A Pilot Study to Examine the Impact of Beta-Alanine Supplementation on Anaerobic Exercise Performance in Collegiate Rugby Athletes
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Approach to the Problem
2.2. Subjects
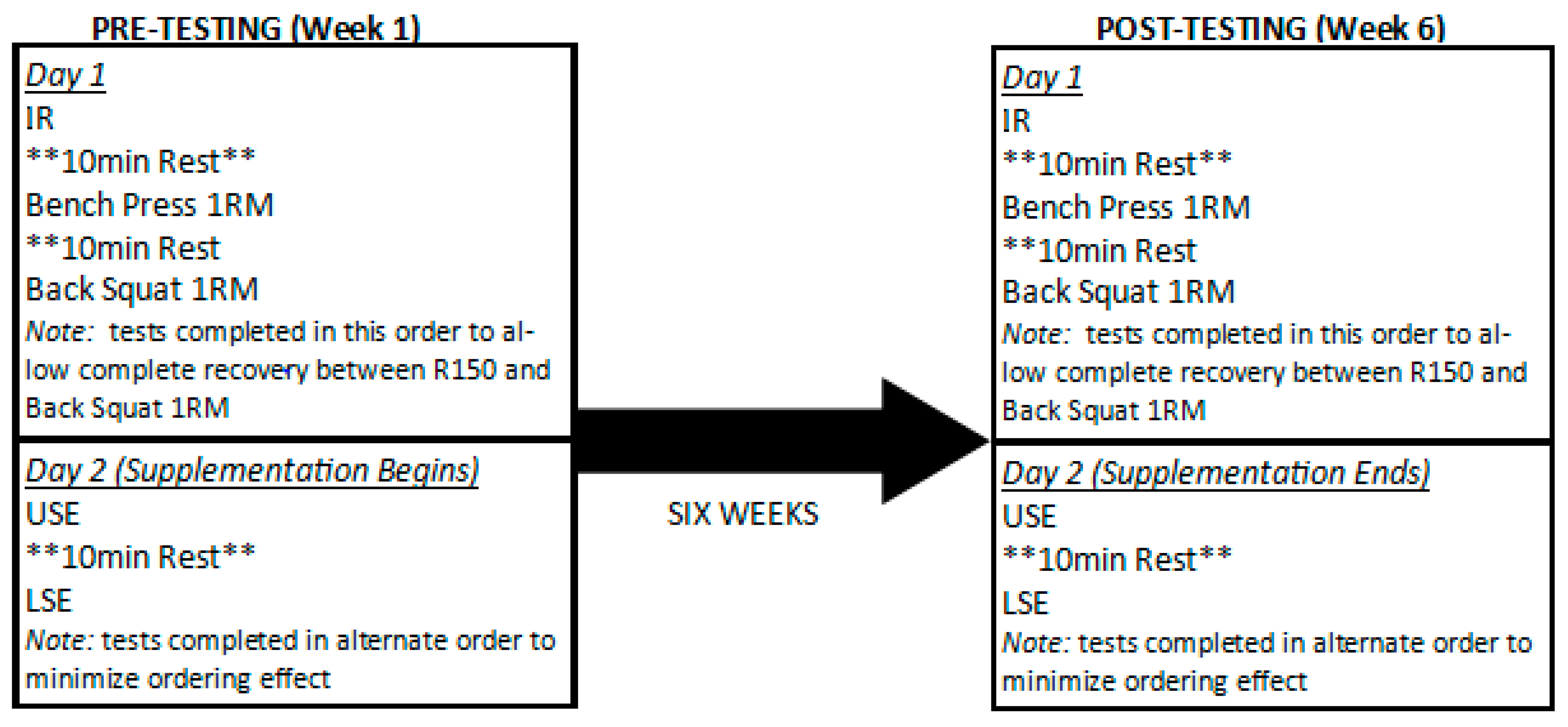
2.3. Procedures
2.3.1. Anthropometry
2.3.2. Dual-Energy X-Ray Absorptiometry (DEXA)
2.3.3. 1 RM Determination
2.3.4. Upper Body and Lower Body Strength Endurance Testing
2.3.5. Intermittent Running Test
2.3.6. Blood Lactate Determination
2.3.7. Heart Rate and Rating of Perceived Exertion (HR and RPE)
2.4. Supplementation
2.5. Training Program
2.6. Statistical Analyses
3. Results
3.1. Dietary Analysis
3.2. Body Composition
3.3. Muscular Strength/Endurance
3.4. Intermittent Sprint Performance and Recovery
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cameron, S.L.; McLay-Cooke, R.T.; Brown, R.C.; Gray, A.R.; Fairbairn, K.A. Increased blood pH but not performance with sodium bicarbonate supplementation in elite rugby union players. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Del Coso, J.; Portillo, J.; Munoz, G.; Abian-Vicen, J.; Gonzalez-Millan, C.; Munoz-Guerra, J. Caffeine-containing energy drink improves sprint performance during an international rugby sevens competition. Amino Acids 2013, 44, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Coutts, A.; Reaburn, P.; Abt, G. Heart rate, blood lactate concentration and estimated energy expenditure in a semi-professional rugby league team during a match: a case study. J. Sports Sci. 2003, 21, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Robergs, R.A.; Ghiasvand, F.; Parker, D. Biochemistry of exercise-induced metabolic acidosis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R502–R516. [Google Scholar] [CrossRef]
- Harris, R.C.; Sale, C. Beta-alanine supplementation in high-intensity exercise. Med. sport sci. 2012, 59, 1–17. [Google Scholar] [CrossRef]
- Blancquaert, L.; Everaert, I.; Derave, W. Beta-alanine supplementation, muscle carnosine and exercise performance. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 63–70. [Google Scholar] [CrossRef]
- Sale, C.; Saunders, B.; Harris, R.C. Effect of beta-alanine supplementation on muscle carnosine concentrations and exercise performance. Amino Acids 2010, 39, 321–333. [Google Scholar] [CrossRef]
- Trexler, E.T.; Smith-Ryan, A.E.; Stout, J.R.; Hoffman, J.R.; Wilborn, C.D.; Sale, C.; Kreider, R.B.; Jager, R.; Earnest, C.P.; Bannock, L.; et al. International society of sports nutrition position stand: Beta-Alanine. J. Int. Soc. Sports Nutr. 2015, 12, 30. [Google Scholar] [CrossRef]
- Artioli, G.G.; Gualano, B.; Smith, A.; Stout, J.; Lancha, A.H., Jr. Role of beta-alanine supplementation on muscle carnosine and exercise performance. Med. Sci. Sports Exerc. 2010, 42, 1162–1173. [Google Scholar] [CrossRef]
- Gardner, M.L.; Illingworth, K.M.; Kelleher, J.; Wood, D. Intestinal absorption of the intact peptide carnosine in man, and comparison with intestinal permeability to lactulose. J. Physiol. 1991, 439, 411–422. [Google Scholar] [CrossRef]
- Harris, R.C.; Tallon, M.J.; Dunnett, M.; Boobis, L.; Coakley, J.; Kim, H.J.; Fallowfield, J.L.; Hill, C.A.; Sale, C.; Wise, J.A. The absorption of orally supplied beta-alanine and its effect on muscle carnosine synthesis in human vastus lateralis. Amino Acids 2006, 30, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.A.; Harris, R.C.; Kim, H.J.; Harris, B.D.; Sale, C.; Boobis, L.H.; Kim, C.K.; Wise, J.A. Influence of beta-alanine supplementation on skeletal muscle carnosine concentrations and high intensity cycling capacity. Amino Acids 2007, 32, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Hobson, R.M.; Saunders, B.; Ball, G.; Harris, R.C.; Sale, C. Effects of beta-alanine supplementation on exercise performance: A meta-analysis. Amino Acids 2012, 43, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Tobias, G.; Benatti, F.B.; de Salles Painelli, V.; Roschel, H.; Gualano, B.; Sale, C.; Harris, R.C.; Lancha, A.H., Jr.; Artioli, G.G. Additive effects of beta-alanine and sodium bicarbonate on upper-body intermittent performance. Amino Acids 2013, 45, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Derave, W.; Ozdemir, M.S.; Harris, R.C.; Pottier, A.; Reyngoudt, H.; Koppo, K.; Wise, J.A.; Achten, E. beta-Alanine supplementation augments muscle carnosine content and attenuates fatigue during repeated isokinetic contraction bouts in trained sprinters. J. Appl. Physiol. 2007, 103, 1736–1743. [Google Scholar] [CrossRef]
- Outlaw, J.J.; Smith-Ryan, A.E.; Buckley, A.L.; Urbina, S.L.; Hayward, S.; Wingfield, H.L.; Campbell, B.; Foster, C.; Taylor, L.W.; Wilborn, C.D. Effects of beta-Alanine on Body Composition and Performance Measures in Collegiate Women. J. Strength. Cond. Res. 2016, 30, 2627–2637. [Google Scholar] [CrossRef]
- Sale, C.; Hill, C.A.; Ponte, J.; Harris, R.C. beta-alanine supplementation improves isometric endurance of the knee extensor muscles. J. Int. Soc. Sports Nutr. 2012, 9, 26. [Google Scholar] [CrossRef]
- Glenn, J.M.; Gray, M.; Stewart, R.W., Jr.; Moyen, N.E.; Kavouras, S.A.; DiBrezzo, R.; Turner, R.; Baum, J.I.; Stone, M.S. Effects of 28-Day Beta-Alanine Supplementation on Isokinetic Exercise Performance and Body Composition in Female Masters Athletes. J. Strength Cond. Res. 2016, 30, 200–207. [Google Scholar] [CrossRef]
- Hoffman, J.R.; Ratamess, N.A.; Faigenbaum, A.D.; Ross, R.; Kang, J.; Stout, J.R.; Wise, J.A. Short-duration beta-alanine supplementation increases training volume and reduces subjective feelings of fatigue in college football players. Nutr. Res. 2008, 28, 31–35. [Google Scholar] [CrossRef]
- Haff, G.; Triplett, N.T. Essentials of Strength Training and Conditioning, 4th ed.; Human Kinetics: Champaign, IL, USA, 2015; p. 752. [Google Scholar]
- Ferguson, T.B.; Syrotuik, D.G. Effects of creatine monohydrate supplementation on body composition and strength indices in experienced resistance trained women. J. Strength Cond. Res. 2006, 20, 939–946. [Google Scholar] [CrossRef]
- Trepanowski, J.F.; Farney, T.M.; McCarthy, C.G.; Schilling, B.K.; Craig, S.A.; Bloomer, R.J. The effects of chronic betaine supplementation on exercise performance, skeletal muscle oxygen saturation and associated biochemical parameters in resistance trained men. J. Strength Cond. Res. 2011, 25, 3461–3471. [Google Scholar] [CrossRef] [PubMed]
- Nana, A.; Slater, G.J.; Stewart, A.D.; Burke, L.M. Methodology review: Using dual-energy X-ray absorptiometry (DXA) for the assessment of body composition in athletes and active people. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 198–215. [Google Scholar] [CrossRef] [PubMed]
- Pyne, D.B.; Boston, T.; Martin, D.T.; Logan, A. Evaluation of the Lactate Pro blood lactate analyser. Eur. J. Appl. Physiol. 2000, 82, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Faraone, S.V. Interpreting Estimates of Treatment Effects: Implications for Managed Care. Pharm. Ther. 2008, 33, 700–711. [Google Scholar]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155. [Google Scholar] [CrossRef]
- Smith, A.E.; Moon, J.R.; Kendall, K.L.; Graef, J.L.; Lockwood, C.M.; Walter, A.A.; Beck, T.W.; Cramer, J.T.; Stout, J.R. The effects of beta-alanine supplementation and high-intensity interval training on neuromuscular fatigue and muscle function. Eur. J. Appl. Physiol. 2009, 105, 357–363. [Google Scholar] [CrossRef]
- Smith, A.E.; Walter, A.A.; Graef, J.L.; Kendall, K.L.; Moon, J.R.; Lockwood, C.M.; Fukuda, D.H.; Beck, T.W.; Cramer, J.T.; Stout, J.R. Effects of beta-alanine supplementation and high-intensity interval training on endurance performance and body composition in men; a double-blind trial. J. Int. Soc. Sports Nutr. 2009, 6, 5. [Google Scholar] [CrossRef]
- Stout, J.R.; Cramer, J.T.; Zoeller, R.F.; Torok, D.; Costa, P.; Hoffman, J.R.; Harris, R.C.; O’Kroy, J. Effects of beta-alanine supplementation on the onset of neuromuscular fatigue and ventilatory threshold in women. Amino Acids 2007, 32, 381–386. [Google Scholar] [CrossRef]
- Culbertson, J.Y.; Kreider, R.B.; Greenwood, M.; Cooke, M. Effects of beta-alanine on muscle carnosine and exercise performance: A review of the current literature. Nutrients 2010, 2, 75–98. [Google Scholar] [CrossRef]
| Outcome | Group | Baseline (95% CI) | Post-Testing (95% CI) | d | Group (p) | Time (p) | G × T (p) |
|---|---|---|---|---|---|---|---|
| Body Mass (kg) | β-alanine | 89.3 ± 14.1 (79.0–99.6) | 88.0 ± 11.7 (79.4–96.7) | 0.02 | 0.416 | 0.064 | 0.895 |
| Placebo | 94.8 ± 12.7 (83.8–105.8) | 93.3 ± 11.0 (84.1–102.6) | |||||
| Percent Bodyfat | β-alanine | 20.9 ± 4.4 (17.4–24.4) | 19.7 ± 4.5 (16.4–23.1) | 0.07 | 0.789 | <0.001 | 0.532 |
| Placebo | 21.7 ± 4.8 (17.9–25.4) | 20.2 ± 4.2 (16.6–23.8) | |||||
| Fat mass (kg) | β-alanine | 18.3 ± 6.7 (13.0–23.6) | 16.9 ± 6.0 (12.5–21.4) | 0.06 | 0.574 | 0.001 | 0.869 |
| Placebo | 20.2 ± 7.2 (14.5–25.9) | 18.4 ± 5.8 (13.6–23.2) | |||||
| Fat-free mass (kg) | β-alanine | 66.7 ± 8.0 (61.0–72.3) | 67.4 ± 6.0 (63.1–71.8) | 0.15 | 0.121 | 0.825 | 0.540 |
| Placebo | 72.7 ± 6.6 (66.7–78.8) | 72.4 ± 5.3 (67.7–77.0) |
| Outcome | Group | Baseline (95% CI) | Post-Testing (95% CI) | d | Group (p) | Time (p) | G × T (p) |
|---|---|---|---|---|---|---|---|
| Bench Press 1 RM (kg) | β-alanine | 104.0 ± 20.6 (87.2–120.9) | 108.7 ± 14.2 (94.7–122.8) | 0.39 | 0.071 | 0.722 | 0.067 |
| Placebo | 128.3 ± 23.8 (110.3–146.3) | 125.1 ± 22.3 (110.1–140.1) | |||||
| Back Squat 1 RM (kg) | β-alanine | 137.8 ± 30.3 (113.9–161.7) | 140.5 ± 27.5 (120.9–160.1) | 0.12 | 0.332 | 0.726 | 0.517 |
| Placebo | 154.2 ± 32.4 (128.7–179.8) | 153.4 ± 23.3 (132.5–174.3) | |||||
| Total Bench Press Repetitions Completed | β-alanine | 50.9 ± 10.2 (45.0–56.7) | 46.9 ± 10.2 (40.7–53.0) | −0.93 | 0.001 | 0.872 | 0.118 |
| Placebo | 32.7 ± 2.3 (26.5–38.9) | 36.0 ± 4.4 (29.4–42.6) | |||||
| Total Back Squat Repetitions Completed | β-alanine | 43.5 ± 14.5 (34.6–52.4) | 47.9 ± 15.8 (38.4–57.3) | 0.33 | 0.589 | 0.150 | 0.231 |
| Placebo | 42.1 ± 6.8 (32.7–51.6) | 42.6 ± 6.2 (32.5–52.7) | |||||
| Bench Press Total Volume Load (kg) | β-alanine | 3654.0 ± 717.1 (3146–4161) | 3485.9 ± 658.6 (3029–3943) | −0.59 | 0.103 | 0.867 | 0.127 |
| Placebo | 2929.2 ± 597.0 (2387–3472) | 3136.6 ± 518.3 (2648–3625) | |||||
| Back Squat Total Volume Load (kg) | β-alanine | 4186.7 ± 1494.9 (3245–5129) | 4626.9 ± 1526.6 (3649–5605) | 0.35 | 0.881 | 0.193 | 0.202 |
| Placebo | 4500.6 ± 830.2 (3493–5508) | 4505.5 ± 911.9 (3460.2–5551) |
| Outcome | Group | Baseline (95% CI) | Post-Testing (95%CI) | d | Group (p) | Time (p) | G × T (p) |
|---|---|---|---|---|---|---|---|
| Total Distance Covered (m) | β-alanine | 648.7 ± 27.5 (620.9–676.4) | 677.2 ± 40.1 (645.2–709.3) | 0.01 | 0.114 | 0.004 | 0.976 |
| Placebo | 617.2 ± 44.5 (587.6–646.9) | 645.3 ± 44.0 (611.0–679.6) | |||||
| Recovery Heart Rate (BPM) | β-alanine | 183.3 ± 9.0 (175.8–190.8) | 180.1 ± 11.6 (172.3–188.0) | 0.29 | 0.263 | 0.120 | 0.623 |
| Placebo | 189.6 ± 9.2 (182.1–197.0) | 183.7 ± 6.9 (175.8–191.6) | |||||
| Recovery Blood Lactate (mmol) | β-alanine | 13.4 ± 2.0 (11.8–15.1) | 10.3 ± 3.5 (8.0–12.6) | −0.94 | 0.655 | 0.029 | 0.126 |
| Placebo | 12.7 ± 2.4 (10.9–14.5) | 12.1 ± 2.4 (9.6–14.6) | |||||
| Recovery RPE (0–10) | β-alanine | 8.8 ± 0.7 (8.2–9.3) | 8.0 ± 1.1 (7.3–8.7) | −1.228 | 0.001 | 0.366 | 0.191 |
| Placebo | 9.4 ± 0.8 (8.8–10.0) | 9.6 ± 0.5 (8.9–10.3) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, C.R.; Harty, P.S.; Stecker, R.A.; Kerksick, C.M. A Pilot Study to Examine the Impact of Beta-Alanine Supplementation on Anaerobic Exercise Performance in Collegiate Rugby Athletes. Sports 2019, 7, 231. https://doi.org/10.3390/sports7110231
Smith CR, Harty PS, Stecker RA, Kerksick CM. A Pilot Study to Examine the Impact of Beta-Alanine Supplementation on Anaerobic Exercise Performance in Collegiate Rugby Athletes. Sports. 2019; 7(11):231. https://doi.org/10.3390/sports7110231
Chicago/Turabian StyleSmith, Charles R., Patrick S. Harty, Richard A. Stecker, and Chad M. Kerksick. 2019. "A Pilot Study to Examine the Impact of Beta-Alanine Supplementation on Anaerobic Exercise Performance in Collegiate Rugby Athletes" Sports 7, no. 11: 231. https://doi.org/10.3390/sports7110231
APA StyleSmith, C. R., Harty, P. S., Stecker, R. A., & Kerksick, C. M. (2019). A Pilot Study to Examine the Impact of Beta-Alanine Supplementation on Anaerobic Exercise Performance in Collegiate Rugby Athletes. Sports, 7(11), 231. https://doi.org/10.3390/sports7110231





