Nutritional Practice and Nitrogen Balance in Elite Japanese Swimmers during a Training Camp
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
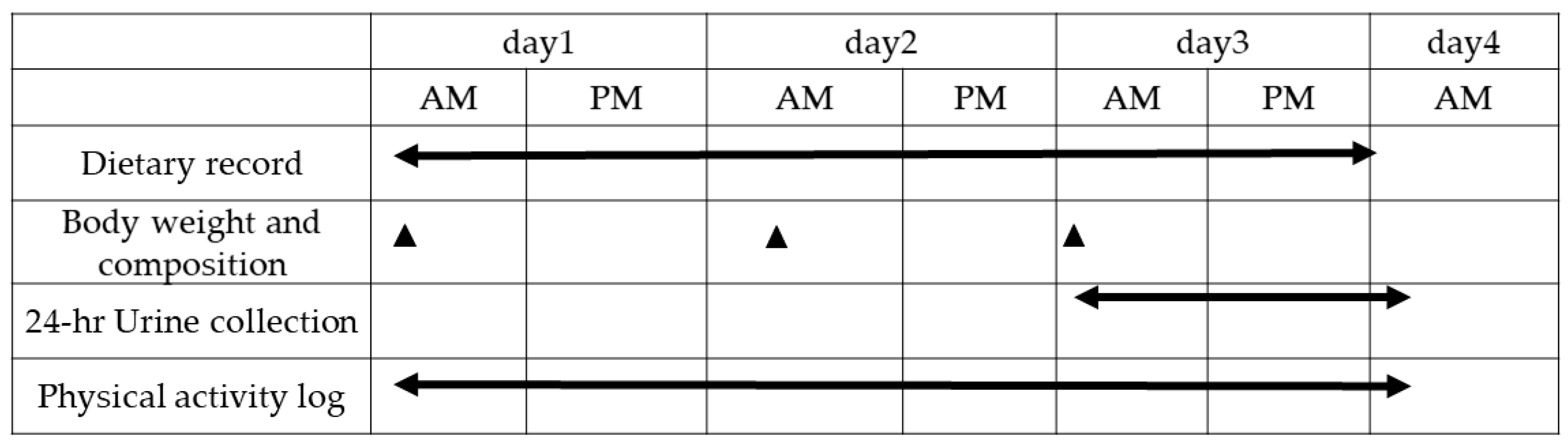
2.2. Overall Experimental Protocol
2.3. Dietary Intake
2.4. Energy Expenditure and Energy Balance
2.5. Nitrogen Balance
2.6. Data Analyses
3. Results
3.1. Subjects Characteristics
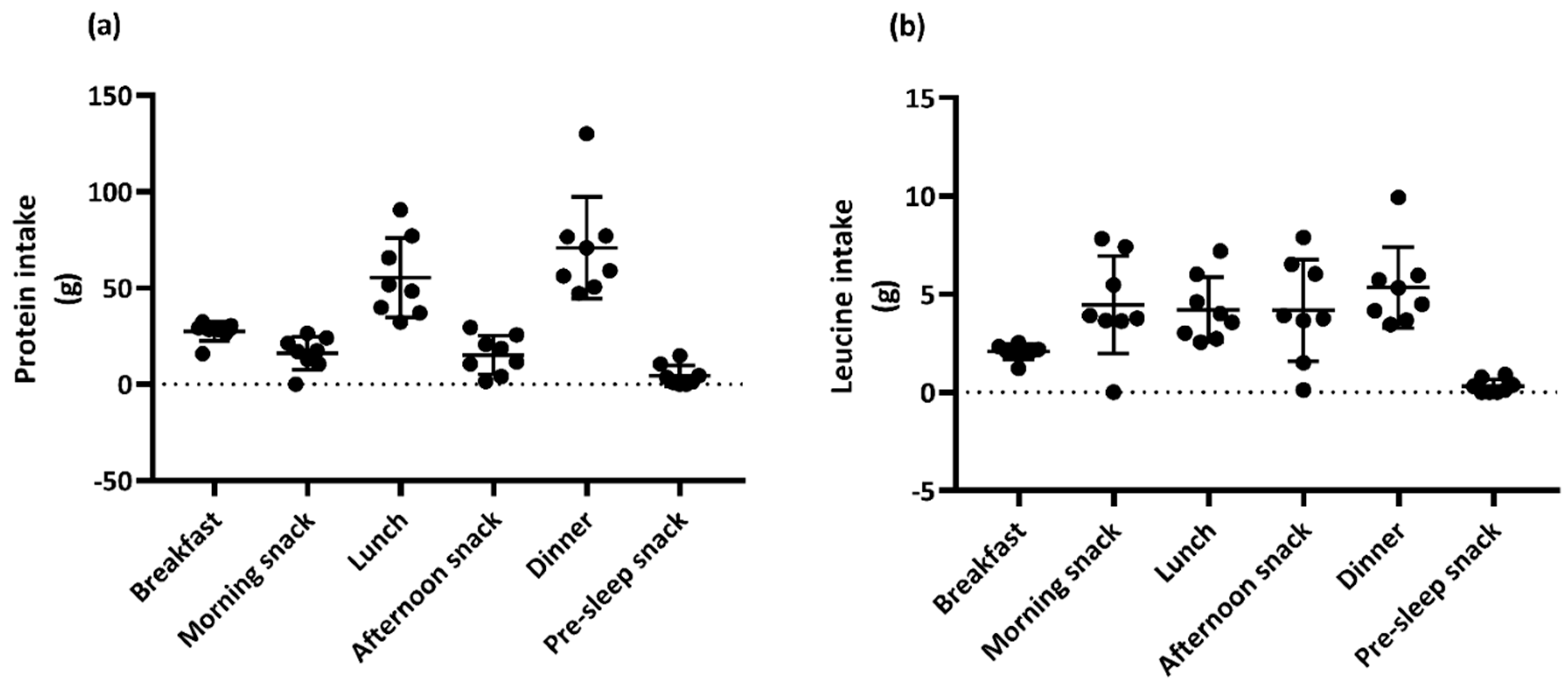
3.2. Dietary Intake
3.3. Energy Expenditure
3.4. Energy Balance
3.5. Nitrogen Balance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. American College of Sports Medicine Joint Position Statement. Nutrition and Athletic Performance. Med. Sci. Sports Exerc. 2016, 48, 543–568. [Google Scholar] [CrossRef]
- Witard, O.C.; Jackman, S.R.; Breen, L.; Smith, K.; Selby, A.; Tipton, K.D. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am. J. Clin. Nutr. 2014, 99, 86–95. [Google Scholar] [CrossRef]
- Biolo, G.; Tipton, K.D.; Klein, S.; Wolfe, R.R. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am. J. Physiol. 1997, 273, E122–E129. [Google Scholar] [CrossRef]
- Moore, D.R.; Camera, D.M.; Areta, J.L.; Hawley, J.A. Beyond muscle hypertrophy: Why dietary protein is important for endurance athletes. Appl. Physiol. Nutr. Metab. 2014, 39, 987–997. [Google Scholar] [CrossRef]
- Phillips, S.M. Dietary protein for athletes: From requirements to metabolic advantage. Appl. Physiol. Nutr. Metab. 2006, 31, 647–654. [Google Scholar] [CrossRef]
- Tarnopolsky, M. Protein requirements for endurance athletes. Nutrition 2004, 20, 662–668. [Google Scholar] [CrossRef]
- Tarnopolsky, M.A.; Atkinson, S.A.; MacDougall, J.D.; Chesley, A.; Phillips, S.; Schwarcz, H.P. Evaluation of protein requirements for trained strength athletes. J. Appl. Physiol. 1992, 73, 1986–1995. [Google Scholar] [CrossRef]
- Kato, H.; Suzuki, K.; Bannai, M.; Moore, D.R. Protein Requirements Are Elevated in Endurance Athletes after Exercise as Determined by the Indicator Amino Acid Oxidation Method. PLoS ONE 2016, 11, e0157406. [Google Scholar] [CrossRef] [PubMed]
- Mazzulla, M.; Sawan, S.A.; Williamson, E.; Hannaian, S.J.; Volterman, K.A.; West, D.W.D.; Moore, D.R. Protein Intake to Maximize Whole-Body Anabolism during Postexercise Recovery in Resistance-Trained Men with High Habitual Intakes is Severalfold Greater than the Current Recommended Dietary Allowance. J. Nutr. 2019, 150, 505. [Google Scholar] [CrossRef]
- Meredith, C.N.; Zackin, M.J.; Frontera, W.R.; Evans, W.J. Dietary protein requirements and body protein metabolism in endurance-trained men. J. Appl. Physiol. 1989, 66, 2850–2856. [Google Scholar] [CrossRef]
- Tarnopolsky, M.A.; MacDougall, J.D.; Atkinson, S.A. Influence of protein intake and training status on nitrogen balance and lean body mass. J. Appl. Physiol. 1988, 64, 187–193. [Google Scholar] [CrossRef]
- Mujika, I.; Stellingwerff, T.; Tipton, K. Nutrition and training adaptations in aquatic sports. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 414–424. [Google Scholar] [CrossRef]
- Dudley, G.A.; Djamil, R. Incompatibility of endurance- and strength-training modes of exercise. J. Appl. Physiol. 1985, 59, 1446–1451. [Google Scholar] [CrossRef]
- Hennessy, L.C.; Watson, A.W. The interference effects of training for strength and endurance simultaneously. J. Strength Cond. Res. 1994, 8, 12–19. [Google Scholar]
- Girold, S.; Calmels, P.; Maurin, D.; Milhau, N.; Chatard, J.C. Assisted and resisted sprint training in swimming. J. Strength Cond. Res. 2006, 20, 547–554. [Google Scholar] [CrossRef]
- Barbosa, T.M.; Fernandes, R.; Keskinen, K.L.; Colaco, P.; Cardoso, C.; Silva, J.; Vilas-Boas, J.P. Evaluation of the energy expenditure in competitive swimming strokes. Int. J. Sports Med. 2006, 27, 894–899. [Google Scholar] [CrossRef]
- Holmer, I. Energy cost of arm stroke, leg kick, and the whole stroke in competitive swimming styles. Eur. J. Appl. Physiol. Occup. Physiol. 1974, 33, 105–118. [Google Scholar] [CrossRef]
- Holmer, I. Physiology of swimming man. Exerc. Sport Sci. Rev. 1979, 7, 87–123. [Google Scholar]
- Nadel, E.R.; Holmer, I.; Bergh, U.; Astrand, P.O.; Stolwijk, J.A. Energy exchanges of swimming man. J. Appl. Physiol. 1974, 36, 465–471. [Google Scholar] [CrossRef]
- Humayun, M.A.; Elango, R.; Ball, R.O.; Pencharz, P.B. Reevaluation of the protein requirement in young men with the indicator amino acid oxidation technique. Am. J. Clin. Nutr. 2007, 86, 995–1002. [Google Scholar] [CrossRef]
- Matsuda, T.; Kato, H.; Suzuki, H.; Mizugaki, A.; Ezaki, T.; Ogita, F. Within-Day Amino Acid Intakes and Nitrogen Balance in Male Collegiate Swimmers during the General Preparation Phase. Nutrients 2018, 10, 1809. [Google Scholar] [CrossRef] [PubMed]
- Jager, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M.; et al. International Society of Sports Nutrition Position Stand: Protein and exercise. J. Int. Soc. Sports Nutr. 2017, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Kerksick, C.M.; Arent, S.; Schoenfeld, B.J.; Stout, J.R.; Campbell, B.; Wilborn, C.D.; Taylor, L.; Kalman, D.; Smith-Ryan, A.E.; Kreider, R.B.; et al. International society of sports nutrition position stand: Nutrient timing. J. Int. Soc. Sports Nutr. 2017, 14, 33. [Google Scholar] [CrossRef] [PubMed]
- Areta, J.L.; Burke, L.M.; Ross, M.L.; Camera, D.M.; West, D.W.; Broad, E.M.; Jeacocke, N.A.; Moore, D.R.; Stellingwerff, T.; Phillips, S.M.; et al. Timing and distribution of protein ingestion during prolonged recovery from resistance exercise alters myofibrillar protein synthesis. J. Physiol. 2013, 591, 2319–2331. [Google Scholar] [CrossRef]
- Mamerow, M.M.; Mettler, J.A.; English, K.L.; Casperson, S.L.; Arentson-Lantz, E.; Sheffield-Moore, M.; Layman, D.K.; Paddon-Jones, D. Dietary protein distribution positively influences 24-h muscle protein synthesis in healthy adults. J. Nutr. 2014, 144, 876–880. [Google Scholar] [CrossRef]
- Tang, J.E.; Moore, D.R.; Kujbida, G.W.; Tarnopolsky, M.A.; Phillips, S.M. Ingestion of whey hydrolysate, casein, or soy protein isolate: Effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J. Appl. Physiol. 2009, 107, 987–992. [Google Scholar] [CrossRef]
- Norton, L.E.; Wilson, G.J.; Layman, D.K.; Moulton, C.J.; Garlick, P.J. Leucine content of dietary proteins is a determinant of postprandial skeletal muscle protein synthesis in adult rats. Nutr. Metab. 2012, 9, 67. [Google Scholar] [CrossRef]
- Schoenfeld, B.J.; Aragon, A.A. How much protein can the body use in a single meal for muscle-building? Implications for daily protein distribution. J. Int. Soc. Sports Nutr. 2018, 15, 10. [Google Scholar] [CrossRef]
- Suzuki, H.; Kato, H.; Ueno, Y.; Takanouchi, T. Nitrogen Balance in Female Japanese National Handball Players During Training Camp. Front. Nutr. 2020, 7, 59. [Google Scholar] [CrossRef]
- Nelson, K.M.; Weinsier, R.L.; Long, C.L.; Schutz, Y. Prediction of resting energy expenditure from fat-free mass and fat mass. Am. J. Clin. Nutr. 1992, 56, 848–856. [Google Scholar] [CrossRef]
- Weststrate, J.A.; Weys, P.J.; Poortvliet, E.J.; Deurenberg, P.; Hautvast, J.G. Diurnal variation in postabsorptive resting metabolic rate and diet-induced thermogenesis. Am. J. Clin. Nutr. 1989, 50, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Keytel, L.R.; Goedecke, J.H.; Noakes, T.D.; Hiiloskorpi, H.; Laukkanen, R.; van der Merwe, L.; Lambert, E.V. Prediction of energy expenditure from heart rate monitoring during submaximal exercise. J. Sports Sci. 2005, 23, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Phillips, W.T.; Ziuraitis, J.R. Energy cost of the ACSM single-set resistance training protocol. J. Strength Cond. Res. 2003, 17, 350–355. [Google Scholar] [CrossRef]
- Costa, M.J.; Balasekaran, G.; Vilas-Boas, J.P.; Barbosa, T.M. Physiological Adaptations to Training in Competitive Swimming: A Systematic Review. J. Hum. Kinet. 2015, 49, 179–194. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, S.; Phillips, S.M.; Carter, S.L.; Lowther, S.; Gibala, M.J.; Tarnopolsky, M.A. Endurance exercise training attenuates leucine oxidation and BCOAD activation during exercise in humans. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E580–E587. [Google Scholar] [CrossRef]
- Moore, D.R.; Del Bel, N.C.; Nizi, K.I.; Hartman, J.W.; Tang, J.E.; Armstrong, D.; Phillips, S.M. Resistance training reduces fasted- and fed-state leucine turnover and increases dietary nitrogen retention in previously untrained young men. J. Nutr. 2007, 137, 985–991. [Google Scholar] [CrossRef]
- Hartman, J.W.; Moore, D.R.; Phillips, S.M. Resistance training reduces whole-body protein turnover and improves net protein retention in untrained young males. Appl. Physiol. Nutr. Metab. 2006, 31, 557–564. [Google Scholar] [CrossRef]
- Hoffer, L.J.; Forse, R.A. Protein metabolic effects of a prolonged fast and hypocaloric refeeding. Am. J. Physiol. 1990, 258, E832–E840. [Google Scholar] [CrossRef]
- Stein, T.P.; Rumpler, W.V.; Leskiw, M.J.; Schluter, M.D.; Staples, R.; Bodwell, C.E. Effect of reduced dietary intake on energy expenditure, protein turnover, and glucose cycling in man. Metabolism 1991, 40, 478–483. [Google Scholar] [CrossRef]
- Helms, E.R.; Zinn, C.; Rowlands, D.S.; Brown, S.R. A systematic review of dietary protein during caloric restriction in resistance trained lean athletes: A case for higher intakes. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 127–138. [Google Scholar] [CrossRef]
- Hector, A.J.; Phillips, S.M. Protein Recommendations for Weight Loss in Elite Athletes: A Focus on Body Composition and Performance. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Lemon, P.W.; Mullin, J.P. Effect of initial muscle glycogen levels on protein catabolism during exercise. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1980, 48, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Howarth, K.R.; Phillips, S.M.; MacDonald, M.J.; Richards, D.; Moreau, N.A.; Gibala, M.J. Effect of glycogen availability on human skeletal muscle protein turnover during exercise and recovery. J. Appl. Physiol. 2010, 109, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Kriengsinyos, W.; Wykes, L.J.; Goonewardene, L.A.; Ball, R.O.; Pencharz, P.B. Phase of menstrual cycle affects lysine requirement in healthy women. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E489–E496. [Google Scholar] [CrossRef]
- Hamadeh, M.J.; Devries, M.C.; Tarnopolsky, M.A. Estrogen supplementation reduces whole body leucine and carbohydrate oxidation and increases lipid oxidation in men during endurance exercise. J. Clin. Endocrinol. Metab. 2005, 90, 3592–3599. [Google Scholar] [CrossRef]
- Lamont, L.S.; Lemon, P.W.; Bruot, B.C. Menstrual cycle and exercise effects on protein catabolism. Med. Sci. Sports Exerc. 1987, 19, 106–110. [Google Scholar] [CrossRef]
- Lariviere, F.; Moussalli, R.; Garrel, D.R. Increased leucine flux and leucine oxidation during the luteal phase of the menstrual cycle in women. Am. J. Physiol. 1994, 267, E422–E428. [Google Scholar] [CrossRef]
- Malowany, J.M.; West, D.W.D.; Williamson, E.; Volterman, K.A.; Abou Sawan, S.; Mazzulla, M.; Moore, D.R. Protein to Maximize Whole-Body Anabolism in Resistance-trained Females after Exercise. Med. Sci. Sports Exerc. 2019, 51, 798–804. [Google Scholar] [CrossRef]
- Packer, J.E.; Wooding, D.J.; Kato, H.; Courtney-Martin, G.; Pencharz, P.B.; Moore, D.R. Variable-Intensity Simulated Team-Sport Exercise Increases Daily Protein Requirements in Active Males. Front. Nutr. 2017, 4, 64. [Google Scholar] [CrossRef]
- Wooding, D.J.; Packer, J.E.; Kato, H.; West, D.W.D.; Courtney-Martin, G.; Pencharz, P.B.; Moore, D.R. Increased Protein Requirements in Female Athletes after Variable-Intensity Exercise. Med. Sci. Sports Exerc. 2017, 49, 2297–2304. [Google Scholar] [CrossRef]
- Moore, D.R.; Robinson, M.J.; Fry, J.L.; Tang, J.E.; Glover, E.I.; Wilkinson, S.B.; Prior, T.; Tarnopolsky, M.A.; Phillips, S.M. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am. J. Clin. Nutr. 2009, 89, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Macnaughton, L.S.; Wardle, S.L.; Witard, O.C.; McGlory, C.; Hamilton, D.L.; Jeromson, S.; Lawrence, C.E.; Wallis, G.A.; Tipton, K.D. The response of muscle protein synthesis following whole-body resistance exercise is greater following 40 g than 20 g of ingested whey protein. Physiol. Rep. 2016, 4. [Google Scholar] [CrossRef]
- Kato, H.; Suzuki, K.; Bannai, M.; Moore, D.R. Branched-Chain Amino Acids Are the Primary Limiting Amino Acids in the Diets of Endurance-Trained Men after a Bout of Prolonged Exercise. J. Nutr. 2018, 148, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Trommelen, J.; van Loon, L.J. Pre-Sleep Protein Ingestion to Improve the Skeletal Muscle Adaptive Response to Exercise Training. Nutrients 2016, 8, 763. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.; Kato, H.; Volterman, K.A.; Suzuki, K.; Moore, D.R. The Effect of Dietary Protein on Protein Metabolism and Performance in Endurance-trained Males. Med. Sci. Sports Exerc. 2019, 51, 352–360. [Google Scholar] [CrossRef]

| Characteristic | Overall | Male | Female |
|---|---|---|---|
| Age (yrs) | 21.9 ± 2.3 | 24.0 ± 0.0 | 21.2 ± 2.2 |
| Height (cm) | 170.9 ± 5.9 | 176 ± 2.8 | 169.2 ± 5.8 |
| Body weight (kg) | 64.2 ± 7.1 | 74.8 ± 2.8 | 60.6 ± 2.9 |
| Fat free mass (kg) | 53.7± 7.4 | 65.4 ± 0.1 | 49.9 ± 1.9 |
| Body fat (%) | 16.4 ± 4.4 | 12.5 ± 3.4 | 17.7 ± 4 |
| N | 8 | 2 | 6 |
| Variable | Day 1 | Day 4 | p Value |
|---|---|---|---|
| Body weight, kg | 64.2 ± 7.0 | 64.2 ± 7.2 | n.s |
| Fat mass, % | 16.4 ± 4.5 | 16.3 ± 4.2 | n.s |
| Fat mass, kg | 10.5 ± 2.9 | 10.4 ± 2.6 | n.s |
| Fat-free mass, kg | 53.7 ± 7.3 | 53.8 ± 7.4 | n.s |
| Variable | Day 1 | Day 2 | Day 3 (Test Day) |
|---|---|---|---|
| Energy | |||
| kcal/day | 3889 ± 1156 | 3803 ± 1128 | 4447 ± 1068 |
| Protein | |||
| g/day | 166 ± 57 | 173 ± 49 | 190 ± 56 |
| g/kg/day | 2.6 ± 0.8 | 2.7 ± 0.7 | 3.0 ± 0.7 |
| Energy % | 17 ± 2 | 18 ± 1 | 17 ± 1 |
| Fat | |||
| g/day | 117.6 ± 23.2 | 110.2 ± 32.2 | 136.3 ± 35.7 |
| g/kg/day | 1.8 ± 0.4 | 1.7 ± 0.5 | 2.1 ± 0.5 |
| Energy % | 28 ± 4.4 | 26.2 ± 2.9 | 27.6 ± 2.8 |
| Carbohydrate | |||
| g/day | 530 ± 208 | 531 ± 166 | 614 ± 136 |
| g/kg/day | 8.3 ± 3.1 | 8.2 ± 2.3 | 9.6 ± 1.9 |
| Energy % | 53 ± 7 | 56 ± 3 | 56 ± 4 |
| Day | Variable | Morning Sessions | Afternoon Sessions | ||
|---|---|---|---|---|---|
| Dry-Land | Swimming | Dry-Land | Swimming | ||
| Day 1 | HR, bpm | 95.2 ± 9.0 | 124.8 ± 4.9 | 86.8 ± 6.1 | 119.6 ± 5.1 |
| Time, min | 58.8 ± 10.0 | 143.0 ± 21.6 | 55.1 ± 1.7 | 148.4 ± 2.7 | |
| Distance, m | 5886 ± 567 | 6833 ± 408 | |||
| Day 2 | HR, bpm | 97.7 ± 15.0 | 139.3 ± 6.1 | ||
| Time, min | 51.3 ± 9.4 | 140.4 ± 3.9 | |||
| Distance, m | 6441 ± 2777 | ||||
| Day 3 | HR, bpm | 89.6 ± 7.9 | 126.6 ± 6.3 | 93.3 ± 4.6 | 122.2 ± 4.9 |
| (Test day) | Time, min | 57.1 ± 13.9 | 156.8 ± 22.6 | 56.1 ± 13.6 | 120.9 ± 18.7 |
| Distance, m | 6844 ± 515 | 5630 ± 765 | |||
| kcal/Day | Day 1 | Day 2 | Day 3 |
|---|---|---|---|
| Resting energy expenditure | 1429 ± 187 | ||
| Diet-induced thermogenesis | 276 ± 82 | 270 ± 80 | 316 ± 76 |
| Exercise-induced energy expenditure | |||
| Normal daily physical activity | 345 ± 97 | 319 ± 68 | 252 ± 98 |
| Exercise sessions | 1742 ± 967 | 1139 ± 286 | 2783 ± 497 |
| Total energy expenditure | 3792 ± 1048 | 3157 ± 429 | 4780 ± 509 |
| Subject No. | Protein Intake (g/kg/day) | Nitrogen Balance (g N/day) |
|---|---|---|
| 1 | 3.59 | 0.47 |
| 2 | 4.39 | 10.34 |
| 3 | 3.16 | 6.76 |
| 4 | 2.57 | 8.32 |
| 5 | 2.42 | 9.46 |
| 6 | 2.25 | 5.77 |
| 7 | 2.37 | 4.73 |
| 8 | 2.89 | 7.96 |
| mean (95% CI range) | 2.96 (2.34–3.57) | 6.73 (4.11–9.34) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizugaki, A.; Kato, H.; Suzuki, H.; Kurihara, H.; Ogita, F. Nutritional Practice and Nitrogen Balance in Elite Japanese Swimmers during a Training Camp. Sports 2021, 9, 17. https://doi.org/10.3390/sports9020017
Mizugaki A, Kato H, Suzuki H, Kurihara H, Ogita F. Nutritional Practice and Nitrogen Balance in Elite Japanese Swimmers during a Training Camp. Sports. 2021; 9(2):17. https://doi.org/10.3390/sports9020017
Chicago/Turabian StyleMizugaki, Ami, Hiroyuki Kato, Haruka Suzuki, Hidefumi Kurihara, and Futoshi Ogita. 2021. "Nutritional Practice and Nitrogen Balance in Elite Japanese Swimmers during a Training Camp" Sports 9, no. 2: 17. https://doi.org/10.3390/sports9020017
APA StyleMizugaki, A., Kato, H., Suzuki, H., Kurihara, H., & Ogita, F. (2021). Nutritional Practice and Nitrogen Balance in Elite Japanese Swimmers during a Training Camp. Sports, 9(2), 17. https://doi.org/10.3390/sports9020017





