Is There a Progressive Withdrawal of Physiological Protections against High-Intensity Exercise-Induced Fatigue during Puberty?
Abstract
:1. Introduction
2. Fatigue and Maturation
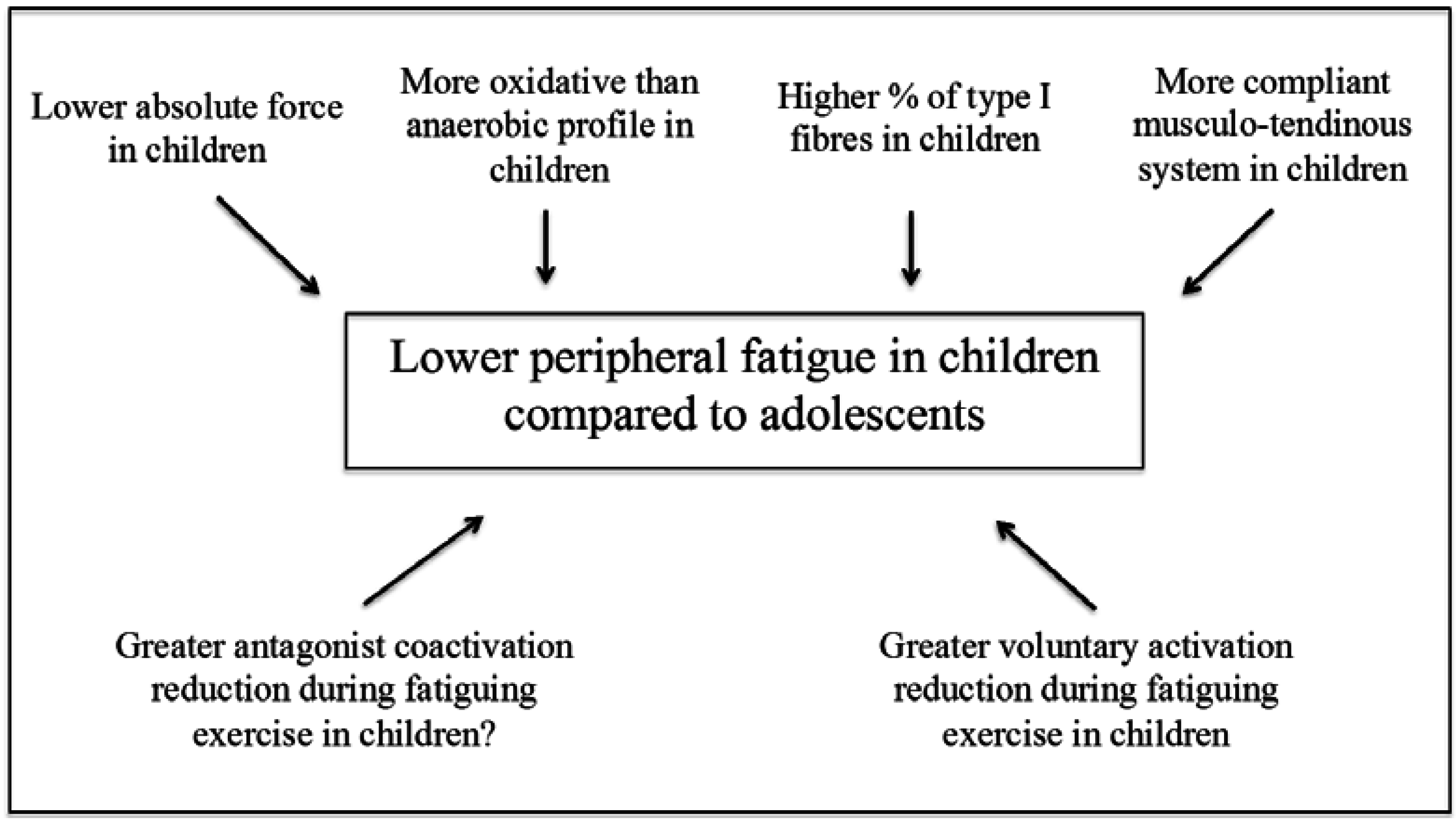
3. Peripheral Factors
3.1. Muscle Mass and Fibre Type Composition
3.2. Energy Metabolism
3.3. Musculo-Tendinous Stiffness
4. Central Factors
4.1. Voluntary Activation
4.2. Antagonist Co-Activation
5. Interplay between Peripheral and Central Factors
6. Conclusions
Author Contributions
Conflicts of Interest
References
- Vollestad, N.K. Measurement of human muscle fatigue. J. Neurosci. Methods 1997, 74, 219–227. [Google Scholar] [CrossRef]
- Ratel, S.; Duche, P.; Hennegrave, A.; van Praagh, E.; Bedu, M. Acid-base balance during repeated cycling sprints in boys and men. J. Appl. Physiol. 2002, 92, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Ratel, S.; Williams, C.A.; Oliver, J.; Armstrong, N. Effects of age and recovery duration on performance during multiple treadmill sprints. Int. J. Sports Med. 2006, 27, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ratel, S.; Bedu, M.; Hennegrave, A.; Dore, E.; Duche, P. Effects of age and recovery duration on peak power output during repeated cycling sprints. Int. J. Sports Med. 2002, 23, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Dipla, K.; Tsirini, T.; Zafeiridis, A.; Manou, V.; Dalamitros, A.; Kellis, E.; Kellis, S. Fatigue resistance during high-intensity intermittent exercise from childhood to adulthood in males and females. Eur. J. Appl. Physiol. 2009, 106, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Zafeiridis, A.; Dalamitros, A.; Dipla, K.; Manou, V.; Galanis, N.; Kellis, S. Recovery during high-intensity intermittent anaerobic exercise in boys, teens, and men. Med. Sci. Sports Exerc. 2005, 37, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.C.; Chen, H.L.; Liu, Y.C.; Nosaka, K. Eccentric exercise-induced muscle damage of pre-adolescent and adolescent boys in comparison to young men. Eur. J. Appl. Physiol. 2014, 114, 1183–1195. [Google Scholar] [CrossRef] [PubMed]
- Van Praagh, E.; Dore, E. Short-term muscle power during growth and maturation. Sports Med. 2002, 32, 701–728. [Google Scholar] [PubMed]
- McCully, K.K.; Faulkner, J.A. Characteristics of lengthening contractions associated with injury to skeletal muscle fibers. J. Appl. Physiol. 1986, 61, 293–299. [Google Scholar] [PubMed]
- Warren, G.L.; Hayes, D.A.; Lowe, D.A.; Armstrong, R.B. Mechanical factors in the initiation of eccentric contraction-induced injury in rat soleus muscle. J. Physiol. 1993, 464, 457–475. [Google Scholar] [CrossRef] [PubMed]
- Colliander, E.B.; Dudley, G.A.; Tesch, P.A. Skeletal muscle fiber type composition and performance during repeated bouts of maximal, concentric contractions. Eur. J. Appl. Physiol. Occup. Physiol. 1988, 58, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Hamada, T.; Sale, D.G.; MacDougall, J.D.; Tarnopolsky, M.A. Interaction of fibre type, potentiation and fatigue in human knee extensor muscles. Acta Physiol. Scand. 2003, 178, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Du Plessis, M.P.; Smit, P.J.; du Plessis, L.A.S.; Geyer, H.J.; Mathews, G.; Louw, H.N.J. The composition of muscle fibers in a group of adolescents. In Children and Exercise xi; Binkhorst, R.A., Kemper, H.C.G., Saris, W.H.M., Eds.; Human Kinetics: Champaign, IL, USA, 1985; pp. 323–328. [Google Scholar]
- Fournier, M.; Ricci, J.; Taylor, A.W.; Ferguson, R.J.; Montpetit, R.R.; Chaitman, B.R. Skeletal muscle adaptation in adolescent boys: Sprint and endurance training and detraining. Med. Sci. Sports Exerc. 1982, 14, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Glenmark, B.; Hedberg, G.; Kaijser, L.; Jansson, E. Muscle strength from adolescence to adulthood—Relationship to muscle fibre types. Eur. J. Appl. Physiol. Occup. Physiol. 1994, 68, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Lexell, J.; Sjostrom, M.; Nordlund, A.S.; Taylor, C.C. Growth and development of human muscle: A quantitative morphological study of whole vastus lateralis from childhood to adult age. Muscle Nerve 1992, 15, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Brockett, C.L.; Morgan, D.L.; Gregory, J.E.; Proske, U. Damage to different motor units from active lengthening of the medial gastrocnemius muscle of the cat. J. Appl. Physiol. 2002, 92, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, B.O.; Karlsson, J.; Saltin, B. Muscle metabolites during exercise in pubertal boys. Acta Paediatr. Scand. Suppl. 1971, 217, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, B.O.; Gollnick, P.D.; Saltin, B. Muscle metabolism and enzyme activities after training in boys 11–13 years old. Acta Physiol. Scand. 1973, 87, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Haralambie, G. Enzyme activities in skeletal muscle of 13–15 years old adolescents. Bull. Eur. Physiopathol. Respir. 1982, 18, 65–74. [Google Scholar] [PubMed]
- Petersen, S.R.; Gaul, C.A.; Stanton, M.M.; Hanstock, C.C. Skeletal muscle metabolism during short-term, high-intensity exercise in prepubertal and pubertal girls. J. Appl. Physiol. 1999, 87, 2151–2156. [Google Scholar] [PubMed]
- Berg, A.; Keul, J. Biochemical changes during exercise in children. In Young Athletes/Biological, Psychological and Educational Perspectives; Malina, R.M., Ed.; Human Kinetics: Champaign, IL, USA, 1988; pp. 61–77. [Google Scholar]
- Beneke, R.; Hutler, M.; Jung, M.; Leithauser, R.M. Modeling the blood lactate kinetics at maximal short-term exercise conditions in children, adolescents, and adults. J. Appl. Physiol. 2005, 99, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Dotan, R.; Ohana, S.; Bediz, C.; Falk, B. Blood lactate disappearance dynamics in boys and men following exercise of similar and dissimilar peak-lactate concentrations. J. Pediatr. Endocrinol. Metab. 2003, 16, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Engel, F.A.; Sperlich, B.; Stockinger, C.; Hartel, S.; Bos, K.; Holmberg, H.C. The kinetics of blood lactate in boys during and following a single and repeated all-out sprints of cycling are different than in men. Appl. Physiol. Nutr. Metab. 2015, 40, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Fleischman, A.; Makimura, H.; Stanley, T.L.; McCarthy, M.A.; Kron, M.; Sun, N.; Chuzi, S.; Hrovat, M.I.; Systrom, D.M.; Grinspoon, S.K. Skeletal muscle phosphocreatine recovery after submaximal exercise in children and young and middle-aged adults. J. Clin. Endocrinol. Metab. 2010, 95, E69–E74. [Google Scholar] [CrossRef] [PubMed]
- Ratel, S.; Tonson, A.; le Fur, Y.; Cozzone, P.; Bendahan, D. Comparative analysis of skeletal muscle oxidative capacity in children and adults: A 31p-mrs study. Appl. Physiol. Nutr. Metab. 2008, 33, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Tonson, A.; Ratel, S.; le Fur, Y.; Vilmen, C.; Cozzone, P.J.; Bendahan, D. Muscle energetics changes throughout maturation: A quantitative 31p-mrs analysis. J. Appl. Physiol. 2010, 109, 1769–1778. [Google Scholar] [CrossRef] [PubMed]
- Kubo, K.; Teshima, T.; Hirose, N.; Tsunoda, N. Growth changes in morphological and mechanical properties of human patellar tendon in vivo. J. Appl. Biomech. 2014, 30, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Hicks, K.M.; Onambele-Pearson, G.L.; Winwood, K.; Morse, C.I. Gender differences in fascicular lengthening during eccentric contractions: The role of the patella tendon stiffness. Acta Physiol. 2013, 209, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Lichtwark, G.A.; Barclay, C.J. A compliant tendon increases fatigue resistance and net efficiency during fatiguing cyclic contractions of mouse soleus muscle. Acta physiol. 2012, 204, 533–543. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, T.D.; Reeves, N.D.; Baltzopoulos, V.; Jones, D.A.; Maganaris, C.N. The effects of agonist and antagonist muscle activation on the knee extension moment-angle relationship in adults and children. Eur. J. Appl. Physiol. 2009, 106, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Kluka, V.; Martin, V.; Vicencio, S.G.; Jegu, A.G.; Cardenoux, C.; Morio, C.; Coudeyre, E.; Ratel, S. Effect of muscle length on voluntary activation level in children and adults. Med. Sci. Sports Exerc. 2015, 47, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Marginson, V.; Eston, R. The relationship between torque and joint angle during knee extension in boys and men. J. Sports Sci. 2001, 19, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Fitch, S.; McComas, A. Influence of human muscle length on fatigue. J. Physiol. 1985, 362, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Desbrosses, K.; Babault, N.; Scaglioni, G.; Meyer, J.P.; Pousson, M. Neural activation after maximal isometric contractions at different muscle lengths. Med. Sci. Sports Exerc. 2006, 38, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Ratel, S.; Kluka, V.; Vicencio, S.G.; Jegu, A.G.; Cardenoux, C.; Morio, C.; Coudeyre, E.; Martin, V. Insights into the mechanisms of neuromuscular fatigue in boys and men. Med. Sci. Sports Exerc. 2015, 47, 2319–2328. [Google Scholar] [CrossRef] [PubMed]
- Streckis, V.; Skurvydas, A.; Ratkevicius, A. Children are more susceptible to central fatigue than adults. Muscle Nerve 2007, 36, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Hatzikotoulas, K.; Patikas, D.; Ratel, S.; Bassa, E.; Kotzamanidis, C. Central and peripheral fatigability in boys and men during maximal contraction. Med. Sci. Sports Exerc. 2014, 46, 1326–1333. [Google Scholar] [CrossRef] [PubMed]
- Belanger, A.Y.; McComas, A.J. Contractile properties of human skeletal muscle in childhood and adolescence. Eur. J. Appl. Physiol. Occup. Physiol. 1989, 58, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Blimkie, C.J. Age- and sex-associated variations in strength duing childhood: Anthopometric, morphologic, neurologic, biomechanic, endocrinologic, genetic and physcial activity correlates. In Perspectives in Exercise Science and Sport Medicine: Youth, Exercise and Sport; Gisolfi, C.V., Lamb, D.R., Eds.; Benchmark Press: Indianapolis, IN, USA, 1989; Volume 2, pp. 99–163. [Google Scholar]
- O’Brien, T.D.; Reeves, N.D.; Baltzopoulos, V.; Jones, D.A.; Maganaris, C.N. In vivo measurements of muscle specific tension in adults and children. Exp. Physiol. 2010, 95, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.; Kluka, V.; Garcia Vicencio, S.; Maso, F.; Ratel, S. Children have a reduced maximal voluntary activation level of the adductor pollicis muscle compared to adults. Eur. J. Appl. Physiol. 2015, 115, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Grosset, J.F.; Mora, I.; Lambertz, D.; Perot, C. Voluntary activation of the triceps surae in prepubertal children. J. Electromyogr. Kinesiol. 2008, 18, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.; Ratel, S. Determining the muscle voluntary activation characteristics in children: A methodological challenge. Commentary on “child-adult differences in muscle activation—A review”. Pediatr. Exerc. Sci. 2014, 26, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Henneman, E.; Somjen, G.; Carpenter, D.O. Excitability and inhibitability of motoneurons of different sizes. J. Neurophysiol. 1965, 28, 599–620. [Google Scholar] [PubMed]
- Nordlund, M.M.; Thorstensson, A.; Cresswell, A.G. Central and peripheral contributions to fatigue in relation to level of activation during repeated maximal voluntary isometric plantar flexions. J. Appl. Physiol. 2004, 96, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Bassa, E.; Patikas, D.; Kotzamanidis, C. Activation of antagonist knee muscles during isokinetic efforts in prepubertal and adult males. Pediatr. Exerc. Sci. 2005, 17, 171–181. [Google Scholar]
- Kellis, E.; Unnithan, V.B. Co-activation of vastus lateralis and biceps femoris muscles in pubertal children and adults. Eur. J. Appl. Physiol. Occup. Physiol. 1999, 79, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Armatas, V.; Bassa, E.; Patikas, D.; Kitsas, I.; Zangelidis, G.; Kotzamanidis, C. Neuromuscular differences between men and prepubescent boys during a peak isometric knee extension intermittent fatigue test. Pediatr. Exerc. Sci. 2010, 22, 205–217. [Google Scholar] [PubMed]
- Falk, B.; Usselman, C.; Dotan, R.; Brunton, L.; Klentrou, P.; Shaw, J.; Gabriel, D. Child-adult differences in muscle strength and activation pattern during isometric elbow flexion and extension. Appl. Physiol. Nutr. Metab. 2009, 34, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Gorianovas, G.; Skurvydas, A.; Streckis, V.; Brazaitis, M.; Kamandulis, S.; McHugh, M.P. Repeated bout effect was more expressed in young adult males than in elderly males and boys. Biomed. Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Frost, G.; Dowling, J.; Dyson, K.; Bar-Or, O. Cocontraction in three age groups of children during treadmill locomotion. J. Electromyogr. Kinesiol. 1997, 7, 179–186. [Google Scholar] [CrossRef]
- Lazaridis, S.; Bassa, E.; Patikas, D.; Giakas, G.; Gollhofer, A.; Kotzamanidis, C. Neuromuscular differences between prepubescents boys and adult men during drop jump. Eur. J. Appl. Physiol. 2010, 110, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, S.N.; Bassa, E.I.; Patikas, D.; Hatzikotoulas, K.; Lazaridis, F.K.; Kotzamanidis, C.M. Biomechanical comparison in different jumping tasks between untrained boys and men. Pediatr. Exerc. Sci. 2013, 25, 101–113. [Google Scholar] [PubMed]
- Noakes, T.D.; st Clair Gibson, A.; Lambert, E.V. From catastrophe to complexity: A novel model of integrative central neural regulation of effort and fatigue during exercise in humans: Summary and conclusions. Br. J. Sports Med. 2005, 39, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Amann, M.; Proctor, L.T.; Sebranek, J.J.; Pegelow, D.F.; Dempsey, J.A. Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J. Physiol. 2009, 587, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Sandrini, G.; Alfonsi, E.; Ruiz, L.; Livieri, C.; Verri, A.P.; Nappi, G. Age-related changes in excitability of nociceptive flexion reflex. An electrophysiological study in school-age children and young adults. Funct. Neurol. 1989, 4, 53–58. [Google Scholar] [PubMed]
- Rowland, T.W.; Cunningham, L.N. Development of ventilatory responses to exercise in normal white children. A longitudinal study. Chest 1997, 111, 327–332. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratel, S.; Martin, V. Is There a Progressive Withdrawal of Physiological Protections against High-Intensity Exercise-Induced Fatigue during Puberty? Sports 2015, 3, 346-357. https://doi.org/10.3390/sports3040346
Ratel S, Martin V. Is There a Progressive Withdrawal of Physiological Protections against High-Intensity Exercise-Induced Fatigue during Puberty? Sports. 2015; 3(4):346-357. https://doi.org/10.3390/sports3040346
Chicago/Turabian StyleRatel, Sébastien, and Vincent Martin. 2015. "Is There a Progressive Withdrawal of Physiological Protections against High-Intensity Exercise-Induced Fatigue during Puberty?" Sports 3, no. 4: 346-357. https://doi.org/10.3390/sports3040346
APA StyleRatel, S., & Martin, V. (2015). Is There a Progressive Withdrawal of Physiological Protections against High-Intensity Exercise-Induced Fatigue during Puberty? Sports, 3(4), 346-357. https://doi.org/10.3390/sports3040346




