Plasma Eicosapentaenoic Acid Is Associated with Muscle Strength and Muscle Damage after Strenuous Exercise
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Design
2.3. Blood Sample
2.4. Maximal Voluntary Contraction Torque
2.5. ECCs
2.6. Muscle Damage Markers
2.7. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dyerberg, J.; Bang, H.O.; Hjorne, N. Fatty acid composition of the plasma lipids in Greenland Eskimos. Am. J. Clin. Nutr. 1975, 28, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, Y.; Nakazato, K.; Ochi, E. Contralateral repeated bout effect after eccentric exercise on muscular activation. Eur. J. Appl. Physiol. 2018, 118, 1997–2005. [Google Scholar] [CrossRef] [PubMed]
- Ochi, E.; Tsuchiya, Y.; Nosaka, K. Differences in post-exercise T2 relaxation time changes between eccentric and concentric contractions of the elbow flexors. Eur. J. Appl. Physiol. 2016, 116, 2145–2154. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, P.M.; Hubal, M.J. Exercise-induced muscle damage in humans. Am. J. Phys. Med. Rehabil. 2002, 81, S52–S69. [Google Scholar] [CrossRef] [PubMed]
- Reinders, I.; Song, X.; Visser, M.; Eiriksdottir, G.; Gudnason, V.; Sigurdsson, S.; Aspelund, T.; Siggeirsdottir, K.; Brouwer, I.A.; Harris, T.B.; et al. Plasma phospholipid PUFAs are associated with greater muscle and knee extension strength but not with changes in muscle parameters in older adults. J. Nutr. 2015, 145, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Rossato, L.T.; De Branco, F.M.S.; Azeredo, C.M.; Rinaldi, A.E.M.; De Oliveira, E.P. Association between omega-3 fatty acids intake and muscle strength in older adults: A study from National Health and Nutrition Examination Survey (NHANES) 1999–2002. Clin. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Gravina, L.; Brown, F.F.; Alexander, L.; Dick, J.; Bell, G.; Witard, O.C.; Galloway, S.D.R. n-3 Fatty Acid Supplementation During 4 Weeks of Training Leads to Improved Anaerobic Endurance Capacity, but not Maximal Strength, Speed, or Power in Soccer Players. Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 305–313. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Macartney, M.J.; Hingley, L.; Brown, M.A.; Peoples, G.E.; McLennan, P.L. Intrinsic heart rate recovery after dynamic exercise is improved with an increased omega-3 index in healthy males. Br. J. Nutr. 2014, 112, 1984–1992. [Google Scholar] [CrossRef] [PubMed]
- Żebrowska, A.; Mizia-Stec, K.; Mizia, M.; Gąsior, Z.; Poprzęcki, S. Omega-3 fatty acids supplementation improves endothelial function and maximal oxygen uptake in endurance-trained athletes. Eur. J. Sport Sci. 2015, 15, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Philpott, J.D.; Witard, O.C.; Galloway, S.D.R. Applications of omega-3 polyunsaturated fatty acid supplementation for sport performance. Res. Sports Med. 2019, 27, 219–237. [Google Scholar] [CrossRef] [PubMed]
- Heileson, J.L.; Funderburk, L.K. The effect of fish oil supplementation on the promotion and preservation of lean body mass, strength, and recovery from physiological stress in young, healthy adults: A systematic review. Nutr. Rev. 2020. [Google Scholar] [CrossRef]
- Ochi, E.; Tsuchiya, Y.; Yanagimoto, K. Effect of eicosapentaenoic acids-rich fish oil supplementation on motor nerve function after eccentric contractions. J. Int. Soc. Sports Nutr. 2017, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, Y.; Yanagimoto, K.; Nakazato, K.; Hayamizu, K.; Ochi, E. Eicosapentaenoic and docosahexaenoic acids-rich fish oil supplementation attenuates strength loss and limited joint range of motion after eccentric contractions: A randomized, double-blind, placebo-controlled, parallel-group trial. Eur. J. Appl. Physiol. 2016, 116, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.C.; Nosaka, K.; Sacco, P. Intensity of eccentric exercise, shift of optimum angle, and the magnitude of repeated-bout effect. J. Appl. Physiol. 2007, 102, 992–999. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, Y.; Yanagimoto, K.; Ueda, H.; Ochi, E. Supplementation of eicosapentaenoic acid-rich fish oil attenuates muscle stiffness after eccentric contractions of human elbow flexors. J. Int. Soc. Sports Nutr. 2019, 16, 19. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, Y.; Hirayama, K.; Ueda, H.; Ochi, E. Two and Four Weeks of β-Hydroxy-β-Methylbutyrate (HMB) Supplementations Reduce Muscle Damage Following Eccentric Contractions. J. Am. Coll. Nutr. 2019, 38, 373–379. [Google Scholar] [CrossRef]
- Ter Borg, S.; Luiking, Y.C.; Van Helvoort, A.; Boirie, Y.; Schols, J.; de Groot, C. Low Levels of Branched Chain Amino Acids, Eicosapentaenoic Acid and Micronutrients Are Associated with Low Muscle Mass, Strength and Function in Community-Dwelling Older Adults. J. Nutr. Health Aging 2019, 23, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: A randomized controlled trial. Am. J. Clin. Nutr. 2011, 93, 402–412. [Google Scholar] [CrossRef]
- Rodacki, C.L.; Rodacki, A.L.; Pereira, G.; Naliwaiko, K.; Coelho, I.; Pequito, D.; Fernandes, L.C. Fish-oil supplementation enhances the effects of strength training in elderly women. Am. J. Clin. Nutr. 2012, 95, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Arkhipova, O.V.; Grishin, S.N.; Sitdikova, G.F.; Zefirov, A.L. The presynaptic effects of arachidonic acid and prostaglandin E2 at the frog neuromuscular junction. Neurosci. Behav. Physiol. 2006, 36, 307–312. [Google Scholar] [CrossRef] [PubMed]
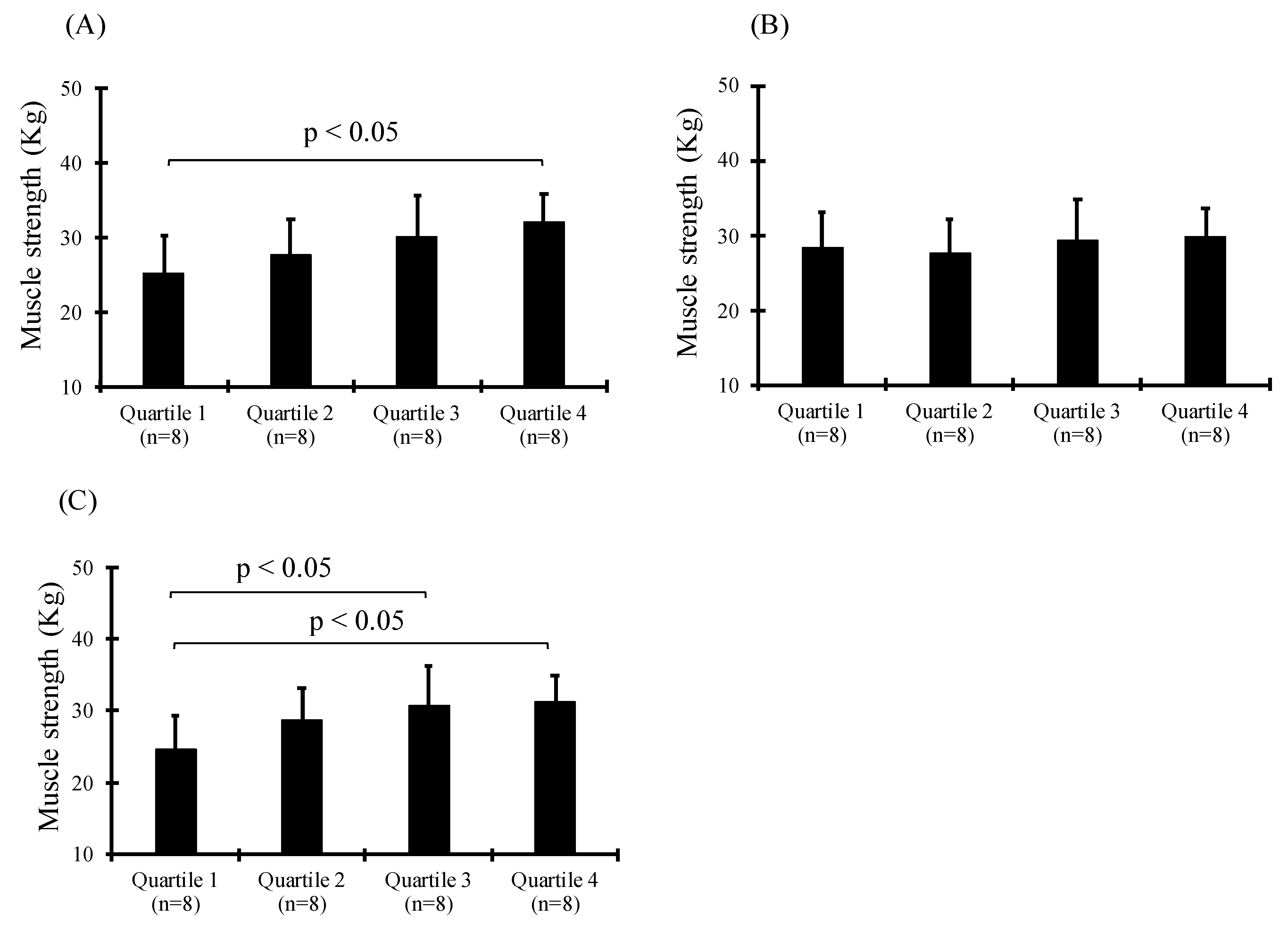
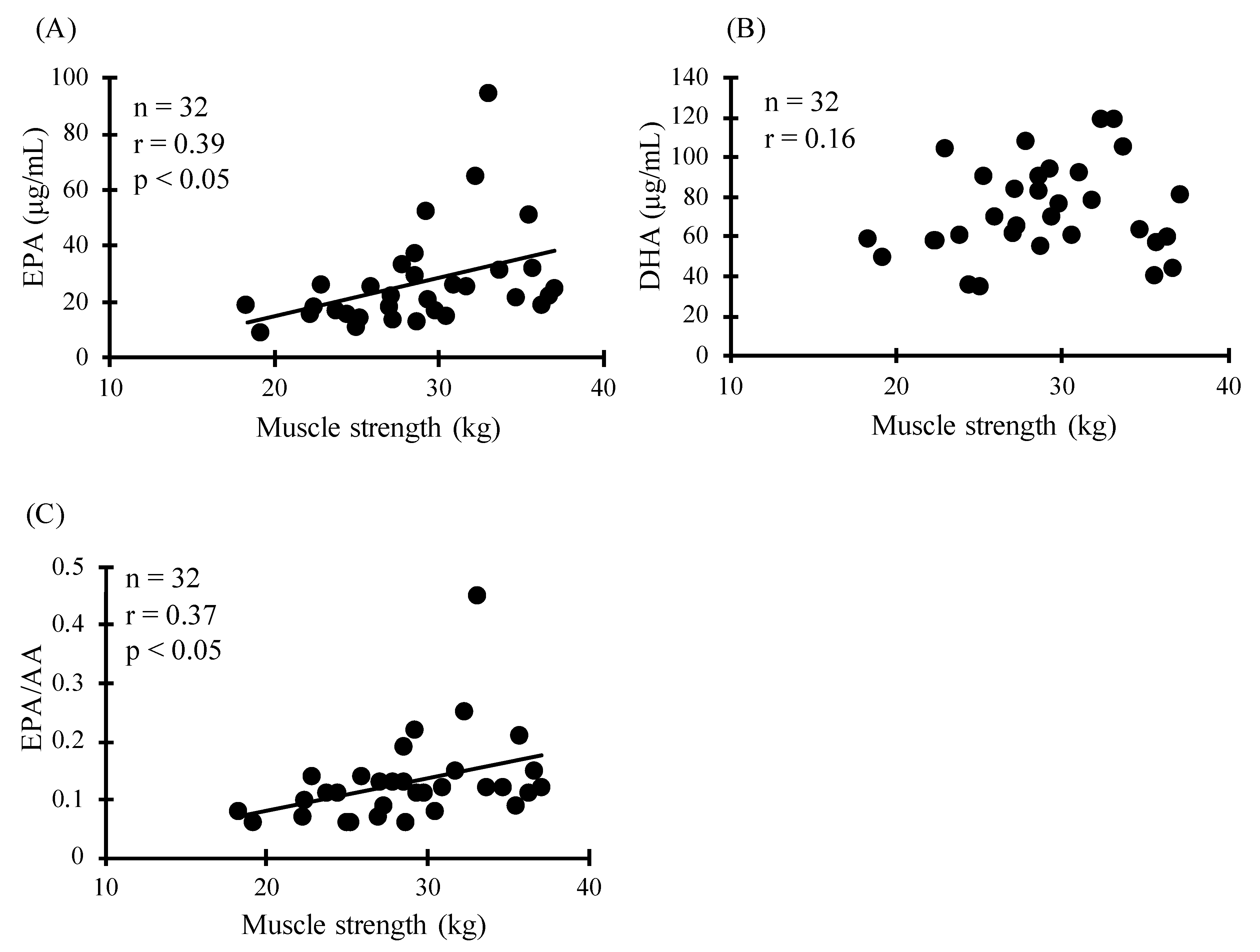
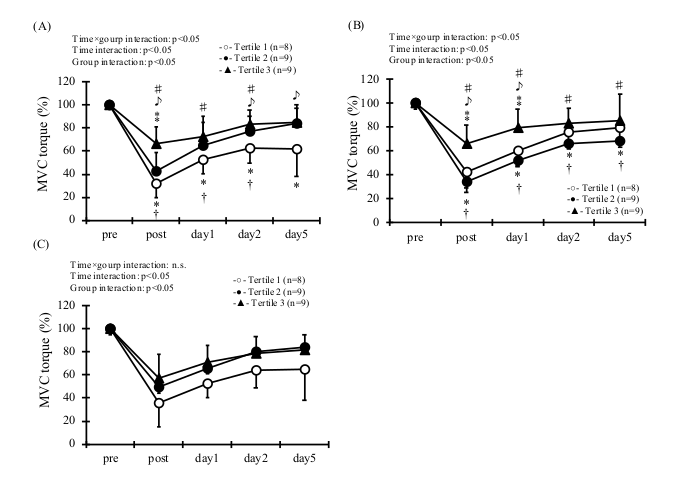
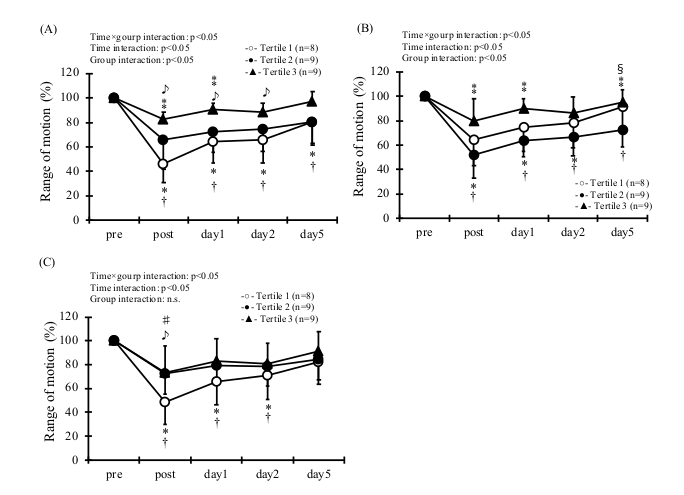
| Quartile | Rage of Concentration (μg/mL) | Age (y) | Height (cm) | Body Mass (kg) | BMI | |
|---|---|---|---|---|---|---|
| EPA | Quartile 1 (n = 8) | 6.9–15.7 | 19.9 ± 1.0 | 170.4 ± 8.9 | 60.2 ± 7.7 | 20.8 ± 2.9 |
| Quartile 2 (n = 8) | 17.3–22.3 | 20.0 ± 1.2 | 171.6 ± 3.9 | 63.9 ± 7.4 | 21.7 ± 2.2 | |
| Quartile 3 (n = 8) | 22.4–29.7 | 20.4 ± 1.1 | 171.7 ± 6.6 | 65.2 ± 6.0 | 22.2 ± 2.3 | |
| Quartile 4 (n = 8) | 30.4–94.5 | 20.0 ± 1.1 | 173.5 ± 7.2 | 73.0 ± 5.3 *† | 24.3 ± 1.8 * | |
| DHA | Quartile 1 (n = 8) | 35.4–58.4 | 19.8 ± 1.0 | 173.3 ± 8.7 | 63.5 ± 9.0 | 21.2 ± 3.0 |
| Quartile 2 (n = 8) | 58.5–66.6 | 19.9 ± 1.1 | 170.0 ± 6.1 | 60.7 ± 6.1 | 21.0 ± 1.5 | |
| Quartile 3 (n = 8) | 70.0–83.7 | 20.3 ± 0.9 | 171.2 ± 5.1 | 65.7 ± 6.6 | 22.4 ± 2.2 | |
| Quartile 4 (n = 8) | 90.8–119.2 | 20.4 ± 1.2 | 172.6 ± 7.0 | 72.6 ± 5.1 † | 24.4 ± 2.1 *† | |
| EPA/AA | Quartile 1 (n = 8) | 0.05–0.08 | 19.9 ± 1.0 | 168.4 ± 7.4 | 60.7 ± 8.6 | 21.4 ± 3.4 |
| Quartile 2 (n = 8) | 0.09–0.12 | 20.0 ± 1.2 | 174.3 ± 5.8 | 65.8 ± 9.0 | 21.6 ± 2.3 | |
| Quartile 3 (n = 8) | 0.12–0.14 | 20.8 ± 0.9 | 170.4 ± 4.2 | 69.2 ± 7.3 | 23.8 ± 2.3 | |
| Quartile 4 (n = 8) | 0.20–0.50 | 19.6 ± 0.9 | 174.1 ± 7.8 | 68.9 ± 5.8 | 22.8 ± 1.9 | |
| All subjects (n = 32) | ― | 20.1 ± 1.0 | 171.8 ± 6.6 | 65.6 ± 7.9 | 22.2 ± 2.6 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochi, E.; Yanagimoto, K.; Tsuchiya, Y. Plasma Eicosapentaenoic Acid Is Associated with Muscle Strength and Muscle Damage after Strenuous Exercise. Sports 2021, 9, 11. https://doi.org/10.3390/sports9010011
Ochi E, Yanagimoto K, Tsuchiya Y. Plasma Eicosapentaenoic Acid Is Associated with Muscle Strength and Muscle Damage after Strenuous Exercise. Sports. 2021; 9(1):11. https://doi.org/10.3390/sports9010011
Chicago/Turabian StyleOchi, Eisuke, Kenichi Yanagimoto, and Yosuke Tsuchiya. 2021. "Plasma Eicosapentaenoic Acid Is Associated with Muscle Strength and Muscle Damage after Strenuous Exercise" Sports 9, no. 1: 11. https://doi.org/10.3390/sports9010011
APA StyleOchi, E., Yanagimoto, K., & Tsuchiya, Y. (2021). Plasma Eicosapentaenoic Acid Is Associated with Muscle Strength and Muscle Damage after Strenuous Exercise. Sports, 9(1), 11. https://doi.org/10.3390/sports9010011





