraction was also an important development in pyrometallurgy. Smirnov [16] first proposed alkaline smelting in molten NaOH to extract Pb from PbS ore at low temperatures of 400–900 °C. Margulis [17] also adopted molten NaOH to smelt metallic Pb scrap at 400 °C. The outstanding advantages of this process were its low temperature, work condition improvement, and reduction of flue gas emissions (95% less than the conventional process).
In the previous alkaline smelting, no sulfur-fixing agent was added, and a single NaOH molten salt was used. Thus, the NaOH participated in the reactions and could not be recovered. Additionally, because the molten salt exhibited excellent properties, including good thermal stability and conductivity [18,19] and a low melting point, it has been widely applied in molten salt electrolysis and solar power cells [20,21]. Therefore, our team investigated a sulfur-fixing smelting method, in which Sb sulfide was smelted in an NaCl-Na2CO3 binary molten salt with ZnO added as a sulfur fixing agent. Sulfur was fixed in the form of ZnS, but the molten salt itself hardly participated in the smelting reaction and could be returned for recycling. A large amount of Zn oxide ash is produced in Pb-Zn smeltery and steel plants. Although it is an important secondary resource, it is difficult to recover because of the presence of high amounts of F and Cl. Thus, using this secondary ZnO ash as a sulfur-fixing agent is a comprehensive way to utilize resources and produce ZnS, which can be returned to the Zn smelter. Our team investigated the molten salt smelting of stibnite in the laboratory, and the Sb generation rate and sulfur-fixing rate were both higher than 90% [22,23]. This new method is a low-carbon and environmentally friendly process.
Generally, stibnite has a variety of associated sulfides and gangues, and the behaviors of these components were unclear in the smelting process. Thus, this study had the goal of understanding the behaviors of the associated minerals, including PbS, FeS2, SiO2, and CaCO3, during the molten salt smelting of stibnite based on thermodynamic calculations and X-ray diffraction (XRD) and Thermogravimetric-differential scanning calorimetry (TG-DSC) detection. Finally, a kilogram-class pilot test was performed in a rotary furnace to further verify the feasibility of the process.
2. Experimental
2.1. Materials
In the experiments conducted during the reaction behavior research, all reagents, including Na2CO3, NaCl, PbS, FeS2, SiO2, CaCO3, and carbon powder, were of analytical grade, and all of them were dried in an oven at 60 °C for 24 h. An Na2CO3-NaCl binary salt eutectic composition was used [22]. The Au-bearing Sb sulfide concentrate used in the kilogram-class pilot experiments was provided by Chenzhou Mining (Hunan, China). It contained 48.08% Sb and Au at 101.05 g/t, and its chemical composition and XRD pattern are provided in Table 1 and Figure 1, respectively. It can be seen that the main phases are Sb2S3, FeS2, and SiO2. Other auxiliary materials used in the pilot test included industrial-grade Na2CO3, NaCl, and ZnO. The main components of reductant coal are listed in Table 2.

Table 1.
Chemical composition of central south stibnite concentrate.
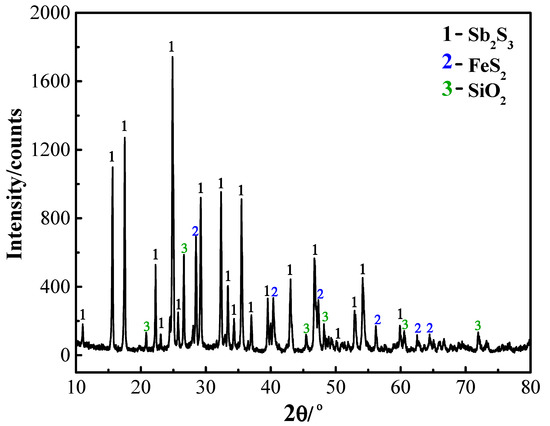
Figure 1.
X-ray diffraction (XRD) pattern of stibnite concentrate.

Table 2.
Chemical composition of reductant coal in pilot scale experiments.
2.2. Methods and Procedures
2.2.1. Laboratory Experiments
The pure and single associated minerals in stibnite were used to reacted with Na2CO3 for reaction behaviors investigation in laboratory, and the sampled amounts were calculated using n(Na2CO3): n(associated minerals): n(carbon) = 1:1:1, and every sample was subjected to TG-DSC analysis after mixing. The reaction temperature was deduced using the endothermic and exothermic peaks in the TG-DSC pattern. Then, 10 g of the mixture was charged in an alumina crucible at the reaction temperature for 2 h. The products were prepared for XRD analyses. All of the experiments were performed using dehydrated high-purity argon gas. The TG-DSC analyses were conducted using a Universal V4.0C thermogravimetric analyzer (TA instrument, New Castle, DE, US) in a nitrogen flow of 100 mL/min and at a heating rate of 10 °C/min. X-ray diffraction (XRD) studies were performed using a D/max 2550 VB + 18 kW powder diffractometer (Rigaku, Akishima-Shi Tokyo, Japan) with a Cu/Kα X-ray source at 40 kV and 300 mA.
2.2.2. Kilogram-ClassPilot Tests
The rotary furnace with the flue gas treatment device used for the kilogram-class pilot tests is shown in Figure 2. In every test, 50 kg of stibnite was used based on the test scale. The external size of the rotary kiln was Ø1620 × 1910 mm2, and it adopted a diesel burner to provide heat. The Sb2S3 concentrate, ZnO, Na2CO3, NaCl, and coal particles were weighed according to the calculated proportions and mixed well. A feeding hopper was used for mixture material addition on the left side of a short kiln. The induced draft fan was turned on when the diesel burner was ignited and was continued to run throughout the smelting process. The furnace was heated by burning diesel fuel and blowing air, and the temperature of the furnace hearth was detected using a thermocouple embedded in the refractory brick and a temperature-measuring gun. During the smelting process, the furnace was rotated several times in a small range every 30 min to increase the material contact. When the smelting concluded after reaching a predetermined time and temperature, the melt was removed using a downspout, leading to a slag charter, and was naturally cooled to indoor temperature. Then, the molten salt and Sb bullion (gathered Au) were separated by hand, and both were sampled and analyzed after grinding with a ball mill and water dissolution. The flue gas discharged from the furnace was cooled using water circulation and a cooling tower, and dust was gathered in a cyclone dust collector, purified by alkali liquor adsorption (3wt% NaOH solution), and was then discharged by the induced draft fan.
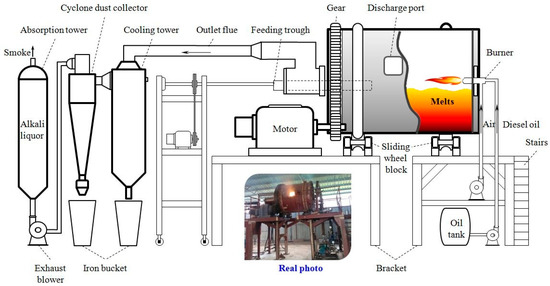
Figure 2.
Rotary kiln and accessories for kilogram-class pilot experiments.
Atomic Absorption Spectroscopy (TAS-990, Purkinje General Instrument Co., Ltd. Beijing, China) was used for the analysis of the Sb in the slag, and the Sb content in the crude Sb was determined by titrimetric analysis (which used ceric sulfate to oxidize Sb(III) in to Sb(V) and excess ceric sulfate faded the color of the methyl orange solution). The Au contents in the Sb bullion and slag were detected by fire assaying. A sulfur analysis was performed using an infrared carbon-sulfur analyzer (CS444, LECO Co., LTD, San Jose, MI, US). The direct recovery rates (DRRs) for the Sb and Au, sulfur-fixing rate (SFR),and volatilization rate (VR) of Sb to smoke were calculated using the following equations, while the DRR of Au was calculated using the same method used for Sb.
where W1 is the initial mass of stibnite (g); W2 is the mass of the crude Sb (g); W3 is the mass of the smelting salt (g); W4 is the mass of the dust from smoke collection (g); x1, x2, and x3 are the Sb contents in the raw ore, crude Sb, and dust (%), respectively; and c1 and c2 are the sulfur contents in the initial stibnite and smelting salt (%), respectively.
3. Results and Discussion
3.1. Thermogravimetric Analysis
Figure 3 shows the TG-DSC results for the different associated minerals and molten salts with carbon addition. Figure 3a shows the reaction results of PbS, where the molar ratio is n(PbS): n(Na2CO3): n(C) = 1:1:2. It can be seen from Figure 3a that there is an apparent endothermic peak at 626.78 °C, where the sharp peak indicates that a strong endothermic reaction occurred with a remarkable mass loss in the system. The eutectic point of Na2CO3-NaCl is 635 °C [24,25,26]. Thus, the endothermic peak at 626.78 °C was attributed to the melting of the mixture, and reaction accelerated because of the liquid-solid reaction after melting. The second exothermic peak emerged at 800 °C. This peak was small and gentle, but the mass loss was further accelerated after 800 °C, which may have been caused by gas emission and flux volatilization. It is noteworthy that Na2CO3 does not decompose before 900 °C [27,28]. Finally, two endothermic peaks appeared around 850 °C, and the mass loss was significant. Based on thermal stability research [29], these can be attributed to the volatilization of the salt.
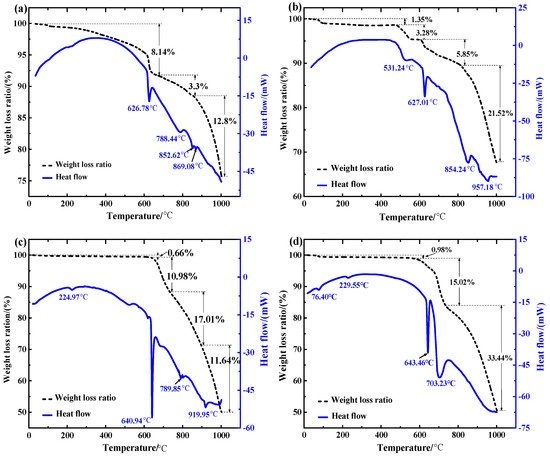
Figure 3.
Thermogravimetric-differential scanning calorimetry (TG-DSC) diagrams of different impurities and molten salts (a) PbS with carbon addition, (b) FeS2 with carbon addition, (c) SiO2 without carbon addition, (d) CaCO3 without carbon addition).
The results of the TG-DSC analysis of FeS2 and eutectic salt are shown in Figure 3b. The experimental materials were prepared with a molar ratio of 1:2:4 (n(FeS2):n(Na2CO3):n(C)). The weight loss curve can be divided into four sections: a loss of approximately 1.35% before 470 °C, 3.28% at 470–600 °C, 5.58% at 600–800 °C, and 21.52% at 800–1000 °C. In addition, four endothermic peaks at 531.24 °C, 627.01 °C, 854.24 °C, and 957.18 °C were observed on the heat flow curve. The first peak and mass loss were caused by dehydration. The second peak, like the one in Figure 3a, was attributed to the melting of the mixture. The last two peaks were relatively gentle and may have been caused by decomposition and interactive reactions.
The TG-DSC results for the SiO2 in the NaCl-Na2CO3 molten salt are shown in Figure 3c. The materials used in the test were prepared according to the mole ratio of n(SiO2):n(Na2CO3) = 1:1, without a carbon addition. There were four endothermic peaks, and the first peak was the dehydration peak at 224.97 °C. The process had a low weightlessness rate of 0.66%. The second peak was still a result of the endothermic melting of the molten salt, at 640.94 °C. The mass loss of the mixture began to accelerate during this process. The third endothermic peak appeared at 789.85 °C, and the peak strength was small, which may have been due to the chemical reaction in the mixture. At the same time, the system mass loss was accelerated. The last weak endothermic peak occurred at 919.95 °C.
In the CaCO3 system, the TG-DSC analysis was also performed at a molar ratio of (n(CaCO3): n(Na2CO3) = 1:1. It can be seen from the results that there were two smaller endothermic peaks in the low-temperature stage, at 76.40 °C and 229.55 °C, and the quality was reduced by 0.98%. These were the dehydration peaks of the materials. Then, there was a sharp endothermic peak and gentle endothermic peak, at 643.46 °C and 703.23 °C, respectively. The third sharp endothermic peak was also a result of the endothermic reaction of the molten salt melting. The fourth peak reaction was relatively gentle, but the mass loss of the system was accelerated. It may have been a result of the decomposition reaction of the CaCO3.
3.2. Thermodynamic Analysis
Based on the previously presented analysis of the thermal reaction behaviors, the possible reactions in different systems and their Gibbs free energy change (ΔGθT) values are listed in Table 3. The ΔGθ-T relationships of these reactions were calculated and are shown in Figure 4, all the data included heat capacity (cp), standard entropy change (ΔSθT) and enthalpy change (ΔHθT) were from the thermodynamic handbook [30]. As can be seen from Figure 4, for PbS, reactions (4)–(7) were all difficult below 800 °C because the ΔGθT values were greater than zero. But the ΔGθT values of reactions (4) and (7) became negative after 800 °C. These were the main reactions of the alkali smelting and reaction smelting of the PbS ore, but the generation of PbSO4 was difficult. The FeS2, which is called pyrite in minerals, was easily decomposed and generated FeS during the smelting of stibnite. The ΔGθT value of reaction (9) also showed that FeS was stable in the molten salt because it could not react with Na2CO3 below 1000 °C. The SiO2, acidic oxide, could easily react with Na2CO3 from 300 °C, because its ΔGθT value became negative. The reaction released CO2 at the same time. Therefore, as shown in Figure 3c, the mass loss of this reaction was larger than those of FeS2 and PbS in front. The ΔGθT values of reaction (11) were less than zero after 870 °C. As is well known, lime begins to decompose and its mass begins to decrease at approximately 1000 °C, which is the temperature that lime is burned in industrial production. However, it is still unclear what reactions occurred between the CaCO3, CaO, and molten salt.

Table 3.
Possible reactions and their ΔGθT values.
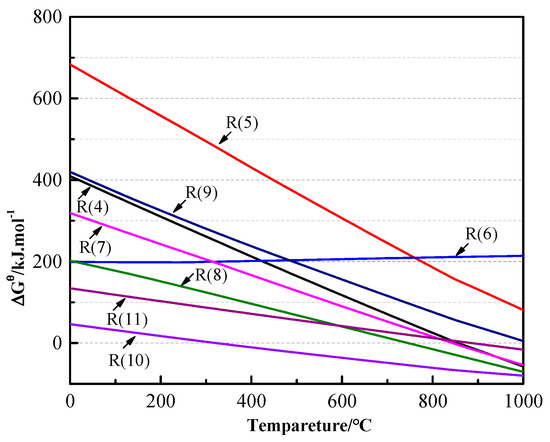
Figure 4.
ΔGTθ-T graph of reactions (4)–(11).
3.3. Process Analysis
To explore the reaction behaviors of the different associated minerals in molten salts, the reaction products of four systems at different temperatures were analyzed using XRD, using the same mole ratio as in the TG-DSC detection. The results are shown in Figure 5. In the PbS-molten salt-C system, four reaction temperatures were selected from the thermogravimetric curve. The results showed that metallic Pb was obtained after 700 °C, with Pb mass values of 3.02, 8.34, and 9.15 g at 700 °C, 850 °C, and 950 °C, respectively, which indicated Pb production rates of 31.85%, 88.04%, and 96.55%, respectively. According to the ΔGθTcalculations for reactions (4) and (7), metallic Pb can be generated by alkali and reaction smelting. An XRD analysis of the smelting salts was performed after the separation of the Pb bullion, and the results are shown in Figure 5a. It can be seen from the XRD results that the main components of the salts were PbS, NaCl, and Na2CO3 at 500 °C. Thus, no reaction occurred in the mixture at this temperature. When the temperature exceeded 700 °C and continued to increase, the main component was changed to Na6(SO4)2CO3, which is a compound of Na2SO4 and Na2CO3. This indicated that Na2SO4 was formed, possibly because part of the PbS reacted with Na2CO3 accorded by reaction (2). The amount of NaCl was basically unchanged.
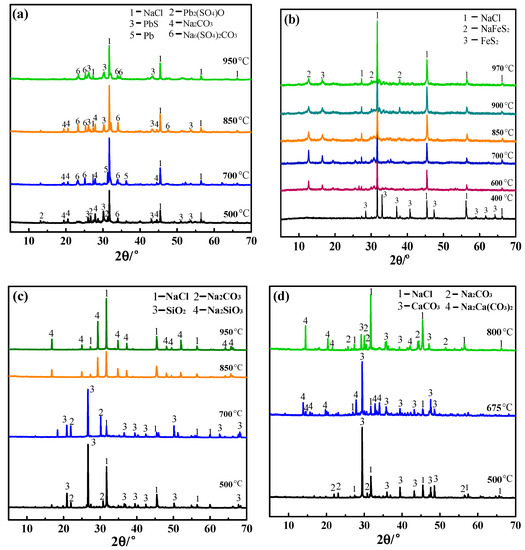
Figure 5.
XRD diagrams of products baked with different impurities and molten salts at different temperatures (a) PbS with carbon addition, (b) FeS2 with carbon addition, (c) SiO2 without carbon addition, (d) CaCO3 without carbon addition.
For FeS2, considering the mass loss and thermal effect, six temperatures were selected for reaction behavior experiments. The XRD results of the products are shown in Figure 5b. It can be seen that no chemical reaction occurred at 400 °C, and the product composition was still FeS2 and molten salt. When the temperature was increased to 600 °C, a large amount of NaFeS2 appeared in the product, which is also a compound of Na2S and FeS. This should have been caused by the decomposition of FeS2 to form FeS and S2, after which the S2 was absorbed to form Na2S, and the Na2S and FeS were easily combined to form NaFeS2. Until the temperature reached 970 °C, the composition of the product was NaFeS2 and NaCl, which could exist stably in the system.
The reactions between SiO2 and binary salt were investigated at four temperatures. It can be seen from Figure 5c that there was no reaction in the mixture below 700 °C. When the temperature exceeded 700 °C, SiO2 and Na2CO3 reacted to form Na2SiO3 following reaction (10), which corresponded to the absorption peak at 789.85 °C in the TG-DSC curves, along with NaCl. Na2SiO3 was stable in the salt until the temperature increased to 950 °C. For CaCO3, reactions were performed at three temperatures. It can be seen from the phase analysis results that the product was a mixture of CaCO3, NaCl, and Na2CO3, and no reaction occurred at 500 °C. When the temperature reached 675 °C, the compound of CaCO3 and Na2CO3 appeared in the product. This showed that the Na2CO3 peaks appeared at 800 °C, which might indicate that Na2Ca(CO3)2 was partially decomposed. Thus, CaCO3 did not decompose but remained stable in the molten salt.
3.4. Kilogram-Class Pilot Tests
In order to verify the feasibility of the molten salt smelting of stibnite and further investigate the behaviors of the associated minerals during smelting, kilogram-class pilot tests were carried out in a rotary kiln. The conditions and results are listed in Table 4. It can be seen from No.1 to No.5 that the DRR values of both Sb and Au increased with the NaCl content. As shown in the phase diagram of NaCl-Na2CO3, the eutectic composition of the system was at 43% NaCl with a eutectic point of 636 °C [29]. The melting point of the mixture decreased with an increase in the NaCl content, and the mobility of flux also improved. Because the price of NaCl is lower than that of Na2CO3, the eutectic composition was adopted for smelting. An increasing the amount of ZnO will reduce the DRR because it has a high melting point and will increase the viscosity of the melt. It can be seen for No.6 to No.8 that the DRR improved with increases in the temperature and reaction time, but the DRR values of Sb and Au were still 80.03% and 85.17%, respectively. Thus, for No.9–No.11, the amount of salt was increased to four or five times the ore mass, and the smelting results obviously increased. The DRR values of Sb and Au were both higher than 90% at 920 °C for 8 h and 870 °C for 10 h, and the lowest Sb content in the slag was 1.28%. It can be seen from Table 4 that the sulfur-fixing rate was high in all the tests, and the highest sulfur-fixing rate reached 96.3%. Even if the reaction was not carried out completely, the sulfur in the ore still existed in the form of sulfide. Thus, the apparent sulfur-fixing rate was high. During the volatilization of Sb and its compounds, which led to a certain volatilization and allowed them to enter the final smoke, but this smoke could return to ingredients.

Table 4.
Smelting results under different experimental conditions in kilogram-class pilot tests.
The delamination of the smelting products and XRD patterns of the molten salt slag from test No.11 before and after water dissolution are shown in Figure 6. It can be seen from Figure 6a that the slag and crude Sb after smelting have obvious stratification, with the crude Sb below the slag. Thus, it would be easy to directly siphon off the molten Sb during industrial production at a high temperature. The XRD results of the molten salt slag before water dissolution showed that the constituents included NaCl, Na2CO3, ZnS, FeS, and Na2ZnSiO4. Thus, the main composition of the molten salt was not changed by smelting. The sulfur phase changed from Sb2S3 and FeS2 to ZnS and FeS, respectively, indicating that the sulfur-fixing reaction was also sufficient.
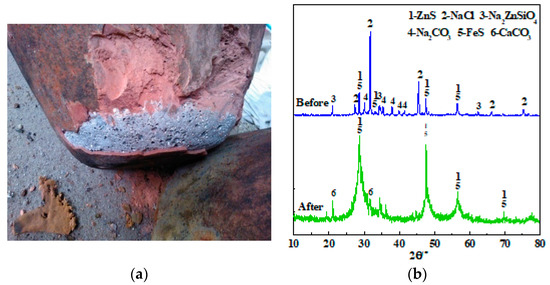
Figure 6.
(a) Stratification of smelting products, and (b) XRD patterns of molten salt residue before and after water dissolution.
Because a large amount of molten salt was added and made the proportion of the reaction products of the associated minerals low, so their characteristic peaks might not be discerned by XRD. Therefore, the soluble NaCl and Na2CO3 in 1000.00 g of molten salt residue were washed away by hot water, and 228.57 g of insoluble residue and 4340.00 mL of lixivium were obtained. An XRD analysis of this insoluble residue was then conducted. The main components of the slag were ZnS, FeS, and CaCO3. Thus, CaCO3 minerals were stable which was consistent with the previously mentioned issues met the front issues. No other phases were detected because of their low content. Therefore, a detailed element balance was conducted based on the quantitative analysis.
The mass of crude Sb obtained from test 11 was 23.23 kg, and the element constituents of this crude Sb, insoluble residue, and lixivium were analyzed, with the results listed in Table 5. The element content of the insoluble residue and lixivium actually represented the soluble and insoluble proportions of the molten salt residue. As can be seen from Table 5, in addition to Sb and Au, 18.80% and 7.43% of the total Fe and As were gathered in the crude Sb. Thus, a portion of the FeS was reduced to metallic Fe. The main components of the insoluble residue were Zn, S, and Fe, which accounted for 38.01%, 25.44%, and 13.14%, respectively. This verified the results of the XRD analysis that the main phases of the residue were FeS and ZnS. The S content in the lixivium reached 1338 mg/L. Therefore, in addition to the soluble sodium salt, soluble sulfur compounds like Na2SO4 and Na2S were also generated, and the total sulfur-fixing rate was high. There was also a portion of soluble silicon in the solution for the formation of Na2SiO3.

Table 5.
Mass of product and its chemical composition obtained from test 11.
According to the element analysis results, the distribution of elements in the crude Sb and molten salt slag could be divided into soluble and insoluble parts based on the proportions of insoluble residue and lixivium, which were calculated and are shown in Figure 7. It can be seen that the Sb, Au, and As elements were the main enrichments in the crude metal, and accounted for 92.37%, 92.77%, and 86.98% of the total quantity of the added ore, respectively. Because of the strong ability of Sb to collect Au, most of the Au was enriched by crude Sb, which facilitated the recovery of Au in the subsequent refining process. Arsenic has properties similar to Sb in nature. Thus, arsenic had the same behavior as Sb and was reduced to a simple substance and gathered in the crude Sb in a reducing atmosphere. In addition, 18.80% Fe and 1.05% Zn were gathered in the crude Sb. Fe could be generated by reaction (9), and its ΔGθ value was negative above 1000 °C. The practice of Zn smelting shows that Zn oxide is difficult to reduce below 1000 °C. Thus, ZnO could be used as a stable sulfur-fixing agent. On the other hand, Fe, Zn, and S were mainly concentrated in the molten salt residue and insoluble. Based on the XRD results in Figure 6b, sulfur was fixed in the form of stable ZnS and FeS in the smelting process, accounting for 96.31% and 81.20%, respectively. However, possibly as a result of the insufficient contact between the materials, some sulfur reacted with alkali to form a soluble salt, accounting for 12.89%, and was not fixed by Zn oxide. Nevertheless, the total sulfur fixation rate (sulfur in the molten salt slag) reached 99.49%. The behaviors of Ca and Si were consistent with the previous analysis results, with 98.76% of the Ca and 81.15% of the Si insoluble in the water dissolution residue. The remainder of the Si might yield soluble silicateby SiO2 and Na2CO3 according to reaction (10).
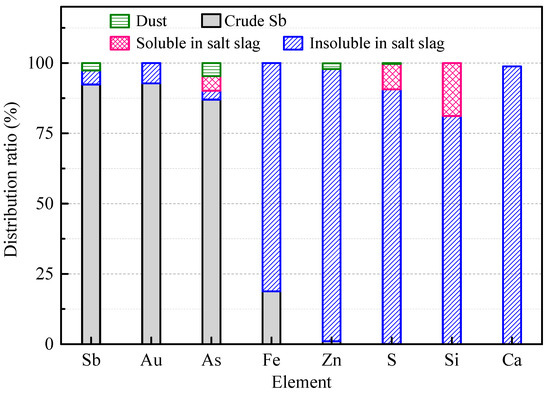
Figure 7.
Element distribution in crude Sb, molten salt slag, and smoke.
4. Conclusions
The reaction behaviors of the associated minerals in stibnite with the Na2CO3-NaCl binary salt were analyzed, and kilogram-class trials of the molten salt smelting of stibnite were conducted. The results showed that PbS could easily participate in reduction reactions with Na2CO3, and carbon and metallic Pb were obtained after 700 °C, with the production rate of Pb reaching 96.55% at 950 °C. FeS2 formed stable NaFeS2 with the molten salt at a high temperature. SiO2 was converted to Na2SiO3 after 850 °C, and CaCO3 form the unstable compound Na2Ca(CO3)2 with the molten salt, which consumed a portion of the molten salt. The results of a pilot test with a scale of 50 kg of concentrate showed that the direct recovery rate of Sb and Au reached 92.37% and 92.77% under the optimum conditions of temperature 920 °C, reaction time 8 h, 1.0 stoichiometric amount of ZnO and eutectic Na2CO3-NaCl molten salt, and Sb bullion was obviously stratified with the molten salt slag after smelting. The element distribution also verified the results of the reaction behavior research, which showed that Sb and Au were mainly distributed in crude Sb, and Fe, Zn, S, Si, and Ca were mainly enriched in the molten salt slag and insoluble. Sulfur was fixed in the form of ZnS and FeS, and a total sulfur-fixing rate of 99.49% was achieved.
Author Contributions
Z.O. and Y.X. carried out the experiments research and L.Y. designed the research and wrote the paper, C.T. reviewed and contributed to the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Nature Science Foundation of China, grant number 51604105.
Acknowledgments
This project was supported financially by the National Nature Science Foundation of China (Grant No. 51604105), for which the authors are grateful.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gierlotka, W. Thermodynamic description of the binary Au-Sb and ternary Au-In-Sb systems. J. Alloys Compd. 2013, 579, 533–539. [Google Scholar] [CrossRef]
- Anderson, C.G. The metallurgy of antimony. Chem. Erde-Geochem. 2012, 72, 3–8. [Google Scholar] [CrossRef]
- Yang, J.G.; Tang, C.B.; Chen, Y.M.; Tang, M.T. Separation of antimony from a stibnite concentrate through a low-temperature smelting process to eliminate SO2 emission. Metall. Mater. Trans. B 2011, 42, 30–36. [Google Scholar] [CrossRef]
- Habashi, F. Handbook of Extractive Metallurgy, Volume II, 1st ed.; WILEY-VCH: Weinheim, Germany, 1997. [Google Scholar]
- Awe, S.A.; Sundkvist, J.E.; Bolin, N.J.; Sandstrom, A. Process flowsheet development for recovering antimony from Sb-bearing copper concentrates. Miner. Eng. 2013, 49, 45–53. [Google Scholar] [CrossRef]
- Awe, S.A.; Sandstrom, A. Selective leaching of arsenic and antimony from a tetrahedrite rich complex sulphide concentrate using alkaline sulphide solution. Miner. Eng. 2010, 23, 1227–1236. [Google Scholar] [CrossRef]
- Lie, X.Q.; Zhong, Q.Y.; Lin, S.Y.; Chu, S.Z. Antimony white production from antimony sulfide ore using acidic solution of antimony pentachloride as lixiviant. J. Cent. South. Inst. Min. Metall. 1990, 21, 615–621. [Google Scholar]
- Yang, J.G.; Wu, Y.T. A hydrometallurgical process for the separation and recovery of antimony. Hydrometallurgy 2014, 143, 68–74. [Google Scholar] [CrossRef]
- Mahlangu, T.; Gudyanga, F.P.; Simbi, D.J. Reductive leaching of stibnite (Sb2S3)flotation concentrates using metallic iron in a hydrochloric acid medium II: Kinetics. Hydrometallurgy 2007, 88, 132–142. [Google Scholar] [CrossRef]
- Xie, Z.F.; Li, B.; Liu, W.L.; Li, H.; Jiang, C.J.; Huang, H.F. A new process for preparation of qualified antimony white from jamesonite. Min. Metall. Eng. 2015, 35, 80–84. [Google Scholar]
- Wang, Q.M.; Guo, X.Y.; Tian, Q.H.; Jiang, T.; Chen, M.; Zhao, B.J. Effects of Matte grade on the distribution of minor elements (Pb, Zn, As, Sb, and Bi) in the bottom blown copper smelting process. Metals 2017, 7, 502. [Google Scholar] [CrossRef]
- Wang, Q.M.; Guo, X.Y.; Tian, Q.H.; Chen, M.; Zhao, B.J. Reaction mechanism and distribution behavior of arsenic in the bottom blown copper smelting process. Metals 2017, 7, 302. [Google Scholar] [CrossRef]
- Liu, W.; Luo, H.L.; Qing, W.Q.; Zheng, Y.X.; Yang, K.; Han, J.W. Investigation into oxygen-enriched bottom-blown stibnite and direct reduction. Metall. Mater. Trans. B 2014, 45, 1281–1290. [Google Scholar] [CrossRef]
- Padilla, R.; Chambi, L.C.; Ruiz, M.C. Antimony production by carbothermic reduction of stibnite in presence of lime. J. Min. Metall. Sect. B-Metall. 2014, 50, 5–13. [Google Scholar] [CrossRef]
- Padilla, R.; Aracena, A.; Ruiz, M.C. Kinetics of stibnite (Sb2S3) oxidation at roasting temperatures. J. Min. Metall. Sect. B-Metall. 2014, 50, 127–132. [Google Scholar] [CrossRef]
- Smirnov, M.P. Direct smelting of lead at low temperature. Nonferr. Met. 1990, 46, 34–36. [Google Scholar]
- Efim, V.M. Low temperature smelting of lead metallic scrap. Erzmetall 2000, 53, 85–89. [Google Scholar]
- Kokoro, I.; Shu, Y.; Masafumi, M. Phase relation and thermodynamic properties of NaCl-Na2CO3 system as a basic system for secondary fly ash incineration processes of municipal wastes. Mater. Trans. 2001, 42, 2480–2486. [Google Scholar]
- Olivarea, R.I.; Chen, C.L.; Wright, S. Thermal stability of molten lithium-sodium-potassium carbonate and influence of additives on the melting point. J. Sol. Energy Eng. 2012, 134, 041002. [Google Scholar] [CrossRef]
- Wang, T.; Mantha, D.; Reddy, R.G. Thermal stability of the eutectic composition in LiNO3-NaNO3-KNO3 ternary system used for thermal energy storage. Sol. Energy Mater. Sol. Cells 2012, 100, 162–168. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Wu, Z.G. Thermal property characterization of a low melting-temperature ternary nitrate salt mixture for thermal energy storage systems. Sol. Energy Mater. Sol. Cells 2011, 96, 3341–3346. [Google Scholar] [CrossRef]
- Ye, L.G.; Tang, C.B.; Chen, Y.M.; Yang, S.H.; Yang, J.G.; Zhang, W.H. One-step extraction of antimony from low-grade stibnite in Sodium Carbonate e Sodium Chloride binary molten salt. J. Clean. Prod. 2015, 93, 134–139. [Google Scholar] [CrossRef]
- Chen, Y.M.; Ye, L.G.; Tang, C.B.; Yang, S.H.; Tang, M.T.; Zhang, W.H. Solubility of Sb in binary Na2CO3-NaCl molten salt. Trans. Nonferr. Met. Soc. China 2015, 25, 3146–3151. [Google Scholar] [CrossRef]
- Jun, Y.; Katsunari, O.; Koichi, A. Thermodynamic assessment of the KCl-K2CO3-NaCl-Na2CO3 system. Calphad 2007, 31, 155–163. [Google Scholar]
- Lu, W.Y.; Zeng, C.L. Preparation of cohesive graphite films by electroreduction of CO32− in molten Na2CO3-NaCl. Surf. Coat. Technol. 2012, 206, 4287–4292. [Google Scholar]
- Jun, Y.; Daisuke, M.; Koichi, A.; Youji, Y.; Hiroshi, Y. Strength of salt core composed of alkali carbonate and alkali chloride mixtures made by casting technique. Mater. Trans. 2007, 48, 1034–1041. [Google Scholar]
- Kim, J.W.; Lee, H.E. Thermal and carbothermic decomposition of Na2CO3 and Li2CO3. Metall. Mater. Trans. B 2001, 32, 17–24. [Google Scholar] [CrossRef]
- Lewis, A.E.; Beautment, C. Prioritising objectives for waste reprocessing: A case study in secondary lead refining. Waste Manag. 2002, 22, 277–285. [Google Scholar] [CrossRef]
- Ye, L.G.; Tang, C.B.; Chen, Y.M.; Yang, S.H.; Tang, M.T. The thermal physical properties and stability of the eutectic composition in a Na2CO3-NaCl binary system. Thermochim. Acta 2014, 596, 14–20. [Google Scholar] [CrossRef]
- Brain, I.; Knacke, O.; Kubaschewski, O. Thermochemical Properties of Inorganic Substance, 1st ed.; Springer: New York, NY, USA, 1972. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).