A Brief Review on Concurrent Training: From Laboratory to the Field
Abstract
1. Introduction
2. The Concurrent Training Effect
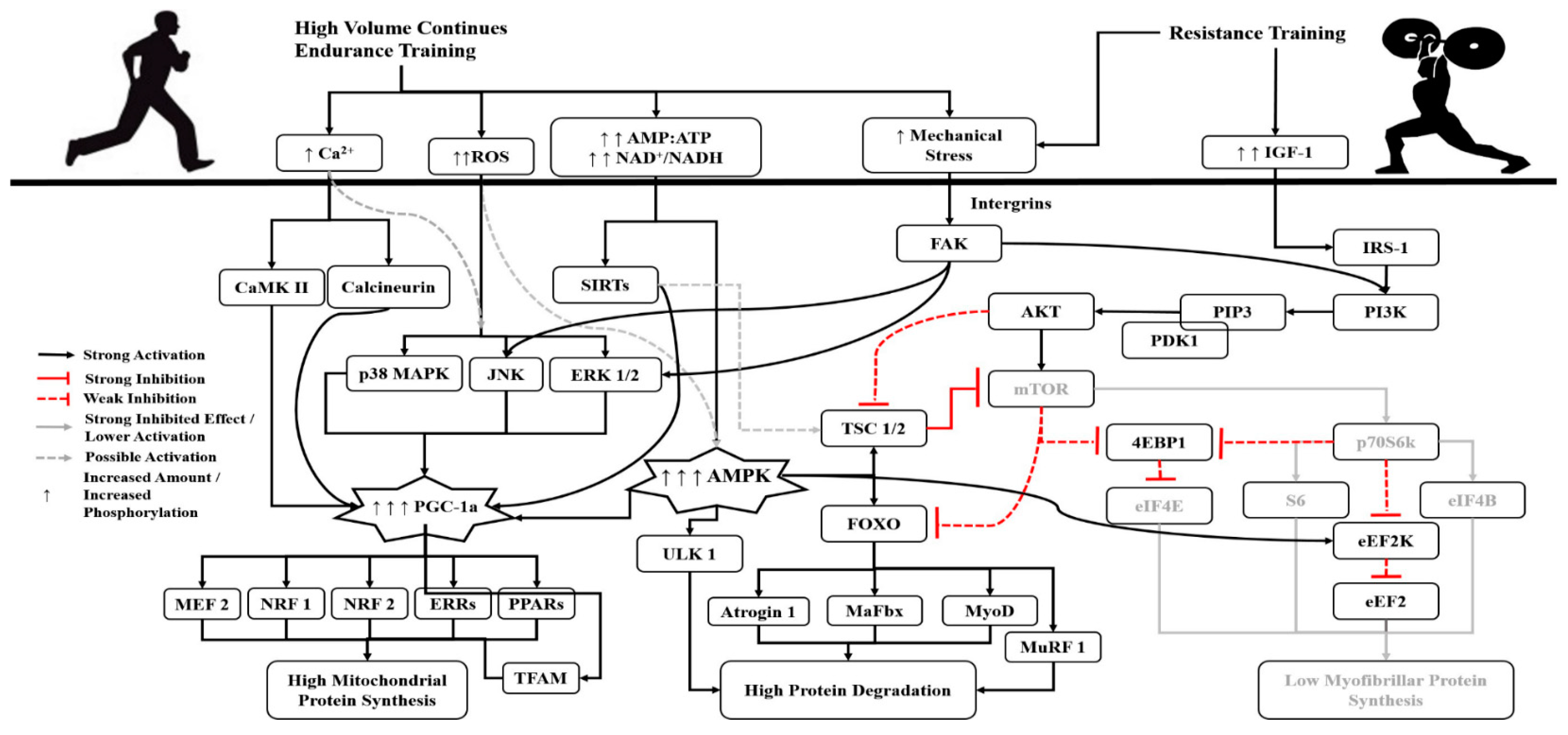
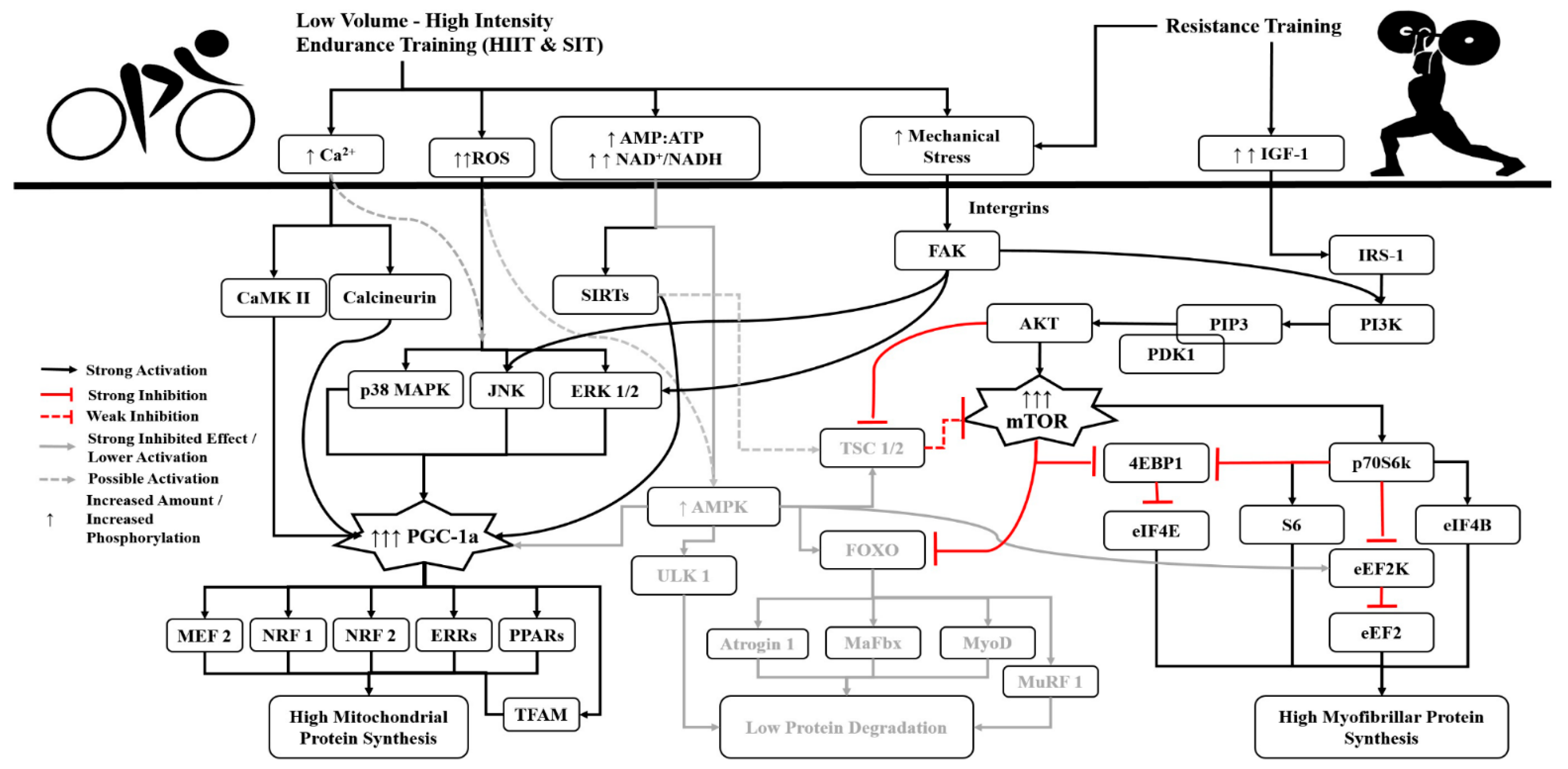
3. The Role of Volume, Intensity and Type of Endurance Training
4. The Role of Training Frequency and Intra-Session Exercise Sequence
5. The Problem of the Acute Studies and the Role of Training History Background
6. Conclusions
- (1)
- The level of fatigue from both modules and the need of inter-stimulus or inter-session time intervals to minimize the training induced overall fatigue.
- (2)
- Consider the training volume of each training regimen of a CT, in an effort to minimize muscle fatigue and energy expenditure.
- (3)
- Incorporate low-volume, high-intensity (maximum and supra-maximum) HIIT or SIT endurance exercises, in an effort to keep low the activation of AMPK.
- (4)
- Where possible, prefer cycling over other types of endurance training.
- (5)
- When the goal is to maximize the resistance training adaptations on muscle mass—strength—power, as well as improve body composition, resistance exercises should be performed prior to endurance exercises.
- (6)
- When the goal of the training is to increase the endurance capacity or when the resistance training adaptations are of lower importance, then endurance exercises should be performed prior to resistance exercises.
- (7)
- Separating training bouts by 3–6 to 24 h, even if this is not always practical to the “real” world of athletes’ training.
- (8)
- Strong consideration of the frequency of each training stimulus. If resistance training-induced adaptations are of importance, consider using a ratio of 2:1 or 3:1 between resistance training sessions per week: endurance training sessions per week. In contrast, it seems that a ratio of 1:1 or 1:2 leads to a better improvement of endurance capacity.
- (9)
- Training experience and background are of high importance for the CTE, which is stronger for experienced participants, while in novice or recreational individuals it is lower. Thus, training plans aiming to maximize performance in well-trained individuals or athletes, through a CT intervention, should be designed very carefully, based on the specific requirement of each sport as well as on the evidence-based suggestions as described above. However, a “new” training stimulus in well-trained individuals, should lead to high and very quick adaptations. Thus, keeping low the volume and the frequency but high the intensity of the new training stimulus may result in increased adaptations from the new training regimen, without, at least in theory, limiting the progression of the commonly used stimuli.
7. Questions to Be Answered in Future Studies
- (1)
- What is the meaning of the acute molecular events that are present after the initial training session for the training-induced adaptations after a longitudinal training intervention?
- (2)
- How is the time-course change of molecular mechanisms responding?
- (3)
- Where is the critical time-point of a training intervention after which the CTE is stronger?
- (4)
- What is the meaning of intra-individuals’ molecular mechanism responses during a CT?
- (5)
- Is there a dose-response relationship between volume—intensity—type—frequency of both endurance and resistance exercise during a CT?
- (6)
- What are the characteristics of the responders and non-responders?
- (7)
- What are the effects of CT, including power and endurance training?
- (8)
- What are the effects of CT in well-trained endurance and/or resistance-trained individuals and/or athletes?
Funding
Acknowledgments
Conflicts of Interest
References
- Issurin, V.B. New horizons for the methodology and physiology of training periodization. Sports Med. 2010, 40, 189–206. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.; Hargreaves, M.; Joyner, M.; Zierath, J. Integrative biology of exercise. Cell 2014, 159, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Schumann, M.; Yli-Peltola, K.; Abbiss, C.; Häkkinen, K. Cardiorespiratory Adaptations during Concurrent Aerobic and Strength Training in Men and Women. PLoS ONE 2015, 10, e0139279. [Google Scholar] [CrossRef] [PubMed]
- Vina, J.; Sanchis-Gomar, F.; Martinez-Bello, V.; Gomez-Cabrera, M. Exercise acts as a drug; the pharmacological benefits of exercise. Br. J. Pharmacol. 2012, 167, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P.; American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef] [PubMed]
- Berryman, N.; Mujika, I.; Arvisais, D.; Roubeix, M.; Binet, C.; Bosquet, L. Strength Training for Middle-and Long-Distance Performance: A Meta-Analysis. Int. J. Sports Physiol. Perform. 2017, 13, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Hickson, R. Interference of strength development by simultaneously training for strength and endurance. Eur. J. Appl. Physiol. 1980, 45, 255–263. [Google Scholar] [CrossRef]
- Wilson, J.M.; Marin, P.J.; Rhea, M.R.; Wilson, S.M.C.; Loenneke, J.P.; Anderson, J.C. Concurrent training: A meta-analysis examining interference of aerobic and resistance exercises. J. Strength Cond. Res. 2012, 26, 2293–2307. [Google Scholar] [CrossRef] [PubMed]
- Dolezal, B.A.; Potteiger, J.A. Concurrent resistance and endurance training influence basal metabolic rate in nondieting individuals. J. Appl. Physiol. 1998, 85, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Hakkinen, K.; Alen, M.; Kraemer, W.J.; Gorostiaga, E.; Izquierdo, M.; Rusko, H.; Mikkola, J.; Hakkinen, A.; Valkeinen, H.; Kaarakainen, E.; et al. Neuromuscular adaptations during concurrent strength and endurance training versus strength training. Eur. J. Appl. Physiol. 2003, 89, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, L.; Watson, A. The interference effects of training for strength and endurance simultaneously. J. Strength Cond. Res. 1994, 8, 12–19. [Google Scholar]
- Hunter, G.; Demment, R.; Miller, D. Development of strength and maximum oxygen uptake during simultaneous training for strength and endurance. J. Sports Med. Phys. Fit. 1987, 27, 269–275. [Google Scholar]
- Kraemer, W.; Patton, J.; Gordon, S.; Harman, E.; Deschenes, M.; Reynolds, K.; Newton, R.; Triplett, N.; Dziados, J. Compatibility of high-intensity strength and endurance training on hormonal and skeletal muscle adaptations. J. Appl. Physiol. 1995, 78, 976–989. [Google Scholar] [CrossRef] [PubMed]
- Leveritt, M.; Abernethy, P. Acute effects of high-intensity endurance exercise on subsequent resistance activity. J. Strength Cond. Res. 1999, 13, 47–51. [Google Scholar]
- McCarthy, J.; Pozniak, M.; Agre, J. Neuromuscular adaptations to concurrent strength and endurance training. Med. Sci. Sports Exerc. 2002, 34, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Terzis, G.; Spengos, K.; Methenitis, S.; Aagaard, P.; Karandreas, N.; Bogdanis, G. Early phase interference between low-intensity running and power training in moderately trained females. Eur. J. Appl. Physiol. 2016, 116, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Tsitkanou, S.; Spengos, K.; Stasinaki, A.N.; Zaras, N.; Bogdanis, G.; Papadimas, G.; Terzis, G. Effects of high-intensity interval cycling performed after resistance training on muscle strength and hypertrophy. Scand. J. Med. Sci. Sports 2017, 27, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Gonzalo, R.; Lundberg, T.R.; Tesch, P.A. Acute molecular responses in untrained and trained muscle subjected to aerobic and resistance exercise training versus resistance training alone. Acta Physiol. 2013, 209, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Fyfe, J.J.; Loenneke, J. Interpreting Adaptation to Concurrent Compared with Single-Mode Exercise Training: Some Methodological Considerations. Sports Med. 2017, 48, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Balabinis, C.; Psarakis, C.; Moukas, M.; Vassiliou, M.; Behrakis, P. Early phase changes by concurrent endurance and strength training. J. Strength Cond. Res. 2003, 17, 393–401. [Google Scholar] [CrossRef]
- McCarthy, J.; Agre, J.; Graf, B.; Pozniak, M.; Vailas, A. Compatibility of adaptive responses with combining strength and endurance training. Med. Sci. Sports Exerc. 1995, 27, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Sillanpää, E.; Häkkinen, A.; Nyman, K.; Mattila, M.; Cheng, S.; Karavirta, L.; Laaksonen, D.; Huuhka, N.; Kraemer, W.; Häkkinen, K. Body composition and fitness during strength and/or endurance training in older men. Med. Sci. Sports Exerc. 2008, 40, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Sillanpää, E.; Laaksonen, D.; Häkkinen, A.; Karavirta, L.; Jensen, B.; Kraemer, W.; Nyman, K.; Häkkinen, K. Body composition, fitness, and metabolic health during strength and endurance training and their combination in middle-aged and older women. Eur. J. Appl. Physiol. 2009, 106, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.F.; Cadore, E.; Kothe, G.; Guedes, M.; Alberton, C.L.; Pinto, S.S.; Pinto, R.; Trindade, G.; Kruel, L. Concurrent training with different aerobic exercises. Int. J. Sports Med. 2012, 33, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Gäbler, M.; Prieske, O.; Hortobágyi, T.; Granacher, U. The Effects of Concurrent Strength and Endurance Training on Physical Fitness and Athletic Performance in Youth: A Systematic Review and Meta-Analysis. Front. Physiol. 2018, 9, 1057. [Google Scholar] [CrossRef] [PubMed]
- Aagaard, P.; Andersen, J. Effects of strength training on endurance capacity in top-level endurance athletes. Scand. J. Med. Sci. Sports 2010, 20, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Paavolainen, L.; Häkkinen, K.; Hämäläinen, I.; Nummela, A.; Rusko, H. Explosive-strength training improves 5-km running time by improving running economy and muscle power. J. Appl. Physiol. 1999, 86, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Aagaard, P.; Andersen, J.L.; Bennekou, M.; Larsson, B.; Olesen, J.L.; Crameri, R.; Magnusson, S.P.; Kjær, M. Effects of resistance training on endurance capacity and muscle fiber composition in young top-level cyclists. Scand. J. Med. Sci. Sports 2011, 21, e298–e307. [Google Scholar] [CrossRef] [PubMed]
- Taipale, R.; Mikkola, J.; Vesterinen, V.; Nummela, A.; Häkkinen, K. Neuromuscular adaptations during combined strength and endurance training in endurance runners: Maximal versus explosive strength training or a mix of both. Eur. J. Appl. Physiol. 2013, 113, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Denadai, B.S.; de Aguiar, R.; de Lima, L.C.R.; Greco, C.C.; Caputo, F. Explosive training and heavy weight training are effective for improving running economy in endurance athletes: A systematic review and meta-analysis. Sports Med. 2017, 47, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Doma, K.; Deakin, G.; Bentley, D. Implications of Impaired Endurance Performance following Single Bouts of Resistance Training: An Alternate Concurrent Training Perspective. Sports Med. (Auckl. NZ) 2017, 47, 2187–2200. [Google Scholar] [CrossRef] [PubMed]
- Karavirta, L.; Häkkinen, K.; Kauhanen, A.; Arija-Blazquez, A.; Sillanpää, E.; Rinkinen, N.; Häkkinen, A. Individual responses to combined endurance and strength training in older adults. Med. Sci. Sports Exerc. 2011, 43, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Coffey, V.; Hawley, J. Concurrent exercise training: Do opposites distract? J. Physiol. 2017, 595, 2883–2896. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.A. Molecular responses to strength and endurance training: Are they incompatible? Appl. Physiol. Nutr. Metab. 2009, 34, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Fyfe, J.J.; Bishop, D.; Stepto, N. Interference between Concurrent Resistance and Endurance Exercise: Molecular Bases and the Role of Individual Training Variables. Sports Med. 2014, 44, 743–762. [Google Scholar] [CrossRef] [PubMed]
- Baar, K. Using molecular biology to maximize concurrent training. Sports Med. 2014, 44, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, D.L.; Philp, A. Can AMPK mediated suppression of mTORC1 explain the concurrent training effect? Cell. Mol. Exerc. Physiol. 2013, 2, e4. [Google Scholar] [CrossRef]
- Hardie, G.; Sakamoto, K. AMPK: A key sensor of fuel and energy status in skeletal muscle. Physiology 2006, 21, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Hardie, G.; Schaffer, B.; Brunet, A. AMPK: An energy-sensing pathway with multiple inputs and outputs. Trends Cell Biol. 2016, 26, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.; Candau, R.; Csibi, A.; Pagano, A.; Raibon, A.; Bernardi, H. The role of AMP-activated protein kinase in the coordination of skeletal muscle turnover and energy homeostasis. Am. J. Physiol. Cell Physiol. 2012, 303, C475–C485. [Google Scholar] [CrossRef] [PubMed]
- Olesen, J.; Kiilerich, K.; Pilegaard, H. PGC-1α-mediated adaptations in skeletal muscle. J. Appl. Physiol. 2010, 460, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Coffey, V.; Pilegaard, H.; Garnham, A.; O’Brien, B.; Hawley, J.A. Consecutive bouts of diverse contractile activity alter acute responses in human skeletal muscle. J. Appl. Physiol. 2009, 106, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.; McCarthy, J.; Zuckerman, P.; Bryan, D.; Bickel, S.; Hunter, G. Frequency of combined resistance and aerobic training in older women. J. Strength Cond. Res. 2013, 27, 1868–1876. [Google Scholar] [CrossRef] [PubMed]
- Fyfe, J.J.; Bishop, D.; Zacharewicz, E.; Russell, A.; Stepto, N. Concurrent exercise incorporating high-intensity interval or continuous training modulates mTORC1 signaling and microRNA expression in human skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R1297–R1311. [Google Scholar] [CrossRef] [PubMed]
- Rønnestad, B.; Hansen, E.; Raastad, T. High volume of endurance training impairs adaptations to 12 weeks of strength training in well-trained endurance athletes. Eur. J. Appl. Physiol. 2012, 112, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Murach, K.; Bagley, J. Skeletal muscle hypertrophy with concurrent exercise training: Contrary evidence for an interference effect. Sports Med. 2016, 46, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Apró, W.; Wang, L.; Pontén, M.; Blomstrand, E.; Sahlin, K. Resistance exercise induced mTORC1 signaling is not impaired by subsequent endurance exercise in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E22–E32. [Google Scholar] [CrossRef] [PubMed]
- De Souza, E.O.; Tricoli, V.; Roschel, H.; Brum, P.C.; Bacurau, A.V.; Ferreira, J.C.; Aoki, M.S.; Neves, M., Jr.; Aihara, A.Y.; da Rocha Correa Fernandes, A. Molecular adaptations to concurrent training. Int. J. Sports Med. 2013, 34, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, T.; Fernandez-Gonzalo, R.; Tesch, P. Exercise-induced AMPK activation does not interfere with muscle hypertrophy in response to resistance training in men. J. Appl. Physiol. 2014, 116, 611–620. [Google Scholar] [CrossRef] [PubMed]
- MacInnis, M.J.; Gibala, M.J. Physiological adaptations to interval training and the role of exercise intensity. J. Physiol. 2016, 595, 2915–2930. [Google Scholar] [CrossRef] [PubMed]
- Gibala, M.J.; Little, J.P.; Macdonald, M.J.; Hawley, J.A. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J. Physiol. 2012, 590, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.; Joo, C.; Jeong, T.S.; Louhelainen, J.; Cochran, A.; Gibala, M.; Gregson, W.; Close, G.; Drust, B.; Morton, J. Matched work high-intensity interval and continuous running induce similar increases in PGC-1α mRNA, AMPK, p38, and p53 phosphorylation in human skeletal muscle. J. Appl. Physiol. 2012, 112, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Batacan, R.; Duncan, M.; Dalbo, V.; Tucker, P.; Fenning, A. Effects of high-intensity interval training on cardiometabolic health: A systematic review and meta-analysis of intervention studies. Br. J. Sports Med. 2017, 51, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Burgomaster, K.; Howarth, K.; Phillips, S.; Rakobowchuk, M.; MacDonald, M.; McGee, S.; Gibala, M. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J. Physiol. 2008, 586, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Gibala, M.J.; McGee, S.L. Metabolic adaptations to short-term high-intensity interval training: A little pain for a lot of gain? Exerc. Sport Sci. Rev. 2008, 36, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Hood, M.; Little, J.; Tarnopolsky, M.; Myslik, F.; Gibala, M. Low-volume interval training improves muscle oxidative capacity in sedentary adults. Med. Sci. Sports Exerc. 2011, 43, 1849–1856. [Google Scholar] [CrossRef] [PubMed]
- Milanović, Z.; Sporiš, G.; Weston, M. Effectiveness of high-intensity interval training (HIT) and continuous endurance training for VO2max improvements: A systematic review and meta-analysis of controlled trials. Sports Med. 2015, 45, 1469–1481. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, S.; Wilson, O.; Taylor, A.; Thøgersen-Ntoumani, C.; Adlan, A.; Wagenmakers, A.; Shaw, C. Low-Volume High-Intensity Interval Training in a Gym Setting Improves Cardio-Metabolic and Psychological Health. PLoS ONE 2015, 10, e0139056. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.W.; Ingham, S.A.; Hunt, J.; Martin, N.; Pringle, J.; Ferguson, R.A. Exercise duration-matched interval and continuous sprint cycling induce similar increases in AMPK phosphorylation, PGC-1α and VEGF mRNA expression in trained individuals. Eur. J. Appl. Physiol. 2016, 116, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Psilander, N.; Tonkonogi, M.; Ding, S.; Sahlin, K. Similar expression of oxidative genes after interval and continuous exercise. Med. Sci. Sports Exerc. 2009, 41, 2136–2144. [Google Scholar] [CrossRef] [PubMed]
- MacInnis, M.; Zacharewicz, E.; Martin, B.; Haikalis, M.; Skelly, L.; Tarnopolsky, M.; Murphy, R.; Gibala, M. Superior mitochondrial adaptations in human skeletal muscle after interval compared to continuous single-leg cycling matched for total work. J. Physiol. 2017, 595, 2955–2968. [Google Scholar] [CrossRef] [PubMed]
- Egan, B.; Carson, B.P.; Garcia-Roves, P.M.; Chibalin, A.V.; Sarsfield, F.M.; Barron, N.; McCaffrey, N.; Moyna, N.M.; Zierath, J.R.; O’Gorman, D.J. Exercise intensity-dependent regulation of peroxisome proliferator-activated receptor coactivator-1 mRNA abundance is associated with differential activation of upstream signalling kinases in human skeletal muscle. J. Physiol. 2010, 588, 1779–1790. [Google Scholar] [CrossRef] [PubMed]
- Combes, A.; Dekerle, J.; Webborn, N.; Watt, P.; Bougault, V.; Daussin, F. Exercise-induced metabolic fluctuations influence AMPK, p38-MAPK and CaMKII phosphorylation in human skeletal muscle. Physiol. Rep. 2015, 3, e12462. [Google Scholar] [CrossRef] [PubMed]
- Pugh, J.; Faulkner, S.; Jackson, A.; King, J.; Nimmo, M. Acute molecular responses to concurrent resistance and high-intensity interval exercise in untrained skeletal muscle. Physiol. Rep. 2015, 3, e12364. [Google Scholar] [CrossRef] [PubMed]
- Lundby, C.; Jacobs, R. Adaptations of skeletal muscle mitochondria to exercise training. Exp. Physiol. 2016, 101, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Lira, V.; Benton, C.; Yan, Z.; Bonen, A. PGC-1α regulation by exercise training and its influences on muscle function and insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E145–E161. [Google Scholar] [CrossRef] [PubMed]
- Rhea, M.; Oliverson, J.; Marshall, G.; Peterson, M.; Kenn, J.; Ayllón, F. Noncompatibility of power and endurance training among college baseball players. J. Strength Cond. Res. 2008, 22, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.; Howatson, G.; Russell, M.; French, D. Performance and neuromuscular adaptations following differing ratios of concurrent strength and endurance training. J. Strength Cond. Res. 2013, 27, 3342–3351. [Google Scholar] [CrossRef] [PubMed]
- Nader, G.A. Concurrent strength and endurance training: From molecules to man. Med. Sci. Sports Exerc. 2006, 38, 1965–1970. [Google Scholar] [CrossRef] [PubMed]
- Enright, K.; Morton, J.; Iga, J.; Drust, B. The effect of concurrent training organisation in youth elite soccer players. Eur. J. Appl. Physiol. 2015, 115, 2367–2381. [Google Scholar] [CrossRef] [PubMed]
- Levin, G.T.; Mcguigan, M.R.; Laursen, P.B. Effect of concurrent resistance and endurance training on physiologic and performance parameters of well-trained endurance cyclists. J. Strength Cond. Res. 2009, 23, 2280–2286. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, T.; Fernandez-Gonzalo, R.; Gustafsson, T.; Tesch, P.A. Aerobic exercise does not compromise muscle hypertrophy response to short-term resistance training. J. Appl. Physiol. 2013, 114, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Millet, G.; Jaouen, B.; Borrani, F.; Candau, R. Effects of concurrent endurance and strength training on running economy and VO2 kinetics. Med. Sci. Sports Exerc. 2002, 34, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Murlasits, Z.; Kneffel, Z.; Thalib, L. The physiological effects of concurrent strength and endurance training sequence: A systematic review and meta-analysis. J. Sports Sci. 2017, 36, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Sabag, A.; Najafi, A.; Michael, S.; Esgin, T.; Halaki, M.; Hackett, D. The compatibility of concurrent high intensity interval training and resistance training for muscular strength and hypertrophy: A systematic review and meta-analysis. J. Sports Sci. 2018, 36, 2472–2483. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.; Marinho, D.; Gil, M.; Izquierdo, M.; Rodríguez-Rosell, D.; Neiva, H.; Marques, M. Concurrent training followed by detraining: Does the resistance training intensity matter? J. Strength Cond. Res. 2017, 32, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Vorup, J.; Tybirk, J.; Gunnarsson, T.; Ravnholt, T.; Dalsgaard, S.; Bangsbo, J. Effect of speed endurance and strength training on performance, running economy and muscular adaptations in endurance-trained runners. Eur. J. Appl. Physiol. 2016, 116, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Hartman, J.; Phillips, S. Increased muscle oxidative potential following resistance training induced fibre hypertrophy in young men. Appl. Physiol. Nutr. Metab. 2006, 31, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Panissa, V.; Tricoli, V.; Julio, U.; Ribeiro, N.; de Azevedo Neto, R.; Carmo, E.; Franchini, E. Acute effect of high-intensity aerobic exercise performed on treadmill and cycle ergometer on strength performance. J. Strength Cond. Res. 2015, 29, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Panissa, V.; Julio, U.; Andreato, L.; Hardt, F.; Franchini, E. Effects of interval time between high-intensity intermittent aerobic exercise on strength performance: Analysis in individuals with different training background. J. Hum. Sport Exer. 2012, 7, 815–825. [Google Scholar] [CrossRef]
- Kazior, Z.; Willis, S.; Moberg, M.; Apró, W.; Calbet, J.; Holmberg, H.C.; Blomstrand, E. Endurance exercise enhances the effect of strength training on muscle fiber size and protein expression of Akt and mTOR. PLoS ONE 2016, 11, e0149082. [Google Scholar] [CrossRef] [PubMed]
- Gergley, J. Comparison of two lower-body modes of endurance training on lower-body strength development while concurrently training. J. Strength Cond. Res. 2009, 23, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.; Walshe, I.; Hamilton, D.; Howatson, G.; Russell, M.; Price, O.; Gibson, A.; French, D. Signaling Responses After Varying Sequencing of Strength and Endurance Training in a Fed State. Int. J. Sports Physiol. Perform. 2016, 11, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Coffey, V.; Jemiolo, B.; Edge, J.; Garnham, A.P.; Trappe, S.W.; Hawley, J.A. Effect of consecutive repeated sprint and resistance exercise bouts on acute adaptive responses in human skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R1441–R1451. [Google Scholar] [CrossRef] [PubMed]
- Eklund, D.; Schumann, M.; Kraemer, W.J.; Izquierdo, M.; Taipale, R.S.; Häkkinen, K. Acute endocrine and force responses and long-term adaptations to same-session combined strength and endurance training in women. J. Strength Cond. Res. 2016, 30, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Taipale, R.; Schumann, M.; Mikkola, J.; Nyman, K.; Kyrolainen, H.; Nummela, A.; Hakkinen, K. Acute neuromuscular and metabolic responses to combined strength and endurance loadings: The “order effect” in recreationally endurance trained runners. J. Sports Sci. 2014, 32, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Chtara, M.; Chamari, K.; Chaouachi, M.; Chaouachi, A.; Koubaa, D.; Feki, Y.; Millet, G.; Amri, M. Effects of intra-session concurrent endurance and strength training sequence on aerobic performance and capacity. Br. J. Sports Med. 2005, 39, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Chtara, M.; Chaouachi, A.; Levin, G.; Chaouachi, M.; Chamari, K.; Amri, M.; Laursen, P. Effect of concurrent endurance and circuit resistance training sequence on muscular strength and power development. J. Strength Cond. Res. 2008, 22, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Eddens, L.; van Someren, K.; Howatson, G. The role of intra-session exercise sequence in the interference effect: A systematic review with meta-analysis. Sports Med. 2017, 48, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Cadore, E.; Izquierdo, M.; Pinto, S.; Alberton, C.; Pinto, R.; Baroni, B.; Vaz, M.; Lanferdini, F.; Radaelli, R.; González-Izal, M. Neuromuscular adaptations to concurrent training in the elderly: Effects of intrasession exercise sequence. Age 2013, 35, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.; Snow, T. Are adaptations to combined endurance and strength training affected by the sequence of training? J. Sports Sci. 1993, 11, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Schumann, M.; Küüsmaa, M.; Newton, R.; Sirparanta, A.; Syväoja, H.; Häkkinen, A.; Häkkinen, K. Fitness and lean mass increases during combined training independent of loading order. Med. Sci. Sports Exerc. 2014, 46, 1758–1768. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, E.; Rech, A.; Minozzo, F.; Botton, C.; Radaelli, R.; Teixeira, B.; Reischak-Oliveira, A.; Pinto, R. Concurrent strength and endurance training exercise sequence does not affect neuromuscular adaptations in older men. Exp. Gerontol. 2014, 60, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.; Saavedra, F.; Simão, R.; Novaes, J.; Rhea, M.; Green, D.; Reis, V.M. Does aerobic and strength exercise sequence in the same session affect the oxygen uptake during and postexercise? J. Strength Cond. Res. 2012, 26, 1872–1878. [Google Scholar] [CrossRef] [PubMed]
- McGawley, K.; Andersson, P. The order of concurrent training does not affect soccer-related performance adaptations. Int. J. Sports Med. 2013, 34, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Tarasi, Z.; Beiki, Y.; Hossini, F.; Malaei, M. The effect of the sequence of concurrent strength and endurance training on aerobic capacity, anaerobic capacity and maximum strength of male adolescents. Aust. J. Basic Appl. Sci. 2011, 5, 1195–1201. [Google Scholar]
- Sale, D.G.; Jacobs, I.; MacDougall, J.D.; Garner, S. Comparison of two regimens of concurrent strength and endurance training. Med. Sci. Sports Exerc. 1990, 22, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Robineau, J.; Babault, N.; Piscione, J.; Lacome, M.; Bigard, A.X. The specific training effects of concurrent aerobic and strength exercises depends on recovery duration. J. Strength Cond. Res. 2016, 30, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Sporer, B.; Wenger, H. Effects of aerobic exercise on strength performance following various periods of recovery. J. Strength Cond. Res. 2003, 17, 638–644. [Google Scholar] [PubMed]
- Coffey, V.G.; Zhong, Z.; Shield, A.; Canny, B.J.; Chibalin, A.V.; Zierath, J.R.; Hawley, J.A. Early signaling responses to divergent exercise stimuli in skeletal muscle from well-trained humans. FASEB J. 2006, 20, 190–192. [Google Scholar] [CrossRef] [PubMed]
- Makanae, Y.; Ogasawara, R.; Fujita, S. Skeletal muscle signaling response to concurrent endurance and resistance exercise. J. Phys. Fit. Sports Med. 2015, 4, 217–221. [Google Scholar] [CrossRef]
- Schumann, M.; Eklund, D.; Taipale, R.; Nyman, K.; Kraemer, W.; Häkkinen, A.; Izquierdo, M.; Häkkinen, K. Acute neuromuscular and endocrine responses and recovery to single-session combined endurance and strength loadings: “Order effect” in untrained young men. J. Strength Cond. Res. 2013, 27, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Apró, W.; Moberg, M.; Hamilton, L.; Ekblom, B.; van Hall, G.; Holmberg, H.C.; Blomstrand, E. Resistance exercise-induced S6K1 kinase activity is not inhibited in human skeletal muscle despite prior activation of AMPK by high-intensity interval cycling. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E470–E481. [Google Scholar] [CrossRef] [PubMed]
- Donges, C.E.; Burd, N.A.; Duffield, R.; Smith, G.C.; West, D.W.; Short, M.J.; Mackenzie, R.; Plank, L.D.; Shepherd, P.R.; Phillips, S.M.; et al. Concurrent resistance and aerobic exercise stimulates both myofibrillar and mitochondrial protein synthesis in sedentary middle-aged men. J. Appl. Physiol. (1985) 2012, 112, 1992–2001. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, N.; Parker, B.; Chaudhuri, R.; Fisher-Wellman, K.; Kleinert, M.; Humphrey, S.; Yang, P.; Holliday, M.; Trefely, S.; Fazakerley, D. Global phosphoproteomic analysis of human skeletal muscle reveals a network of exercise-regulated kinases and AMPK substrates. Cell Metab. 2015, 22, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Benziane, B.; Burton, T.J.; Scanlan, B.; Galuska, D.; Canny, B.J.; Chibalin, A.V.; Zierath, J.R.; Stepto, N.K. Divergent cell signaling after short-term intensified endurance training in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1427–E1438. [Google Scholar] [CrossRef] [PubMed]
- Perry, C.; Lally, J.; Holloway, G.; Heigenhauser, G.; Bonen, A.; Spriet, L.L. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J. Physiol. 2010, 588, 4795–4810. [Google Scholar] [CrossRef] [PubMed]
- Nader, G.A.; von Walden, F.; Liu, C.; Lindvall, J.; Gutmann, L.; Pistilli, E.; Gordon, P. Resistance exercise training modulates acute gene expression during human skeletal muscle hypertrophy. J. Appl. Physiol. 2014, 116, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Atherton, P.J.; Babraj, J.; Smith, K.; Singh, J.; Rennie, M.J.; Wackerhage, H. Selective activation of AMPK-PGC-1alpha or PKB-TSC2-mTOR signaling can explain specific adaptive responses to endurance or resistance training-like electrical muscle stimulation. FASEB J. 2005, 19, 786–788. [Google Scholar] [CrossRef] [PubMed]
- Camera, D.; Edge, J.; Short, M.; Hawley, J.; Coffey, V. Early time course of Akt phosphorylation after endurance and resistance exercise. Med. Sci. Sports Exerc. 2010, 42, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Mascher, H.; Andersson, H.; Nilsson, P.A.; Ekblom, B.; Blomstrand, E. Changes in signalling pathways regulating protein synthesis in human muscle in the recovery period after endurance exercise. Acta Physiol. 2007, 191, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Nader, G.A.; Esser, K.A. Intracellular signaling specificity in skeletal muscle in response to different modes of exercise. J. Appl. Physiol. 2001, 90, 1936–1942. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Goodyear, L. Invited review: Intracellular signaling in contracting skeletal muscle. J. Appl. Physiol. 2002, 93, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Stuart, C.; Howell, M.; Baker, J.; Dykes, R.; Duffourc, M.; Ramsey, M.; Stone, M. Cycle training increased GLUT4 and activation of mTOR in fast twitch muscle fibers. Med. Sci. Sports Exerc. 2010, 42, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.B.; Phillips, S.M.; Atherton, P.J.; Patel, R.; Yarasheski, K.E.; Tarnopolsky, M.A.; Rennie, M.J. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J. Physiol. 2008, 586, 3701–3717. [Google Scholar] [CrossRef] [PubMed]
- Konopka, A.; Harber, M. Skeletal muscle hypertrophy after aerobic exercise training. Exerc. Sport Sci. Rev. 2014, 42, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, H.C.; Fujita, S.; Cadenas, J.G.; Chinkes, D.L.; Volpi, E.; Rasmussen, B.B. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J. Physiol. 2006, 576, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Koopman, R.; Zorenc, A.H.; Gransier, R.J.; Cameron-Smith, D.; van Loon, L.J. Increase in S6K1 phosphorylation in human skeletal muscle following resistance exercise occurs mainly in type II muscle fibers. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E1245–E1252. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Methenitis, S. A Brief Review on Concurrent Training: From Laboratory to the Field. Sports 2018, 6, 127. https://doi.org/10.3390/sports6040127
Methenitis S. A Brief Review on Concurrent Training: From Laboratory to the Field. Sports. 2018; 6(4):127. https://doi.org/10.3390/sports6040127
Chicago/Turabian StyleMethenitis, Spyridon. 2018. "A Brief Review on Concurrent Training: From Laboratory to the Field" Sports 6, no. 4: 127. https://doi.org/10.3390/sports6040127
APA StyleMethenitis, S. (2018). A Brief Review on Concurrent Training: From Laboratory to the Field. Sports, 6(4), 127. https://doi.org/10.3390/sports6040127




