Abstract
Gastrocnemius medialis (GM) architecture and ankle angle were compared between flexibility trained (n = 10) and not trained (n = 6) female athletes, aged 8–10 years. Ankle angle, fascicle length, pennation angle and muscle thickness were measured at the mid-belly and the distal part of GM, at rest and at the end of one min of static stretching. Flexibility trained (FT) and not trained athletes (FNT) had similar fascicle length at the medial (4.19 ± 0.37 vs. 4.24 ± 0.54 cm, respectively, p = 0.841) and the distal part of GM (4.25 ± 0.35 vs. 4.18 ± 0.65 cm, respectively, p = 0.780), similar pennation angles, and muscle thickness (p > 0.216), and larger ankle angle at rest (120.9 ± 4.2 vs. 110.9 ± 5.8°, respectively, p = 0.001). During stretching, FT displayed greater fascicle elongation compared to FNT at the medial (+1.67 ± 0.37 vs. +1.28 ± 0.22 cm, respectively, p = 0.048) and the distal part (+1.84 ± 0.67 vs. +0.97 ± 0.97 cm, respectively, p = 0.013), larger change in joint angle and muscle tendon junction displacement (MTJ) (p < 0.001). Muscle thickness was similar in both groups (p > 0.053). Ankle dorsiflexion angle significantly correlated with fascicle elongation at the distal part of GM (r = −0.638, p < 0.01) and MTJ displacement (r = −0.610, p < 0.05). Collectively, FT had greater fascicle elongation at the medial and distal part of GM and greater MTJ displacement during stretching than FNT of similar age.
1. Introduction
Triceps-surae (gastrocnemius medialis, gastrocnemius lateralis, and soleus) muscles architecture is an important functional characteristic in athletes, patients, and the elderly [1,2], as these muscles are prime movers of the ankle joint during locomotion [3,4]. Longer and less pennate gastrocnemius muscle fascicles increase muscle shortening velocity and thus power output [5] while pennation angle and muscle thickness are positively correlated with muscle force production [6,7].
Maturational growth, from infant to adult, and mechanical stimuli alter triceps-surae muscle architecture [8,9]. During growth, muscles are continuously stretched due to skeletal development [10], but data on gastrocnemius architectural properties in developing children are limited [8,10,11]. Moreover, it is largely unknown how gastrocnemius muscles architecture is related to functional properties in youth athletes [8] although athletes participating in sports like gymnastics, figure skating or ballet, are submitted to regular flexibility training [12].
Various training modes, such as resistance and eccentric training, provide the mechanical stimulus to induce morphological changes in the muscle-tendon unit by altering fascicle length, muscle thickness and volume, and pennation angle [13,14]. The effect of these types of training on muscle structure is well documented however, evidence is limited on stretching interventions [15], although flexibility is considered a major component of physical fitness [16]. Long-term static stretching interventions in humans, examining differences in muscle architecture and joint range of motion (ROM), presented equivocal results [17,18]. For example, Freitas and Mil-Homens [17] found a significant increase in biceps femoris fascicle length (+12.3 mm, p = 0.04) in physically active participants, following 8 weeks of intensive static stretching training (450 sec of stretching repeated 3 times per week). In contrast, Lima et al. [18] did not observe any changes in biceps femoris and vastus lateralis muscles architecture after 8 weeks of training in 12 physically active participants, using a short-duration, static stretching (3 sets of 30 sec of stretching, 3 times per week). The discrepant results between studies may be due to the different stretching training volume and intensity, combined with the short duration of the interventions (~8 weeks) [15]. Along this line, recent cross-sectional studies that examined populations with a chronic flexibility training background (>15 years of systematic stretching) reported that professional ballet dancers [11] and elite level rhythmic gymnasts [19] had longer resting fascicle length in gastrocnemius medialis compared to controls or not trained in flexibility athletes. These studies highlight that muscle architecture differs between athletes with different flexibility-training history; although sport-specific selection criteria or heredity may also be reflected in the dissimilarities in muscle architecture observed. Therefore, examining differences in muscle architecture in youth athletes submitted to different training load characteristics, provides useful information on muscle longitudinal growth in typically developing children and allows for the definition of exercise prescription in clinical populations. To this end, this study examined differences in gastrocnemius medialis (GM) architectural properties at rest and during stretching between child rhythmic gymnasts who trained and competed for at least 2 years, with same age girls participating in volleyball training. Rhythmic gymnasts were selected for their large joint ROM and compliant muscles and also due to their extensive flexibility training [20] while volleyball players were selected because their training included much less stretching training volume [21]. It was hypothesized that flexibility trained child female athletes would have longer fascicles at rest and during stretching compared to same age, flexibility not trained athletes.
2. Materials and Methods
2.1. Subjects
Ten female rhythmic gymnasts and six volleyball players, aged 8–10 years, took part in this study. Rhythmic gymnasts who competed at Hellenic, age-group (8–10 years old) all-around competitions were recruited from different gymnastics clubs and represented the flexibility trained group (FT). Volleyball players were participating in volleyball training in one club and were considered as not trained in flexibility athletes (FNT). Both sports, rhythmic gymnastics and volleyball, involve weight-bearing activities, but, rhythmic gymnastics training includes systematic stretching (≈40–60 min per session), while volleyball training includes <10 min of stretching exercises per session [22,23,24,25]. Athletes’ characteristics are shown in Table 1. Maturity offset was calculated according to Mirwald et al. [26]. Before participating in the study, the athletes and their parents were informed about the aim and procedures of the study and provided written informed consent. The athletes had no injuries of the lower limbs for the past six months. The study design and procedures were in accordance with the declaration of Helsinki. The Institutional Ethics Committee approved the study (registration number: 1040, 14 February 2018).

Table 1.
Anthropometric characteristics of the participants (means ± standard deviation).
2.2. Experimental Design
In order to examine differences in gastrocnemius medialis (GM) architectural properties and ankle angle between child athletes with different flexibility training history, all participants were tested over two sessions. In the first, familiarization session, athletes became familiar with the study protocol. Anthropometric characteristics of the athletes were also assessed during this session. In the main testing session, athletes’ ankle angle and GM architectural characteristics were assessed in two conditions: (a) at rest and (d) during stretching. Resting ankle joint angle and GM architecture (fascicle length, pennation angle, muscle thickness) were assessed with the athletes lying in prone position on a physiotherapy bed for 20 min. (for detailed information, see description below). Following measurements of ankle angle and GM architecture at rest, athletes were standing for two min. Then, athletes performed a 1-min standing ankle dorsiflexion. Five seconds before the end of the stretching intervention a pause was imposed to obtain still ultrasound images. At the end of the 1 min stretching maximal ankle dorsiflexion was also assessed (for detailed information, see description below). Νo intense exercise or stretching was allowed in the 48 h preceding testing.
Anthropometric Characteristics
Height was measured without shoes with the use of a stadiometer, and body mass was measured with a calibrated digital scale (Seca 208 and Seca 710, Hamburg, Germany). Body mass index was calculated as the ratio of body weight to the squared standing height (kg/m2). The length of each lower extremity was measured as the distance between trochanter major to the floor with the participants in standing position. The distance between tibiofemoral joint cleft and medial malleolus was determined as calf length.
2.3. Gastrocnemius Medialis Architecture and Ankle Joint Angle at Rest
In order to avoid trigonometric estimations or multiple scans along the muscle length to be assembled [27], in the present study, panoramic ultrasound images were obtained, via extended-field-of view imaging, along the fascicle length of GM.
All ultrasound measurements were performed in the morning and after athletes remained in a prone position on the examination bed, with their ankles hanging loosely on the outside of the bed for at least 20 min [28]. Muscle architecture of the right leg GM, (dominant leg that is in stance while kicking a ball) was obtained with a 10 MHz linear probe (38 mm) via extended field of view mode (Product model Z5, Shenzhen, Mindray Bio-Medical Electronics Co., Ltd., Shenzhen, China). Ultrasound images were recorded at the medial and the distal part of the GM muscle belly: one-third and half of the distance from the popliteal crease to the center of the medial malleolus, respectively. These points were marked on the skin using an echo-absorptive tape that served as reference marker [28] (Figure 1). In order to measure musculotendinous junction (MTJ) displacement, MTJ was located by real-time static ultrasound imaging and marked on the skin by an echo-absorptive tape, as well. The transducer was orientated perpendicular to the skin and parallel to the fascicles to minimize perspective and parallax measurement errors [29]. A probe path (dashed line) was drawn on the skin with a permanent pen by using static ultrasound according to the fascicle path seen from the ultrasound image. A single view was taken by moving continuously the probe in a slow and steady rate along the marked path. For each part of the muscle (medial and distal), three different fascicle lengths were measured from the deep aponeurosis to the superficial aponeurosis with a linear trace. Where the muscle fascicles met the lower aponeurosis the respective pennation angles were measured. The average of the lengths and angles of the three fascicles was used for statistical analysis for each part of the GM. The distance between the superficial and deep aponeuroses was determined as muscle thickness. Two consecutive measurements for each part of the muscle were assessed, and the average value was used for further analysis. All images were analyzed with image analysis software (Motic Images Plus 2.0, Motic, Hong Kong, China). Test-retest reliability was determined by using the intraclass correlation coefficient on 6 participants, on two separate days. The ICC (two-way random effects) for muscle fascicle length was 0.93 (95% CI: 0.576–0.990, p = 0.000), for muscular thickness it was 0.90 (95% CI: 0.474–0.984, p = 0.001), and for pennation angle, 0.95 (95% CI: 0.689–0.993, p = 0.001).
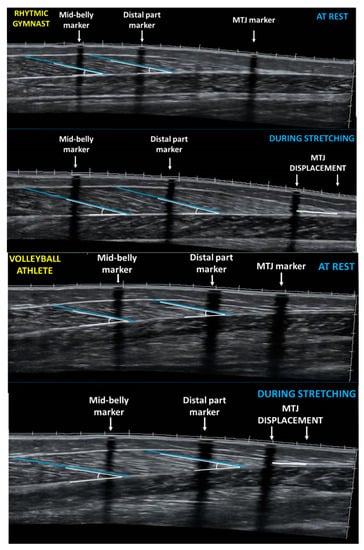
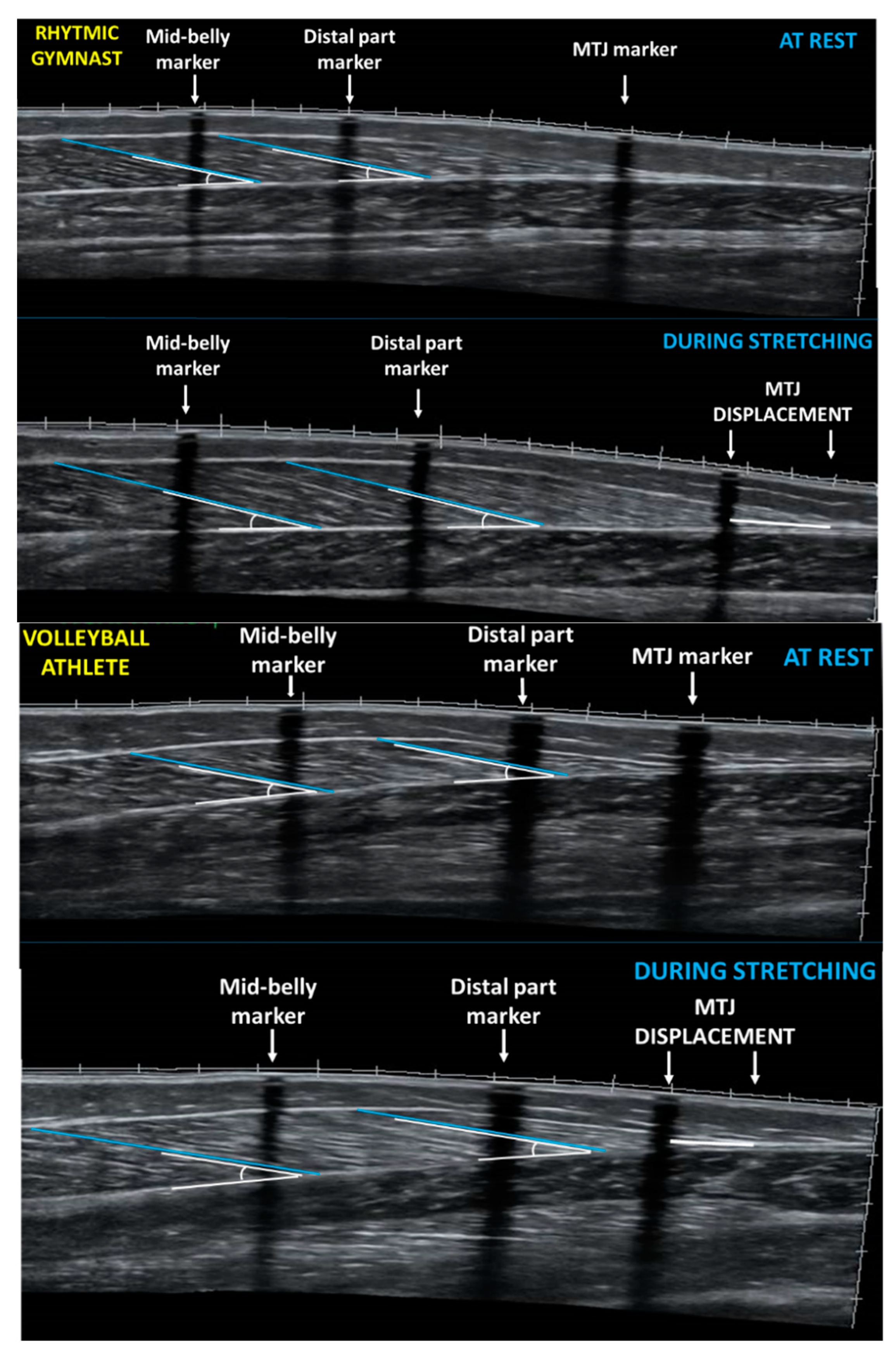
Figure 1.
Panoramic sonographic image of gastrocnemius medialis of a rhythmic gymnast (top panel) and a volleyball player (bottom panel) at rest and during stretching showing fascicle length and pennation angle at the mid-belly and at the distal part of GM. MTJ: muscle-tendon junction.
Ankle joint angle at rest was also measured with the athletes lying in a prone position, with their ankles hanging loosely off the bed. Resting ankle joint angle was defined as the angle created by the intersection of the femur-tibia to lateral malleolus line and lateral malleolus to fifth metatarsal line [19]. Reflective markers were placed on these anatomical points in order to define the ankle angle using a digital camera (Casio Exilim Pro EX-F1, Shibuya, Tokyo, Japan). Image analysis was performed via a free software (Tracker 4.91© 2016 Douglas Brown). Intra-class correlation coefficients for resting ankle angle was 0.98 (95% Confidence Intervals (CI): 0.833–0.998, p = 0.000).
2.4. Gastrocnemius Medialis Architecture and Ankle Joint Angle during Ankle Dorsiflexion Stretching
Panoramic ultrasound images from the two parts of the GM muscle belly (medial and distal) were obtained following the method described above. Reflective motion analysis markers, and echo-absorptive tapes remained on the skin, and the drawn path (dashed line) of the resting measurements was used, to re-assess the regions of interest. Following two minutes of standing, all athletes performed a slow, passive standing dorsiflexion stretching, for one minute. Five seconds before the end of the stretching intervention a pause was imposed to capture still images. To obtain GM ultrasound images during stretching, the probe was placed 38 mm above the skin marker that identified the middle part of the muscle belly. In addition, ‘MTJ displacement’ was defined as the difference between the MTJ marker at rest and MTJ point during stretching (Figure 1).
Ankle dorsiflexion stretching while standing is commonly performed in sport practice [30], and the athletes were familiar with it. Stretching was performed with the athletes barefoot. Athletes were instructed to relax while they passively stretched their ankle plantar flexors, in a slow and continuous manner. The foot to be tested (right) was placed on the midline of a marked area on the floor, and the left foot was placed forward at step-length distance. The end point of standing dorsiflexion stretching was defined as the point that the athletes felt discomfort without lifting their heel and with no pelvic rotation. The athletes put their hands against the wall to maintain balance and were asked to keep the extended position of their hip and knee joints, during stretching [30]. Stretch intensity was indicated by the athletes using the 0–10 Wong-Baker FACES Pain Scale for children [31] to ensure that stretch achieved the point of discomfort (~8 on a scale of 0–10). During the execution of the stretch, participants were instructed to reach a pain of discomfort level of 8 in the scale of 0–10, and thus they held the stretch at exactly this perceived intensity. The psychometric properties of this commonly used pictorial scale assessing acute pain have been found to be appropriate for children over the age of 3 [31]. Six faces depict different expressions, ranging from “no hurt” to “extremely upset from pain”. A digital camera (Casio Exilim Pro EX-F1, Shibuya, Tokyo, Japan) was placed perpendicular to the plane of motion of the right leg in order to record the standing ankle dorsiflexion angle. Stretching ankle joint angle was analyzed using reflective markers placed on the knee, ankle and fifth metatarsal and calculated using free software (Tracker 4.91© 2016 Douglas Brown). Maximal standing dorsiflexion was defined as the intersection of a line joining the knee and ankle markers and horizontal (a line crossing the heel and the fifth metatarsal).
2.5. Statistical Procedures
Descriptive statistics were calculated. Shapiro-Wilks test checked for normality of data distribution. Pearson correlations coefficient (r) detected linear relations between the examined variables. Unpaired T-test examined differences between groups in anthropometry and architectural characteristics of GM at rest. A two-way ANOVA (time x group) with repeated measures for time (rest or stretch) and group (flexibility trained vs. not trained) was conducted separately for the medial and the distal part of the muscle, to examine the effect of stretching on fascicle lengths, pennation angles and thicknesses, and ankle joint angle. A Tukey post-hoc test was performed when a significant main effect or interaction was observed (p < 0.05). Effect sizes calculation for pairwise comparisons was performed with Cohen’s d [32]. To assess test-retest reliability the intra-class correlation coefficients (ICCs) were used. Statistical significance was set at p < 0.05. Statistical analyzes were conducted using SPSS (SPSS Statistics Version 25.0, IBM corporation, Armonk, NY, USA).
3. Results
3.1. Gastrocnemius Medialis Architecture and Ankle Joint Angle at Rest
Resting fascicle length of FT athletes was similar to FNT at the mid-belly (4.19 ± 0.37 vs. 4.24 ± 0.54 cm, respectively, t14 = −0.204, p = 0.841) and the distal part of gastrocnemius medialis (4.25 ± 0.35 vs. 4.18 ± 0.65 cm, respectively, t14 = 0.284, p = 0.780). FT and FNT athletes displayed also similar pennation angle and muscle thickness at the mid-belly (t14 = 0.661, p = 0.519 and t14 = 0.002, p = 0.998, respectively) and the distal part of the gastrocnemius medialis (t14 = −1.297, p = 0.216 and t14 = 0.807, p = 0.433, respectively) (Table 2). Resting ankle angle was larger in FT by 8% compared with FNT (120.86 ± 4.19° vs. 110.95 ± 5.79°, respectively, t14 = 3.982, p = 0.001) (Table 2).

Table 2.
Changes in muscle architecture characteristics and ankle angle following stretching for the flexibility trained (FT) (n = 10) and not trained athletes (FNT) (n = 6).
3.2. Gastrocnemius Medialis Architecture and Ankle Joint Angle during Ankle Dorsiflexion Stretching
During stretching, the elongation of fascicles was greater in FT athletes compared to the FNT at the mid-belly of the muscle by 23% (+1.67 ± 0.37 cm vs. +1.28 ± 0.22 cm, p = 0.048) and the distal part by 47% (+1.84 ± 0.67 vs. +0.97 ± 0.29 cm, p = 0.013). Furthermore, FT athletes displayed greater maximal ankle dorsiflexion by 13% (p < 0.001), as well as greater muscle tendon junction displacement by 33% (p < 0.001) (Table 2). No differences were found between groups in muscle thickness at mid-belly and at the distal part (p > 0.053). However, FNT athletes displayed greater pennation angle at the mid-belly of gastrocnemius medialis (−2.90 ± 1.29 vs. −4.93 ± 1.91 vs., p = 0.048), but not at the distal part (p = 0.362) (Table 2).
3.3. Correlations Between Fascicle Length, Ankle Angles and MTJ Displacement
When all athletes were considered as a group, significant correlations were found between fascicle elongation at the distal part of GM and MTJ displacement (r = 0.752, p < 0.01) and ankle angle during stretching (r =−0.638, p < 0.01). Moreover, a significant correlation was found between MTJ displacement and ankle angle during stretching (r = −0.610, p < 0.05).
4. Discussion
This study examined differences in GM architectural properties at the middle and the distal part of the muscle belly, at rest and during stretching, between flexibility trained and not trained female athletes (rhythmic gymnasts and volleyball players, respectively), aged 8–10 years. The main finding of this study was that, at rest, the two groups displayed similar GM architectural properties, but during stretching FT displayed greater fascicle elongation at the middle and the distal part of GM, and greater MTJ displacement. In addition, FT had larger ankle joint angles at rest and larger change in ankle angle during stretching, compared with FNT, athletes. Significant correlations were found between fascicle elongation at the distal part of GM, MTJ displacement and ankle angle during dorsiflexion.
Gastrocnemius muscle is a prime mover in ankle plantar flexion and thus its architecture is related to force/power production and range of motion [5]. However, chronic modifications to gastrocnemius muscles architecture because of exercise or training in children are currently unknown [33,34,35]. The results of this study indicated that the two groups had similar resting fascicle length at the medial and the distal part of GM. This finding is interesting because recent cross-sectional studies found longer resting fascicle length in flexibility trained, compared with untrained adult participants. For example, a previous study [11] compared professional ballet dancers to controls and found that ballet dancers had longer fascicles in GM in resting prone position (55 ± 5 vs. 47 ± 6 mm, respectively). Another study that examined elite rhythmic gymnasts and female volleyball players, also reported that gymnasts had longer fascicle length at rest at the mid-belly and the distal part of GM compared to volleyball players, by 20 and 18%, respectively [19]. Resting fascicle length has been recently linked with plantar flexion torque and work in healthy adults [34] and was related to the muscle’s force-length relationship [35]. The participants of the present study were growing children, aged 8–10 years. During growth, muscle-tendon units are increased in length, to keep up with increases in bone length [10,35]. Benard et al. [10] examined how maturational growth and skeletal development imparts changes in muscle architecture and found that GM muscle length increases (through an increase in muscle, tendon and fascicle length) approximately 6% per year from age 5 to 12, in proportion with increases in tibia length. That study also reported that the length component of the physiological cross-sectional area of GM as well as muscle fascicles, increased in length [10]. Thus, even if long-term systematic and extensive flexibility training might increase muscle fascicle length, it is plausible that the mechanical stimulus of stretching training is not adequate to induce changes additional to maturational growth in developing children. Nevertheless, cross-sectional study-designs do not imply causation and, at present, the chronic effect of static stretching training on joint range of motion and muscle architecture in humans is not sufficiently documented.
In the present study, fascicle elongation was measured at mid-belly and at the distal part of GM during maximal ankle dorsiflexion. This was because the mid-belly might not accurately reflect muscle architecture across the entire gastrocnemius muscle [36]. The results of this study indicated that fascicle elongation was greater in FT athletes compared to FNT at the middle (p = 0.048, d = 1.21) and the distal part of GM, (p = 0.013, d = 1.59) by 23 and 47%, respectively. This result is in line with previous research reporting greater elongation of GM fascicles in flexible compared to inflexible subjects [19,37]. Importantly, an almost twofold greater elongation was observed in FT athletes at the distal part of GM compared with FNT (+1.84 ± 0.70 vs. 0.97 ± 0.32 cm, respectively, p = 0.013) and significantly greater MTJ displacement (p = 0.001, d = 2.24). Simpson et al. [38] examined adaptations in architectural characteristics of gastrocnemius medialis and lateralis following 6 weeks of stretching training, in adult participants. The authors reported that muscle fascicles in the belly increased by 5.1% by week 6 whereas fascicles in the junction were 25% longer [38]. A previous study also reported that adult rhythmic gymnasts displayed greater fascicle elongation at the distal part compared to volleyball players (45 vs. 39%, respectively, p = 0.026) [19]. This finding highlights that there may be non-uniform morphological adaptations along the length of a bi-articular muscle, like GM, depending on training history. Chronic flexibility training and/or other components of sport-specific training may induce muscle architectural adaptations that differ between the muscle belly and the region near the musculotendinous junction. Previous animal studies identified higher levels of myosin heavy chain mRNA at the MTJ of fibers stretched for 4 days [39] and suggested that fiber lengthening, following stretching, created a need for contractile protein synthesis and assembly into myofibrils at the MTJ [40]. A recent study in humans also reported that following a 4-weeks resistance training intervention, the remodeling of muscle fibres near the MTJ was very high [41].
Enhanced joint ROM following static stretching training has been shown with various stretching protocols in youth athletes or in physical education settings [42,43]. This study examined ankle angle at rest lying in prone position, and during maximal ankle dorsiflexion. The results of the present study indicated that at rest, rhythmic gymnasts had greater ankle angle by 8%, compared with volleyball players (p = 0.001, d = 1.21) (Table 2). A similar finding was reported in a previous study with adult rhythmic gymnasts, and the authors assumed that different resting ankle joint angle between groups may imply a different slack length in the muscles surrounding the ankle joint due to long-term, extensive flexibility training [19]. It is not known whether flexibility training and/or other components of sport-specific training may alter the “neutral”, resting ankle joint angle [11]. Previous studies in adults reported similar ankle joint angles at rest between flexibility trained and not trained subjects [11,37]; however, further research is required on the impact of chronic flexibility training on body tissues determining joints range of motions.
Ankle joint dorsiflexion angle was also significantly greater in FT compared to FNT athletes by 13% (p = 0.001, d = 2.88), and muscle tendon junction displacement by 33% (p < 0.001, d = 2.24) (Table 2). Moltubakk et al. [11] and Donti et al. [19] also found larger ankle dorsiflexion angle in adult ballet dancers and gymnasts compared to controls, a fact mirroring their regular, intensive stretching training. Acute increases in joint ROM following stretching are mainly due to an increased tolerance to stretch [44]. The association of chronic increases in joint ROM with adaptations in muscle architecture has not been clearly established [17,18,37]. Some previous long-term stretching interventions in adults, indicated enhanced joint ROM followed by concomitant increases in fascicle length [15,38] while other long-term stretching interventions failed to detect changes in muscle architecture [18,37]. Amongst the factors determining joint ROM, maximal fascicle elongation at the distal part of the muscle belly and MTJ displacement in the present study, were strongly associated with larger maximal ankle dorsiflexion angle (r = −0.638, p < 0.01, and r = −0.610, p = 0.05, respectively). However, the cross-sectional design of this study limits interpretation of these findings. In addition, available studies indicate that there is considerable variation in GM muscle architecture associated with chronological age [8]. Thus, the small number of participants in this study is a limitation that should be acknowledged. Chronic intervention studies are required in developing athletes, to distinguish genetic or acquired through years of sport-specific training changes in muscle architecture in order to examine the contribution of changes in fascicle length to the increase in muscle length in typically developing children. It should be noted that the time frame of middle childhood (6–11 years) has been proposed as a ‘window of opportunity’ for developing flexibility and as a sensitive period for morphological changes [45,46].
5. Conclusions
Collectively, greater muscle elongation at the mid-belly and the distal part of GM during static stretching, and greater ankle angles at rest and during dorsiflexion were observed in FT compared to FNT female athletes, aged 8–10 years. These findings indicate that between children with different flexibility training history, muscle architecture differs only during stretching, and that there are non-uniform adaptations along GM length depending on training history. Albeit speculative, increased muscle fascicle elongation may represent an early-stage adaptation to stretching-induced increases in resting fascicle length found in flexibility trained, female adult athletes compared with not trained controls.
Author Contributions
Conceptualization, O.D., I.P., and G.C.B.; methodology, I.P., O.D., G.C.B., P.S., and G.T.; data curation, I.P., O.D., V.G.; writing—original draft preparation, O.D., I.P., A.D., writing—review and editing, O.D., G.C.B., and G.T.; supervision, O.D. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no external funding.
Acknowledgments
The authors would like to thank the athletes for their dedicated time and collaboration.
Conflicts of Interest
The authors have no conflict of interest to declare
References
- Nesser, T.W.; Latin, R.W.; Berg, K.; Prentice, E. Physiological determinants of 40-m sprint performance in young male athletes. J. Strength Cond. Res. 1996, 10, 263–267. [Google Scholar] [CrossRef]
- Graf, A.; Judge, J.O.; Õunpuu, S.; Thelen, D.G. The effect of walking speed on lower-extremity joint powers among elderly adults who exhibit low physical performance. Arch. Phys. Med. Rehabil. 2005, 86, 2177–2183. [Google Scholar] [CrossRef]
- Bobbert, M.F.; Mackay, M.; Schinkelshoek, D.; Huijing, P.A.; van Ingen Schenau, G.J. Biomechanical analysis of drop and countermovement jumps. Eur. J. Appl. Physiol. Occup. Physiol. 1986, 54, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.G.; Sumner, B.J.; Kram, R. Muscle contributions to propulsion and braking during walking and running: Insight from external force perturbations. Gait Posture 2014, 40, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Baxter, J.R.; Hullfish, T.J.; Chao, W. Functional deficits may be explained by plantarflexor remodeling following Achilles tendon rupture repair: Preliminary findings. J. Biomech. 2018, 79, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, A.; Wakeling, J.M. Associations between muscle structure and contractile performance in seniors. Clin. Biomech. 2013, 28, 705–711. [Google Scholar] [CrossRef]
- Morse, C.I.; Thom, J.M.; Reeves, N.D.; Birch, K.M.; Narici, M.V. In vivo physiological cross-sectional area and specific force are reduced in the gastrocnemius of elderly men. J. Appl. Physiol. 2005, 99, 1050–1055. [Google Scholar] [CrossRef]
- Legerlotz, K.; Smith, H.K.; Hing, W.A. Variation and reliability of ultrasonographic quantification of the architecture of the medial gastrocnemius muscle in young children. Clin. Physiol. Funct. Imaging 2010, 30, 198–205. [Google Scholar] [CrossRef]
- Lichtwark, G.A.; Wilson, A.M. Optimal muscle fascicle length and tendon stiffness for maximising gastrocnemius efficiency during human walking and running. J. Theor. Biol. 2008, 252, 662–673. [Google Scholar] [CrossRef]
- Benard, M.R.; Harlaar, J.; Becher, J.G.; Huijing, P.A.; Jaspers, R.T. Effects of growth on geometry of gastrocnemius muscle in children: A three-dimensional ultrasound analysis. J. Anat. 2011, 219, 388–402. [Google Scholar] [CrossRef]
- Moltubakk, M.M.; Magulas, M.M.; Villars, F.O.; Seynnes, O.R.; Bojsen-Møller, J. Specialized properties of the triceps surae muscle-tendon unit in professional ballet dancers. Scand. J. Med. Sci. Sports 2018, 28, 2023–2034. [Google Scholar] [CrossRef] [PubMed]
- Sands, W.A.; McNeal, J.R.; Panitente, G.; Murray, S.R.; Nassar, L.; Jemni, M.; Mizuguchi, S.; Stone, M.H. Stretching the spines of gymnasts: A review. Sports Med. 2016, 46, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Blazevich, A.J. Effects of physical training and detraining, immobilisation, growth and aging on human fascicle geometry. Sports Med. 2006, 36, 1003–1017. [Google Scholar] [CrossRef] [PubMed]
- Franchi, M.V.; Reeves, N.D.; Narici, M.V. Skeletal muscle remodeling in response to eccentric vs. concentric loading: Morphological, molecular, and metabolic adaptations. Front. Physiol. 2017, 8, 447. [Google Scholar] [CrossRef]
- Freitas, S.R.; Mendes, B.; Le Sant, G.; Andrade, R.J.; Nordez, A.; Milanovic, Z. Can chronic stretching change the muscle-tendon mechanical properties? A review. Scand. J. Med. Sci. Sport. 2018, 28, 794–806. [Google Scholar] [CrossRef]
- Nuzzo, J.L. The Case for Retiring Flexibility as a Major Component of Physical Fitness. Sports Med. 2019, 1–18. [Google Scholar] [CrossRef]
- Freitas, S.R.; Mil-Homens, P. Effect of 8-week high-intensity stretching training on biceps femoris architecture. Scand. J. Med. Sci. Sports 2015, 29, 1737–1740. [Google Scholar] [CrossRef]
- Ε Lima, K.M.; Carneiro, S.P.; Alves, D.D.S.; Peixinho, C.C.; de Oliveira, L.F. Assessment of muscle architecture of the biceps femoris and vastus lateralis by ultrasound after a chronic stretching program. J. Sport Med. 2015, 25, 55–60. [Google Scholar] [CrossRef]
- Donti, O.; Panidis, I.; Terzis, G.; Bogdanis, G.C. Gastrocnemius Medialis Architectural Properties at Rest and During Stretching in Female Athletes with Different Flexibility Training Background. Sports 2019, 7, 39. [Google Scholar] [CrossRef]
- Donti, O.; Tsolakis, C.; Bogdanis, G.C. Effects of baseline levels of flexibility and vertical jump ability on performance following different volumes of static stretching and potentiating exercises in elite gymnasts. J. Sports Sci. Med. Sports 2014, 13, 105. [Google Scholar]
- Malliaras, P.; Cook, J.L.; Kent, P. Reduced ankle dorsiflexion range may increase the risk of patellar tendon injury among volleyball players. J. Sci. Med. Sport. 2006, 9, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Moltubakk, M.M.H. Effects of Long-Term Stretching Training on Muscle-Tendon Morphology, Mechanics and Function. Ph.D. Thesis, Norges Idrettshøgskole, Oslo, Norway, 2019. [Google Scholar]
- Douda, H.T.; Toubekis, A.G.; Avloniti, A.A.; Tokmakidis, S.P. Physiological and anthropometric determinants of rhythmic gymnastics performance. Int. J. Sports Physiol. Perform. 2008, 3, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Simenz, C.J.; Dugan, C.A.; Ebben, W.P. Strength and conditioning practices of National Basketball Association strength and conditioning coaches. J. Strength Cond. Res. 2005, 19, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Gabbett, T.; Georgieff, B. Physiological and anthropometric characteristics of Australian junior national, state and novice volleyball players. J. Strength Cond. Res. 2007, 21, 902–908. [Google Scholar] [CrossRef]
- Mirwald, R.L.; Baxter-Jones, A.D.; Bailey, D.A.; Beunen, G.P. An assessment of maturity from anthropometric measurements. Med. Sci. Sports Exerc. 2002, 34, 689–694. [Google Scholar] [CrossRef]
- Kawakami, Y.; Ichinose, Y.; Fukunaga, T. Architectural and functional features of human triceps surae muscles during contraction. J. Appl. Physiol. 1998, 85, 398–404. [Google Scholar] [CrossRef]
- Reeves, N.D.; Maganaris, C.N.; Narici, M.V. Ultrasonographic assessment of human skeletal muscle size. Eur. J. Appl. Physiol. 2004, 91, 116–118. [Google Scholar] [CrossRef]
- Noorkoiv, M.; Stavnsbo, A.; Aagaard, P.; Blazevich, A.J. In vivo assessment of muscle fascicle length by extended field-of-view ultrasonography. J. Physiol. 2010, 109, 1974–1979. [Google Scholar] [CrossRef]
- Jung, D.Y.; Koh, E.K.; Kwon, O.Y.; Yi, C.H.; Oh, J.S.; Weon, J.H. Effect of medial arch support on displacement of the myotendinous junction of the gastrocnemius during standing wall stretching. J. Orthop. Sports Phys. Ther. 2009, 39, 867–874. [Google Scholar] [CrossRef]
- Wong, D.L.; Hockenberry-Eaton, M.; Wilson, D.; Winkelstein, M.L.; Schwartz, P. Wong’s Essentials of Pediatric Nursing, 6th ed.; Mosby: St. Louis, MO, USA, 2001; p. 1301. [Google Scholar]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Lieber, R.L.; Friden, J. Functional and clinical significance of skeletal muscle architecture. Muscle Nerve 2000, 23, 1647–1666. [Google Scholar] [CrossRef]
- Ema, R.; Akagi, R.; Wakahara, T.; Kawakami, Y. Training-induced changes in architecture of human skeletal muscles: Current evidence and unresolved issues. J. Sports Med. Phys. Fit. 2016, 5, 37–46. [Google Scholar] [CrossRef]
- O’Brien, T.D.; Reeves, N.D.; Baltzopoulos, V.; Jones, D.A.; Maganaris, C.N. Muscle–tendon structure and dimensions in adults and children. J. Anat. 2010, 216, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.K.; Fortuna, R.; Herzog, W. Distal and proximal fascicle length changes in active and passive human gastrocnemius muscle. J. Undergrad. Res. 2015, 5. [Google Scholar]
- Blazevich, A.J.; Cannavan, D.; Waugh, C.M.; Fath, F.; Miller, S.C.; Kay, A.D. Neuromuscular factors influencing the maximum stretch limit of the human plantar flexors. J. Appl. Physiol. 2012, 113, 1446–1455. [Google Scholar] [CrossRef]
- Simpson, C.L.; Kim, B.D.H.; Bourcet, M.R.; Jones, G.R.; Jakobi, J.M. Stretch training induces unequal adaptation in muscle fascicles and thickness in medial and lateral gastrocnemii. Scand. J. Med. Sci. Sports 2017, 27, 1597–1604. [Google Scholar] [CrossRef]
- Dix, D.J.; Eisenburg, B.R. Myosin mRNA accumulation and myofibrillar genesis at the myotendinous junction of stretched muscle fibres. J. Cell Biol. 1990, 111, 1885–1894. [Google Scholar] [CrossRef]
- Antin, P.B.; Tokunaka, S.; Nachmias, V.T.; Holtzer, H. Role of stress fiber-like structures in assembling nascent myofibrils in myosheets recovering from exposure to ethyl methanesulfonate. J. Cell Biol. 1986, 102, 1464–1479. [Google Scholar] [CrossRef]
- Jakobsen, J.R.; Jakobsen, N.R.; Mackey, A.L.; Koch, M.; Kjaer, M.; Krogsgaard, M.R. Remodeling of muscle fibers approaching the human myotendinous junction. Scand. J. Med. Sci. Sports 2018, 28, 1859–1865. [Google Scholar] [CrossRef]
- Donti, O.; Papia, K.; Toubekis, A.; Donti, A.; Sands, W.A.; Bogdanis, G.C. Flexibility training in preadolescent female athletes: Acute and long-term effects of intermittent and continuous static stretching. J. Sports Sci. 2018, 36, 1453–1460. [Google Scholar] [CrossRef]
- Mayorga Vega, D.; Merino-Marban, R.; Redondo-Martín, F.J.; Viciana, J. Effect of a one-session-per-week physical education-based stretching program on hamstring extensibility in schoolchildren. Kinesiol. Ιnt. J. Fundam. Appl. 2017, 49, 101–108. [Google Scholar] [CrossRef][Green Version]
- Weppler, C.H.; Magnusson, S.P. Increasing muscle extensibility: A matter of increasing length or modifying sensation? Phys. Ther. 2010, 90, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, R.S.; Oliver, J.L. The youth physical development model: A novel approach to long-term athletic development. Strength Cond. J. 2012, 34, 61–72. [Google Scholar] [CrossRef]
- Malina, R.M.; Bouchard, C.; Bar-Or, O. Growth, Maturation, and Physical Activity, 2nd ed.; Human Kinetics: Champaign, IL, USA, 2004; pp. 215–220. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).