Therapeutic Exercise for Hospitalized Sarcopenic Patients: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Registration
2.2. Search Strategy
2.3. Exclusion Criteria
2.4. Study Selection
2.5. Data Extraction
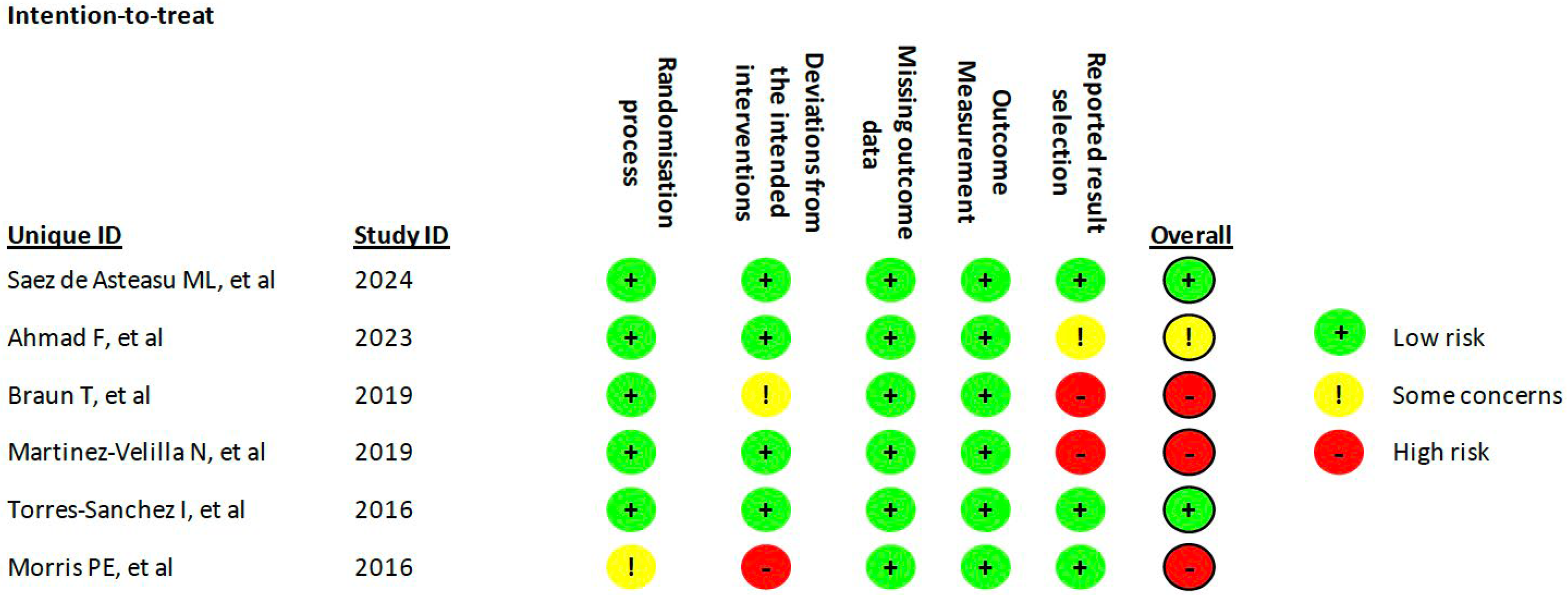
2.6. Quality Assessment
2.7. Meta-Analysis
3. Results
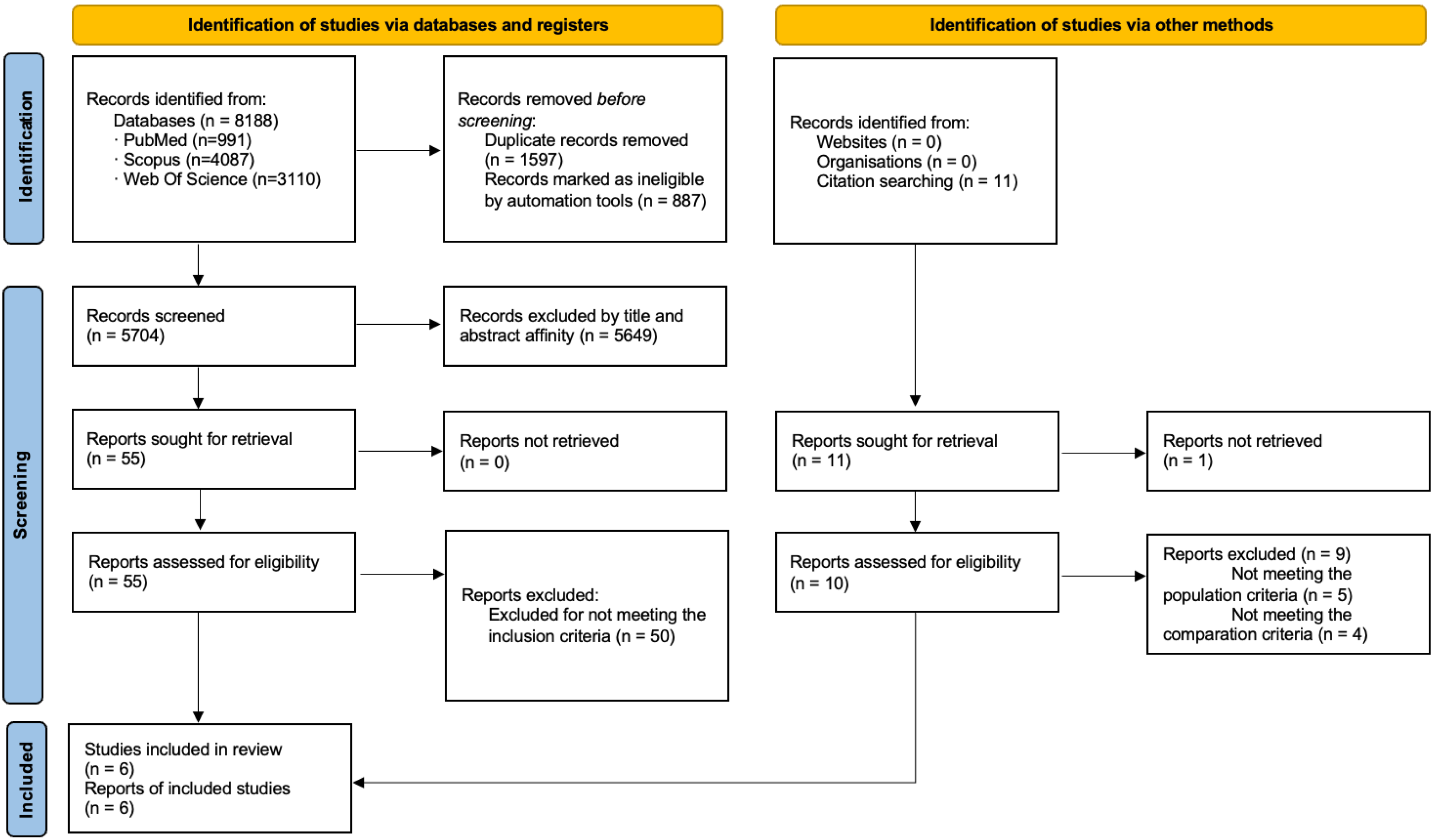
3.1. Study Selection
3.2. Study Characteristic
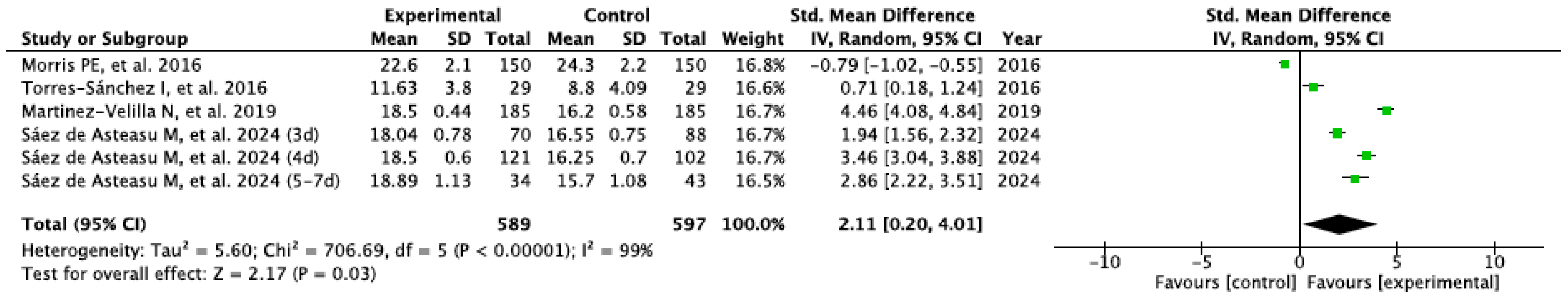
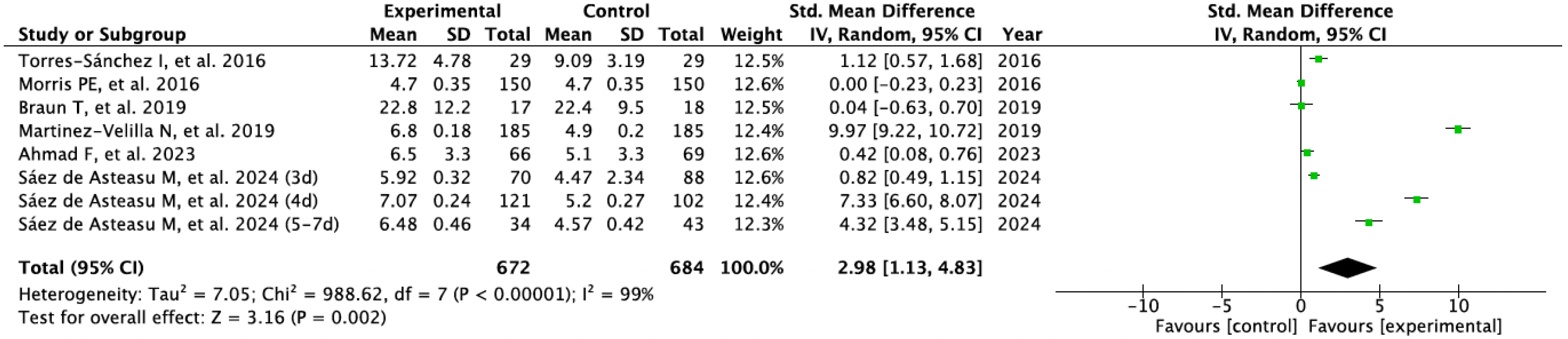
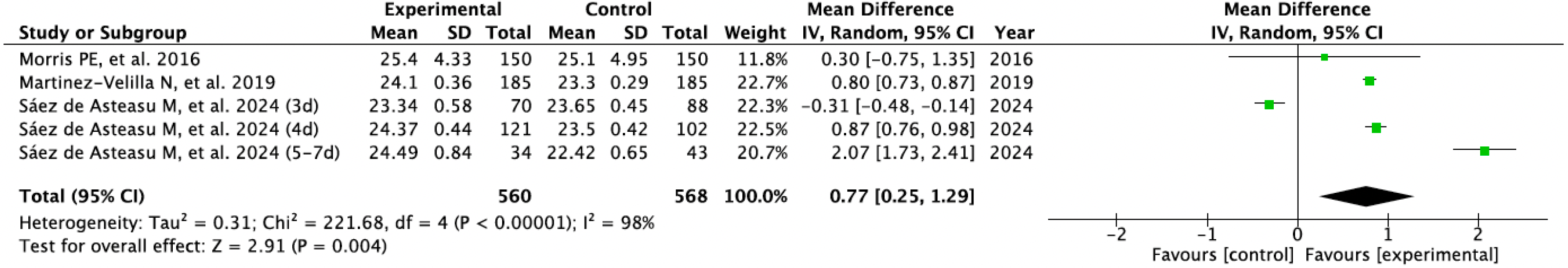
3.3. Results Obtained in Meta-Analysis
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RCT | Randomized Controlled Trial |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROSPERO | Prospective Register of Systematic Reviews |
| EWGSOP | European Working Group on Sarcopenia in Older People |
| RoB-2 | Cochrane Risk-of-Bias tool version 2.0 |
| TIDieR | Template for Intervention Description and Replication |
| MD | Mean differences |
| SMD | Standardized mean differences |
| MMSE | Mini-Mental State Examination |
References
- Ethgen, O.; Beaudart, C.; Buckinx, F.; Bruyère, O.; Reginster, J.Y. The future prevalence of sarcopenia in Europe: A claim for public health action. Calcif. Tissue Int. 2017, 100, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Bone, A.E.; Hepgul, N.; Kon, S.; Maddocks, M. Sarcopenia and frailty in chronic respiratory disease. Chronic Respir. Dis. 2017, 14, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.V.; Hsu, T.H.; Wu, W.T.; Huang, K.C.; Han, D.S. Association between sarcopenia and cognitive impairment: A systematic review and meta-analysis. J. Am. Med. Dir. Assoc. 2016, 17, 1164.e7–1164.e15. [Google Scholar] [CrossRef]
- Dhar, M.; Kapoor, N.; Suastika, K.; Khamseh, M.E.; Selim, S.; Kumar, V.; Raza, S.A.; Azmat, U.; Pathania, M.; Mahadeb, Y.P.; et al. South Asian Working Action Group on Sarcopenia (SWAG-SARCO)—A consensus document. Osteoporos. Sarcopenia 2022, 8, 35–57. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.; Wang, C.; Tao, W.; Dou, Q.; Yang, Y. Sarcopenia as a predictor of hospitalization among older people: A systematic review and meta-analysis. BMC Geriatr. 2018, 18, 188. [Google Scholar] [CrossRef]
- Langlois, F.; Vu, T.T.M.; Kergoat, M.J.; Chassé, K.; Dupuis, G.; Bherer, L. The multiple dimensions of frailty: Physical capacity, cognition, and quality of life. Int. Psychogeriatr. 2012, 24, 1429–1436. [Google Scholar] [CrossRef]
- Maeda, K.; Shamoto, H.; Wakabayashi, H.; Akagi, J. Sarcopenia is highly prevalent in older medical patients with mobility limitation: Comparisons according to ambulatory status. Nutr. Clin. Pract. 2017, 32, 110–115. [Google Scholar] [CrossRef]
- Pasco, J.A.; Williams, L.J.; Jacka, F.N.; Stupka, N.; Brennan Olsen, S.L.; Holloway, K.L.; Berk, M. Sarcopenia and the common mental disorders: A potential regulatory role of skeletal muscle on brain function? Curr. Osteoporos. Rep. 2015, 13, 351–357. [Google Scholar] [CrossRef]
- Zengarini, E.; Giacconi, R.; Mancinelli, L.; Riccardi, G.R.; Castellani, D.; Vetrano, D.L.; Onder, G.; Volpato, S.; Ruggiero, C.; Fabbietti, P.; et al. Prognosis and interplay of cognitive impairment and sarcopenia in older adults discharged from acute care hospitals. J. Clin. Med. 2019, 8, 1693. [Google Scholar] [CrossRef]
- Sato, Y.; Yoshimura, Y.; Abe, T.; Nagano, F.; Matsumoto, A. Hospital-associated sarcopenia and the preventive effect of high energy intake along with intensive rehabilitation in patients with acute stroke. Nutrition 2023, 116, 112181. [Google Scholar] [CrossRef]
- Coelho-Junior, H.J.; Calvani, R.; Azzolino, D.; Picca, A.; Tosato, M.; Landi, F.; Cesari, M.; Marzetti, E. Protein intake and sarcopenia in older adults: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2022, 19, 8718. [Google Scholar] [CrossRef]
- Sakuma, K.; Yamaguchi, A. Recent advances in pharmacological, hormonal, and nutritional intervention for sarcopenia. Pflügers Arch. 2018, 470, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Abdellaoui, A.; Heraud, N.; Courbon, M. La stimulation électrique neuromusculaire au cœur des soins intensifs. Réanimation 2012, 21, 511–519. [Google Scholar] [CrossRef]
- Beckwée, D.; Delaere, A.; Aelbrecht, S.; Baert, V.; Beaudart, C.; Bruyere, O.; de Saint-Hubert, M.; Bautmans, I. Exercise interventions for the prevention and treatment of sarcopenia: A systematic umbrella review. J. Nutr. Health Aging 2019, 23, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Mao, L.; Feng, Y.; Ainsworth, B.E.; Liu, Y.; Chen, N. Effects of different exercise training modes on muscle strength and physical performance in older people with sarcopenia: A systematic review and meta-analysis. BMC Geriatr. 2021, 21, 708. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Shi, Q.; Nong, K.; Li, S.; Yue, J.; Huang, J.; Dong, B.; Beauchamp, M.; Hao, Q. Exercise for sarcopenia in older people: A systematic review and network meta-analysis. J. Cachexia Sarcopenia Muscle 2023, 14, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
- Negm, A.M.; Lee, J.; Hamidian, R.; Jones, C.A.; Khadaroo, R.G. Management of sarcopenia: A network meta-analysis of randomized controlled trials. J. Am. Med. Dir. Assoc. 2022, 23, 707–714. [Google Scholar] [CrossRef]
- Wang, H.; Huang, W.Y.; Zhao, Y. Efficacy of exercise on muscle function and physical performance in older adults with sarcopenia: An updated systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2022, 19, 8212. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions, Version 6.2 (Updated February 2021). 2021. Available online: www.training.cochrane.org/handbook (accessed on 15 November 2024).
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Centre for Reviews & Dissemination. Systematic Reviews: CRD’s Guidance for Undertaking Systematic Reviews in Healthcare; University of York: York, UK, 2009. [Google Scholar]
- Kalinkovich, A.; Livshits, G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res. Rev. 2017, 35, 200–221. [Google Scholar] [CrossRef]
- Barbat Artigas, S.; Pion, C.H.; Leduc Gaudet, J.P.; Rolland, Y.; Aubertin Leheudre, M. Exploring the role of muscle mass, obesity, and age in the relationship between muscle quality and physical function. J. Am. Med. Dir. Assoc. 2014, 15, 303.e13–303.e20. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomized trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Hoffmann, T.C.; Glasziou, P.P.; Boutron, I.; Milne, R.; Perera, R.; Moher, D.; Altman, D.G.; Barbour, V.; Macdonald, H.; Johnston, M.; et al. Better reporting of interventions: Template for intervention description and replication (TiDieR) checklist and guide. BMJ 2014, 348, g1687. [Google Scholar] [CrossRef] [PubMed]
- Yamato, T.P.; Maher, C.G.; Saragiotto, B.T.; Catley, M.J.; Moseley, A.M. Rasch analysis suggested that items from the template for intervention description and replication (TiDieR) checklist can be summed to create a score. J. Clin. Epidemiol. 2018, 101, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Briani, R.V.; Ferreira, A.S.; Pazzinatto, M.F.; Pappas, E.; Silva, D.D.; de Azevedo, F.M. What interventions can improve quality of life or psychosocial factors of individuals with knee osteoarthritis? A systematic review with meta-analysis of primary outcomes from randomized controlled trials. Br. J. Sports Med. 2018, 52, 1031–1038. [Google Scholar] [CrossRef]
- Sáez de Asteasu, M.L.; Martínez-Velilla, N.; Zambom-Ferraresi, F.; Galbete, A.; Ramírez-Vélez, R.; Cadore, E.L.; Abizanda, P.; Gómez-Pavón, J.; Izquierdo, M. Dose response relationship between exercise duration and enhanced function and cognition in acutely hospitalized older adults: A secondary analysis of a randomized clinical trial. Innov. Aging 2024, 8, igae053. [Google Scholar] [CrossRef]
- Ahmad, F.; Fountotos, R.; Goldfarb, M.; Bharaj, N.; Munir, H.; Marsala, J.; Rudski, L.G.; Afilalo, J. De frailing intervention for hospitalized cardiovascular patients in the TARGET EFT randomized clinical trial. Eur. Heart J. Qual. Care Clin. Outcomes 2023, 9, 482–489. [Google Scholar] [CrossRef]
- Braun, T.; Grüneberg, C.; Süßmilch, K.; Wiessmeier, M.; Schwenk, I.; Eggert, S.; Machleit Ebner, A.; Harras, I.; Thiel, C. An augmented prescribed exercise program (APEP) to improve mobility of older acute medical patients—A randomized, controlled pilot and feasibility trial. BMC Geriatr. 2019, 19, 240. [Google Scholar] [CrossRef]
- Martínez-Velilla, N.; Casas-Herrero, A.; Zambom-Ferraresi, F.; de Asteasu, M.L.; Lucia, A.; Galbete, A.; García-Baztán, A.; Alonso-Renedo, J.; González-Glaría, B.; Gonzalo-Lázaro, M.; et al. Effect of exercise intervention on functional decline in very elderly patients during acute hospitalization: A randomized clinical trial. JAMA Intern. Med. 2019, 179, 28–36. [Google Scholar] [CrossRef]
- Torres Sánchez, I.; Valenza, M.C.; Cabrera Martos, I.; López Torres, I.; Benítez Feliponi, Á.; Conde Valero, A. Effects of an exercise intervention in frail older patients with chronic obstructive pulmonary disease hospitalized due to an exacerbation: A randomized controlled trial. COPD 2017, 14, 37–42. [Google Scholar] [CrossRef]
- Morris, P.E.; Berry, M.J.; Files, D.C.; Thompson, J.C.; Hauser, J.; Flores, L.; Dhar, S.; Chmelo, E.; Lovato, J.; Case, L.D.; et al. Standardized rehabilitation and hospital length of stay among patients with acute respiratory failure: A randomized clinical trial. JAMA 2016, 315, 2694–2702. [Google Scholar] [CrossRef]
- Gao, Q.; Hu, K.; Yan, C.; Zhao, B.; Mei, F.; Chen, F.; Zhao, L.; Shang, Y.; Ma, Y.; Ma, B. Associated factors of sarcopenia in community-dwelling older adults: A systematic review and meta-analysis. Nutrients 2021, 13, 4291. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e302. [Google Scholar] [CrossRef]
- Izquierdo, M.; Rodriguez Mañas, L.; Sinclair, A.J. Editorial: What is new in exercise regimes for frail older people—How does the Erasmus Vivifrail project take us forward? J. Nutr. Health Aging 2016, 20, 736–737. [Google Scholar] [CrossRef]
- García Pérez de Sevilla, G.; Sánchez Pinto, B. Effectiveness of physical exercise and neuromuscular electrical stimulation interventions for preventing and treating intensive care unit-acquired weakness: A systematic review of randomized controlled trials. Intensive Crit. Care Nurs. 2023, 74, 103333. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 2015, 25, 1–72. [Google Scholar] [CrossRef] [PubMed]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zou, L.; Chen, S.T.; Bae, J.H.; Kim, D.Y.; Liu, X.; Song, W. Effects and moderators of exercise on sarcopenic components in sarcopenic elderly: A systematic review and meta-analysis. Front. Med. 2021, 8, 649748. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Yakabe, M.; Akishita, M. Age related sarcopenia and its pathophysiological bases. Inflamm. Regen. 2016, 36, 17. [Google Scholar] [CrossRef]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; Van Kan, G.A.; Andrieu, S.; Bauer, J.; Breuille, D.; et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef]
- Gao, X.; Chen, Y.; Cheng, P. Unlocking the potential of exercise: Harnessing myokines to delay musculoskeletal aging and improve cognitive health. Front. Physiol. 2024, 15, 1338875. [Google Scholar] [CrossRef]
- Wang, B.; Liang, J.; Lu, C.; Lu, A.; Wang, C. Exercise Regulates Myokines in Aging-Related Diseases through Muscle-Brain Crosstalk. Gerontology 2024, 70, 193–209. [Google Scholar] [CrossRef]
- Denkinger, M.D.; Lukas, A.; Nikolaus, T.; Hauer, K. Factors associated with fear of falling and associated activity restriction in community-dwelling older adults: A systematic review. Am. J. Geriatr. Psychiatry 2015, 23, 72–86. [Google Scholar] [CrossRef]
- Sartini, M.; Cristina, M.L.; Spagnolo, A.M.; Cremonesi, P.; Costaguta, C.; Monacelli, F.; Garau, J.; Odetti, P. The epidemiology of domestic injurious falls in a community dwelling elderly population: An outgrowing economic burden. Eur. J. Public Health 2010, 20, 604–606. [Google Scholar] [CrossRef]
| Study ID | Setting | Population | N Age (% Females) | Reason of Hospitalization | Exclusion Criteria | Risk of Bias |
|---|---|---|---|---|---|---|
| Saéz de Asteasu ML et al., 2024 [29] | Hospital Geriatric Unit (Spain) | Elderly patients (>75 y) | 570 EG: 87.4 ± 4.6 (50%) CG: 87.3 ± 5.1 (54%) | Cardiovascular (33%) Infectious (25%) Pulmonary (12%) Digestive (9%) Neurological (5%) Other (16%) | - Planned hospital stay < 6 days - Severe cognitive impairment - Inability to cooperate - Terminal illness - Uncontrolled heart disease - Major surgery < 3 months - Bone Fracture < 3 months | LOW |
| Ahmad F et al., 2023 [30] | Hospital Cardiovascular Unit (Canada) | Elderly Cardiovascular patients (>65 y) | 135 EG: 78.2 ± 8.0 (53%) CG: 80.2 ± 7.3 (55%) | Cardiovascular (80%) Other (20%) | - Planned hospital stay < 3 days - Cardiac surgery < 3 days - Clinically or mental unstable - Neurological disease - Palliative care | SOME CONCERNS |
| Braun T et al., 2019 [31] | Hospital Geriatric Unit (Germany) | Elderly patients (>65 y) | 35 EG: 78.6 ± 7.5 (76%) CG: 83.1 ± 7.4 (72%) | Musculoskeletal (34%) Cardiovascular (17%) Pulmonary (8.5%) Digestive (2.8%) Other (37.7%) | - Cognitive impairment - Inability to cooperate - Language barrier - Psychiatric condition - Palliative care - Medical restriction for exercise | HIGH |
| Martinez-Velilla N et al., 2019 [32] | Hospital Geriatric Unit (Spain) | Elderly patients (>75 y) | 370 EG: 87.6 ± 4.6 (54%) CG: 87.1 ± 7.2 (59%) | Cardiovascular (35%) Infectious (18%) Pulmonary (13%) Digestive (10%) Neurological (5%) Other (19%) | - Planned hospital stay < 6 days - Severe cognitive impairment - Palliative care - Uncontrolled heart disease - Recent surgery - Bone fracture | HIGH |
| Torres-Sánchez I et al., 2016 [33] | Hospital Pulmonary Unit (Spain) | Frail Elderly patients (>65 y) with COPD | 58 EG: 75.65 ± 6.25 (24%) CG: 72.12 ± 8.2 (31%) | Pulmonary (100%) | - Inability to cooperate - Cognitive impairment - Psychiatric condition - Neurological disorders - Musculoskeletal disorders - Organ failure - Cancer - Recent exacerbation of COPD | LOW |
| Morris PE et al., 2016 [34] | Medical Center Intensive Care Unit (USA) | Adult patients with acute respiratory failure | 300 EG: 55 ± 17 (56%) CG: 58 ± 14 (54%) | Pulmonary (100%) | - Cognitive impairment - Obesity - Neurological disease - Bone fracture | HIGH |
| Study ID | Experimental Intervention | TIDIER | Control Intervention | Variable | Relevant Results |
|---|---|---|---|---|---|
| Saéz de Asteasu ML et al., 2024 [29] | · Usual Care · Therapeutic Exercise: | 20 | Usual Care · Medical care · can included PT | - Physical Performance (SPPB) - Muscle Strength (handgrip) - Gate Speed (GVT) - Cognitive Function (MMSE) | EG improved compared to CG (p < 0.001). The 4-day program showed most significant benefits in physical performance. The 4-day program showed most significant benefits in muscle strength and cognition. No adverse events |
| Resistance Training From 2 × 10 sets 30% RM Day 1 to 3 × 8 sets 60% RM Day 5–7 (>10% RM/day) 2–3 sets × 8–10 reps Rises chair—Chess and leg press—Leg extension Balance Training Semi-tandem foot standing—Line walking—Stepping practice—Walking with obstacles—Unstable surfaces—Base of support variations—Leg Weight transfers Unsupervised Functional Training 0.5–1 kg anklets and handgrip ball Knee extension/flexion—Hip abduction—Walking 20 min session 2 sessions/day 3 to 7 days | |||||
| Ahmad F et al., 2023 [30] | · Usual Care · Cognitive stimulation · Protein supplementation · Anemia treatment · Therapeutic Exercise: | 18 | Usual Care · Medical care · PT 2–3 sessons/week · Nutritional care · Anemia treatment | - Physical Performance (SPPB) - Sarcopenia Screening (SARC-F) | EG improved physical performance (p < 0.001) and SARC-F (p < 0.02) compared to CG. No adverse events. |
| Strength Training NR Flexibility Training NR Balance Training NR 20 min session 2 sessions/day | |||||
| Braun T et al., 2019 [31] | · Usual Care · Therapeutic Exercise: | 22 | Usual Care · Medical care · Multidisciplinary rehabilitation | - Physical Performance (TUG; 6 MWT) - Mobility (DEMMI & HABAM) - Walking Ability (Gate speed) | No statistically significant differences. No severe adverse events. |
| Resistance Training 12–14/20 BORG 3 sets × 13–15 reps Progression individually directed by physiotherapist Chair rise—Heel raises—Partial squats—Stepping forward—Sideways up onto block Balance Training Clossed feet stance- Semitandem stance—Walking—Walking back—Stairclimbing 20–30 min session 4–5 sessions/week 1–3 weeks | |||||
| Martinez-Velilla N et al., 2019 [32] | · Usual Care · Therapeutic Exercise: | 20 | Usual Care · Medical care · PT (Walking exercises) | - Physical Performance (SPPB; Barthel) - Muscle Strength (handgrip) - Cognitive Function (MMSE) | CG showed impairment in physical performance after hospitalization, whereas EG reversed this trend (p < 0.001). Significant intervention benefits were also found at the cognitive function over the CG (p < 0.001). No adverse events. |
| Resistance Training 30–60%RM 2–3 sets × 8–10 reps Progression Not Specified Squats rises chair—Leg press—Bilateral knee extension—Bench press Balance Training Semi-tandem foot standing—Line walking—Stepping practice—Walking with obstacles—Unstable surfaces—Base of support variations—Leg Weight transfers Unsupervised Functional Training 0.5–1 kg anklets and handgrip ball Knee extension/flexion—Hip abduction—Walking 20 min session 2 sessions/day 5–7 days | |||||
| Torres-Sánchez I et al., 2016 [33] | · Usual Care · Therapeutic Exercise: | 18 | Usual care · Medical care | - Physical Performance (30 STS) - Muscle Strength (quadriceps dynamometer) - Balance (One leg stance) | Significant between-group differences were observed in muscle strength (p = 0.028) and balance (p = 0.013) after the intervention. All the variables improved significantly (p < 0.05) in the EG. All the variables showed impairment in the CG. No adverse events. |
| Aerobic Training + Oxygen Therapy Cycling with pedal increasing daily cycling intensity and progression adapted to the patients’ levels of dyspnea and fatigue (<6/10 BORG). 1 session/day 7 days/week During hospitalization | |||||
| Morris PE et al., 2016 [34] | · Usual Care · Conventional PT · Therapeutic Exercise: | 18 | Usual Care · Medical care · Weekly PT | - Physical Performance (SPPB; FPI) - Muscle Strength (handgrip; dynamometry) - Cognitive Function (MMSE) | No significant between group effects, but EG improve at follow-up with significant with-in group differences. No between differences in adverse events. There was an episode of asymptomatic bradycardia during resistance training. |
| Resistance Training NR 3 sets × 8 reps Intensity maintained throughout intervention Joint mobility with elastic resistance bands, mainly focus on lower limbs Balance Training Seated balance—Forward and lateral weight shifting—Marching in place—Ambulation 3 sessions/day 7 days/week |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan-Fook, O.; Martin-Núñez, J.; Raya-Benítez, J.; Navas-Otero, A.; Cabrera-Martos, I.; Valenza, M.C.; Heredia-Ciuró, A. Therapeutic Exercise for Hospitalized Sarcopenic Patients: A Systematic Review and Meta-Analysis. Sports 2025, 13, 326. https://doi.org/10.3390/sports13090326
Chan-Fook O, Martin-Núñez J, Raya-Benítez J, Navas-Otero A, Cabrera-Martos I, Valenza MC, Heredia-Ciuró A. Therapeutic Exercise for Hospitalized Sarcopenic Patients: A Systematic Review and Meta-Analysis. Sports. 2025; 13(9):326. https://doi.org/10.3390/sports13090326
Chicago/Turabian StyleChan-Fook, Olivier, Javier Martin-Núñez, Julia Raya-Benítez, Alba Navas-Otero, Irene Cabrera-Martos, Marie Carmen Valenza, and Alejandro Heredia-Ciuró. 2025. "Therapeutic Exercise for Hospitalized Sarcopenic Patients: A Systematic Review and Meta-Analysis" Sports 13, no. 9: 326. https://doi.org/10.3390/sports13090326
APA StyleChan-Fook, O., Martin-Núñez, J., Raya-Benítez, J., Navas-Otero, A., Cabrera-Martos, I., Valenza, M. C., & Heredia-Ciuró, A. (2025). Therapeutic Exercise for Hospitalized Sarcopenic Patients: A Systematic Review and Meta-Analysis. Sports, 13(9), 326. https://doi.org/10.3390/sports13090326