Gastrocnemius Medialis Architectural Properties in Flexibility Trained and Not Trained Child Female Athletes: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Experimental Design
Anthropometric Characteristics
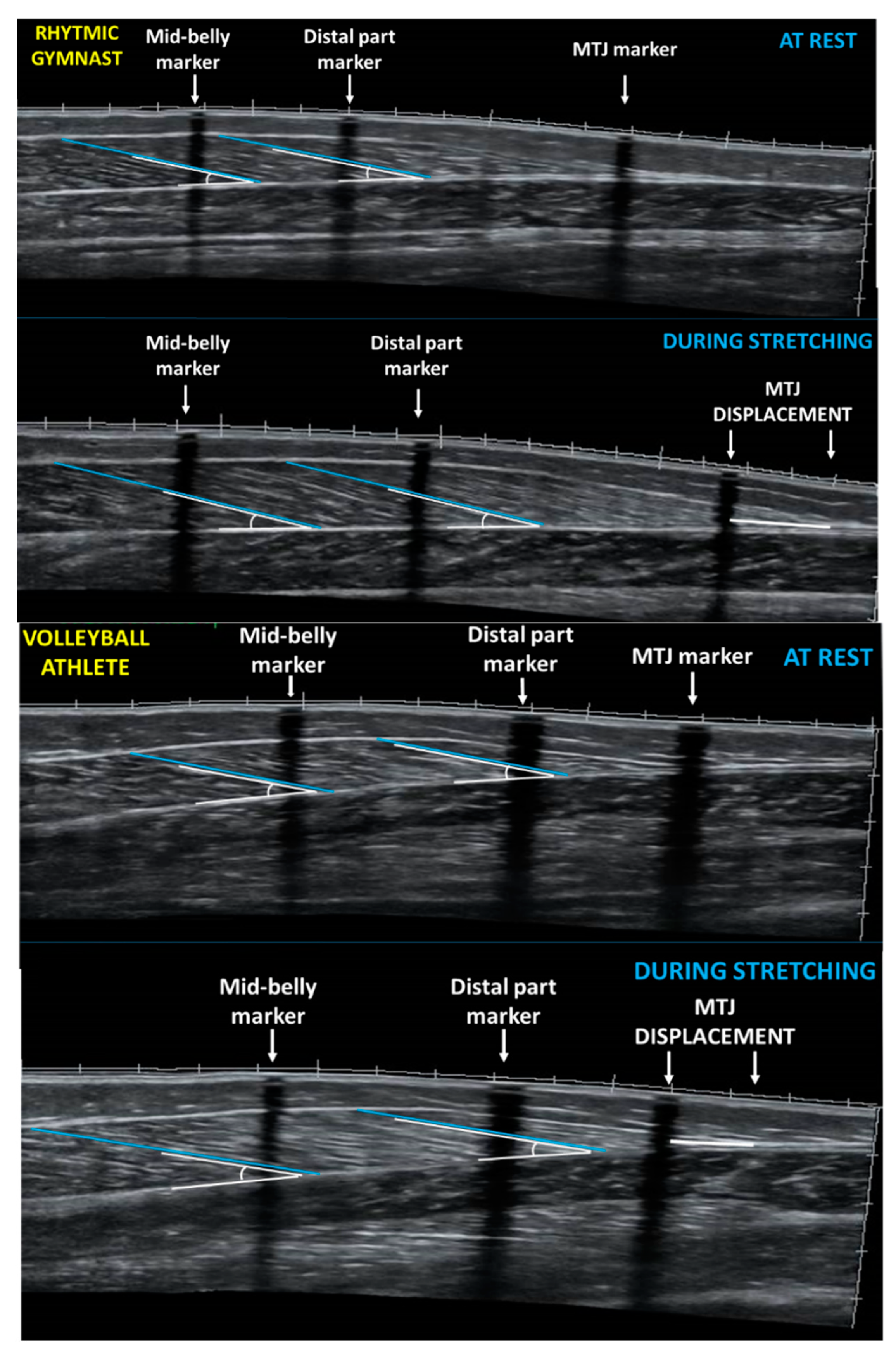
2.3. Gastrocnemius Medialis Architecture and Ankle Joint Angle at Rest
2.4. Gastrocnemius Medialis Architecture and Ankle Joint Angle during Ankle Dorsiflexion Stretching
2.5. Statistical Procedures
3. Results
3.1. Gastrocnemius Medialis Architecture and Ankle Joint Angle at Rest
3.2. Gastrocnemius Medialis Architecture and Ankle Joint Angle during Ankle Dorsiflexion Stretching
3.3. Correlations Between Fascicle Length, Ankle Angles and MTJ Displacement
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nesser, T.W.; Latin, R.W.; Berg, K.; Prentice, E. Physiological determinants of 40-m sprint performance in young male athletes. J. Strength Cond. Res. 1996, 10, 263–267. [Google Scholar] [CrossRef]
- Graf, A.; Judge, J.O.; Õunpuu, S.; Thelen, D.G. The effect of walking speed on lower-extremity joint powers among elderly adults who exhibit low physical performance. Arch. Phys. Med. Rehabil. 2005, 86, 2177–2183. [Google Scholar] [CrossRef]
- Bobbert, M.F.; Mackay, M.; Schinkelshoek, D.; Huijing, P.A.; van Ingen Schenau, G.J. Biomechanical analysis of drop and countermovement jumps. Eur. J. Appl. Physiol. Occup. Physiol. 1986, 54, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.G.; Sumner, B.J.; Kram, R. Muscle contributions to propulsion and braking during walking and running: Insight from external force perturbations. Gait Posture 2014, 40, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Baxter, J.R.; Hullfish, T.J.; Chao, W. Functional deficits may be explained by plantarflexor remodeling following Achilles tendon rupture repair: Preliminary findings. J. Biomech. 2018, 79, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, A.; Wakeling, J.M. Associations between muscle structure and contractile performance in seniors. Clin. Biomech. 2013, 28, 705–711. [Google Scholar] [CrossRef]
- Morse, C.I.; Thom, J.M.; Reeves, N.D.; Birch, K.M.; Narici, M.V. In vivo physiological cross-sectional area and specific force are reduced in the gastrocnemius of elderly men. J. Appl. Physiol. 2005, 99, 1050–1055. [Google Scholar] [CrossRef]
- Legerlotz, K.; Smith, H.K.; Hing, W.A. Variation and reliability of ultrasonographic quantification of the architecture of the medial gastrocnemius muscle in young children. Clin. Physiol. Funct. Imaging 2010, 30, 198–205. [Google Scholar] [CrossRef]
- Lichtwark, G.A.; Wilson, A.M. Optimal muscle fascicle length and tendon stiffness for maximising gastrocnemius efficiency during human walking and running. J. Theor. Biol. 2008, 252, 662–673. [Google Scholar] [CrossRef]
- Benard, M.R.; Harlaar, J.; Becher, J.G.; Huijing, P.A.; Jaspers, R.T. Effects of growth on geometry of gastrocnemius muscle in children: A three-dimensional ultrasound analysis. J. Anat. 2011, 219, 388–402. [Google Scholar] [CrossRef]
- Moltubakk, M.M.; Magulas, M.M.; Villars, F.O.; Seynnes, O.R.; Bojsen-Møller, J. Specialized properties of the triceps surae muscle-tendon unit in professional ballet dancers. Scand. J. Med. Sci. Sports 2018, 28, 2023–2034. [Google Scholar] [CrossRef] [PubMed]
- Sands, W.A.; McNeal, J.R.; Panitente, G.; Murray, S.R.; Nassar, L.; Jemni, M.; Mizuguchi, S.; Stone, M.H. Stretching the spines of gymnasts: A review. Sports Med. 2016, 46, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Blazevich, A.J. Effects of physical training and detraining, immobilisation, growth and aging on human fascicle geometry. Sports Med. 2006, 36, 1003–1017. [Google Scholar] [CrossRef] [PubMed]
- Franchi, M.V.; Reeves, N.D.; Narici, M.V. Skeletal muscle remodeling in response to eccentric vs. concentric loading: Morphological, molecular, and metabolic adaptations. Front. Physiol. 2017, 8, 447. [Google Scholar] [CrossRef]
- Freitas, S.R.; Mendes, B.; Le Sant, G.; Andrade, R.J.; Nordez, A.; Milanovic, Z. Can chronic stretching change the muscle-tendon mechanical properties? A review. Scand. J. Med. Sci. Sport. 2018, 28, 794–806. [Google Scholar] [CrossRef]
- Nuzzo, J.L. The Case for Retiring Flexibility as a Major Component of Physical Fitness. Sports Med. 2019, 1–18. [Google Scholar] [CrossRef]
- Freitas, S.R.; Mil-Homens, P. Effect of 8-week high-intensity stretching training on biceps femoris architecture. Scand. J. Med. Sci. Sports 2015, 29, 1737–1740. [Google Scholar] [CrossRef]
- Ε Lima, K.M.; Carneiro, S.P.; Alves, D.D.S.; Peixinho, C.C.; de Oliveira, L.F. Assessment of muscle architecture of the biceps femoris and vastus lateralis by ultrasound after a chronic stretching program. J. Sport Med. 2015, 25, 55–60. [Google Scholar] [CrossRef]
- Donti, O.; Panidis, I.; Terzis, G.; Bogdanis, G.C. Gastrocnemius Medialis Architectural Properties at Rest and During Stretching in Female Athletes with Different Flexibility Training Background. Sports 2019, 7, 39. [Google Scholar] [CrossRef]
- Donti, O.; Tsolakis, C.; Bogdanis, G.C. Effects of baseline levels of flexibility and vertical jump ability on performance following different volumes of static stretching and potentiating exercises in elite gymnasts. J. Sports Sci. Med. Sports 2014, 13, 105. [Google Scholar]
- Malliaras, P.; Cook, J.L.; Kent, P. Reduced ankle dorsiflexion range may increase the risk of patellar tendon injury among volleyball players. J. Sci. Med. Sport. 2006, 9, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Moltubakk, M.M.H. Effects of Long-Term Stretching Training on Muscle-Tendon Morphology, Mechanics and Function. Ph.D. Thesis, Norges Idrettshøgskole, Oslo, Norway, 2019. [Google Scholar]
- Douda, H.T.; Toubekis, A.G.; Avloniti, A.A.; Tokmakidis, S.P. Physiological and anthropometric determinants of rhythmic gymnastics performance. Int. J. Sports Physiol. Perform. 2008, 3, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Simenz, C.J.; Dugan, C.A.; Ebben, W.P. Strength and conditioning practices of National Basketball Association strength and conditioning coaches. J. Strength Cond. Res. 2005, 19, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Gabbett, T.; Georgieff, B. Physiological and anthropometric characteristics of Australian junior national, state and novice volleyball players. J. Strength Cond. Res. 2007, 21, 902–908. [Google Scholar] [CrossRef]
- Mirwald, R.L.; Baxter-Jones, A.D.; Bailey, D.A.; Beunen, G.P. An assessment of maturity from anthropometric measurements. Med. Sci. Sports Exerc. 2002, 34, 689–694. [Google Scholar] [CrossRef]
- Kawakami, Y.; Ichinose, Y.; Fukunaga, T. Architectural and functional features of human triceps surae muscles during contraction. J. Appl. Physiol. 1998, 85, 398–404. [Google Scholar] [CrossRef]
- Reeves, N.D.; Maganaris, C.N.; Narici, M.V. Ultrasonographic assessment of human skeletal muscle size. Eur. J. Appl. Physiol. 2004, 91, 116–118. [Google Scholar] [CrossRef]
- Noorkoiv, M.; Stavnsbo, A.; Aagaard, P.; Blazevich, A.J. In vivo assessment of muscle fascicle length by extended field-of-view ultrasonography. J. Physiol. 2010, 109, 1974–1979. [Google Scholar] [CrossRef]
- Jung, D.Y.; Koh, E.K.; Kwon, O.Y.; Yi, C.H.; Oh, J.S.; Weon, J.H. Effect of medial arch support on displacement of the myotendinous junction of the gastrocnemius during standing wall stretching. J. Orthop. Sports Phys. Ther. 2009, 39, 867–874. [Google Scholar] [CrossRef]
- Wong, D.L.; Hockenberry-Eaton, M.; Wilson, D.; Winkelstein, M.L.; Schwartz, P. Wong’s Essentials of Pediatric Nursing, 6th ed.; Mosby: St. Louis, MO, USA, 2001; p. 1301. [Google Scholar]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Lieber, R.L.; Friden, J. Functional and clinical significance of skeletal muscle architecture. Muscle Nerve 2000, 23, 1647–1666. [Google Scholar] [CrossRef]
- Ema, R.; Akagi, R.; Wakahara, T.; Kawakami, Y. Training-induced changes in architecture of human skeletal muscles: Current evidence and unresolved issues. J. Sports Med. Phys. Fit. 2016, 5, 37–46. [Google Scholar] [CrossRef]
- O’Brien, T.D.; Reeves, N.D.; Baltzopoulos, V.; Jones, D.A.; Maganaris, C.N. Muscle–tendon structure and dimensions in adults and children. J. Anat. 2010, 216, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.K.; Fortuna, R.; Herzog, W. Distal and proximal fascicle length changes in active and passive human gastrocnemius muscle. J. Undergrad. Res. 2015, 5. [Google Scholar]
- Blazevich, A.J.; Cannavan, D.; Waugh, C.M.; Fath, F.; Miller, S.C.; Kay, A.D. Neuromuscular factors influencing the maximum stretch limit of the human plantar flexors. J. Appl. Physiol. 2012, 113, 1446–1455. [Google Scholar] [CrossRef]
- Simpson, C.L.; Kim, B.D.H.; Bourcet, M.R.; Jones, G.R.; Jakobi, J.M. Stretch training induces unequal adaptation in muscle fascicles and thickness in medial and lateral gastrocnemii. Scand. J. Med. Sci. Sports 2017, 27, 1597–1604. [Google Scholar] [CrossRef]
- Dix, D.J.; Eisenburg, B.R. Myosin mRNA accumulation and myofibrillar genesis at the myotendinous junction of stretched muscle fibres. J. Cell Biol. 1990, 111, 1885–1894. [Google Scholar] [CrossRef]
- Antin, P.B.; Tokunaka, S.; Nachmias, V.T.; Holtzer, H. Role of stress fiber-like structures in assembling nascent myofibrils in myosheets recovering from exposure to ethyl methanesulfonate. J. Cell Biol. 1986, 102, 1464–1479. [Google Scholar] [CrossRef]
- Jakobsen, J.R.; Jakobsen, N.R.; Mackey, A.L.; Koch, M.; Kjaer, M.; Krogsgaard, M.R. Remodeling of muscle fibers approaching the human myotendinous junction. Scand. J. Med. Sci. Sports 2018, 28, 1859–1865. [Google Scholar] [CrossRef]
- Donti, O.; Papia, K.; Toubekis, A.; Donti, A.; Sands, W.A.; Bogdanis, G.C. Flexibility training in preadolescent female athletes: Acute and long-term effects of intermittent and continuous static stretching. J. Sports Sci. 2018, 36, 1453–1460. [Google Scholar] [CrossRef]
- Mayorga Vega, D.; Merino-Marban, R.; Redondo-Martín, F.J.; Viciana, J. Effect of a one-session-per-week physical education-based stretching program on hamstring extensibility in schoolchildren. Kinesiol. Ιnt. J. Fundam. Appl. 2017, 49, 101–108. [Google Scholar] [CrossRef][Green Version]
- Weppler, C.H.; Magnusson, S.P. Increasing muscle extensibility: A matter of increasing length or modifying sensation? Phys. Ther. 2010, 90, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, R.S.; Oliver, J.L. The youth physical development model: A novel approach to long-term athletic development. Strength Cond. J. 2012, 34, 61–72. [Google Scholar] [CrossRef]
- Malina, R.M.; Bouchard, C.; Bar-Or, O. Growth, Maturation, and Physical Activity, 2nd ed.; Human Kinetics: Champaign, IL, USA, 2004; pp. 215–220. [Google Scholar]
| Anthropometric Characteristics | Flexibility Trained Athletes (n = 10) | Flexibility Untrained Athletes (n = 6) | t (14) | p |
|---|---|---|---|---|
| Age (y) | 9.00 ± 0.56 | 9.00 ± 0.63 | 0.000 | 1.000 |
| Training experience (y) | 3.70 ± 1.25 | 2.67 ± 0.52 | 1.906 | 0.770 |
| Height (m) | 1.34 ± 0.61 | 1.38 ± 0.3 | −1.388 | 0.187 |
| Body mass (kg) | 27.57 ± 3.44 | 40.15 ± 5.89 | −5.450 | 0.000 |
| Body Mass Index(kg/m2) | 15.29 ± 1.15 | 21.05 ± 2.39 | −6.565 | 0.000 |
| Knee height (cm) | 29.20 ± 2.03 | 30.32 ± 2.08 | −1.057 | 0.308 |
| Leg length (cm) | 69.30 ± 3.62 | 70.33 ± 2.34 | −0.622 | 0.544 |
| Maturity offset | −5.52 ± 0.17 | −4.99 ± 0.26 | −5.023 | 0.000 |
| Variables | Athletes | Pre−Stretching Measurements | Δ Values (Pre−vs. Stretching) | p | Cohens’ d (Pre–Post Stretching) | Cohens’ d of Δ Values between Groups |
|---|---|---|---|---|---|---|
| Fascicle Length Mid−belly (cm) | FT | 4.19 ± 0.37 | +1.67 ± 0.39 | 0.048 | 5.30 | 1.21 |
| FNT | 4.24 ± 0.54 | +1.28 ± 0.24 | 2.60 | |||
| Fascicle Length Distal part (cm) | FT | 4.25 ± 0.35 | +1.84 ± 0.70 | 0.013 | 4.58 | 1.59 |
| FNT | 4.18 ± 0.65 | +0.97 ± 0.32 | 1.62 | |||
| Thickness Mid−belly (cm) | FT | 1.53 ± 0.12 | +0.23 ± 0.06 | 0.053 | 1.94 | 1.25 |
| FNT | 1.53 ± 0.24 | +0.15 ± 0.10 | 0.73 | |||
| Thickness Distal part (cm) | FT | 0.74 ± 0.22 | +0.46 ± 0.16 | 0.061 | 2.31 | 1.14 |
| FNT | 0.64 ± 0.25 | +0.29 ± 0.16 | 1.20 | |||
| Pennation angle Mid−belly (°) | FT | 21.76 ± 1.76 | −4.93 ± 2.01 | 0.048 | 3.57 | 1.19 |
| FNT | 21.19 ± 1.43 | −2.90 ± 1.41 | 2.09 | |||
| Pennation angle Distal part (°) | FT | 18.00 ± 1.96 | −2.48 ± 2.60 | 0.362 | 1.44 | 0.52 |
| FNT | 19.46 ± 2.51 | −1.35 ± 1.70 | 0.69 | |||
| Ankle angle (°) | FT | 120.9 ± 4.2 | −62.7 ± 6.7 | 0.001 | 13.25 | 2.88 |
| FNT | 111.0 ± 5.8† | −44.9 ± 6.3 | 8.92 | |||
| MTJ Displacement (cm) | FT | +2.31 ± 0.40 | 0.001 | 2.24 | ||
| FNT | +1.54 ± 0.30 † |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panidi, I.; Bogdanis, G.C.; Gaspari, V.; Spiliopoulou, P.; Donti, A.; Terzis, G.; Donti, O. Gastrocnemius Medialis Architectural Properties in Flexibility Trained and Not Trained Child Female Athletes: A Pilot Study. Sports 2020, 8, 29. https://doi.org/10.3390/sports8030029
Panidi I, Bogdanis GC, Gaspari V, Spiliopoulou P, Donti A, Terzis G, Donti O. Gastrocnemius Medialis Architectural Properties in Flexibility Trained and Not Trained Child Female Athletes: A Pilot Study. Sports. 2020; 8(3):29. https://doi.org/10.3390/sports8030029
Chicago/Turabian StylePanidi, Ioli, Gregory C. Bogdanis, Vasiliki Gaspari, Polyxeni Spiliopoulou, Anastasia Donti, Gerasimos Terzis, and Olyvia Donti. 2020. "Gastrocnemius Medialis Architectural Properties in Flexibility Trained and Not Trained Child Female Athletes: A Pilot Study" Sports 8, no. 3: 29. https://doi.org/10.3390/sports8030029
APA StylePanidi, I., Bogdanis, G. C., Gaspari, V., Spiliopoulou, P., Donti, A., Terzis, G., & Donti, O. (2020). Gastrocnemius Medialis Architectural Properties in Flexibility Trained and Not Trained Child Female Athletes: A Pilot Study. Sports, 8(3), 29. https://doi.org/10.3390/sports8030029






