Does High-Intensity Interval Training Increase Muscle Strength, Muscle Mass, and Muscle Endurance? A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Information Sources and Search Strategy
2.4. Selection Process
2.5. Data Collection Process Data Items
2.6. Risk of Bias Assessment
2.7. Certainty of Evidence
2.8. Data Synthesis and Analysis
2.9. Post Hoc Protocol Deviations
3. Results
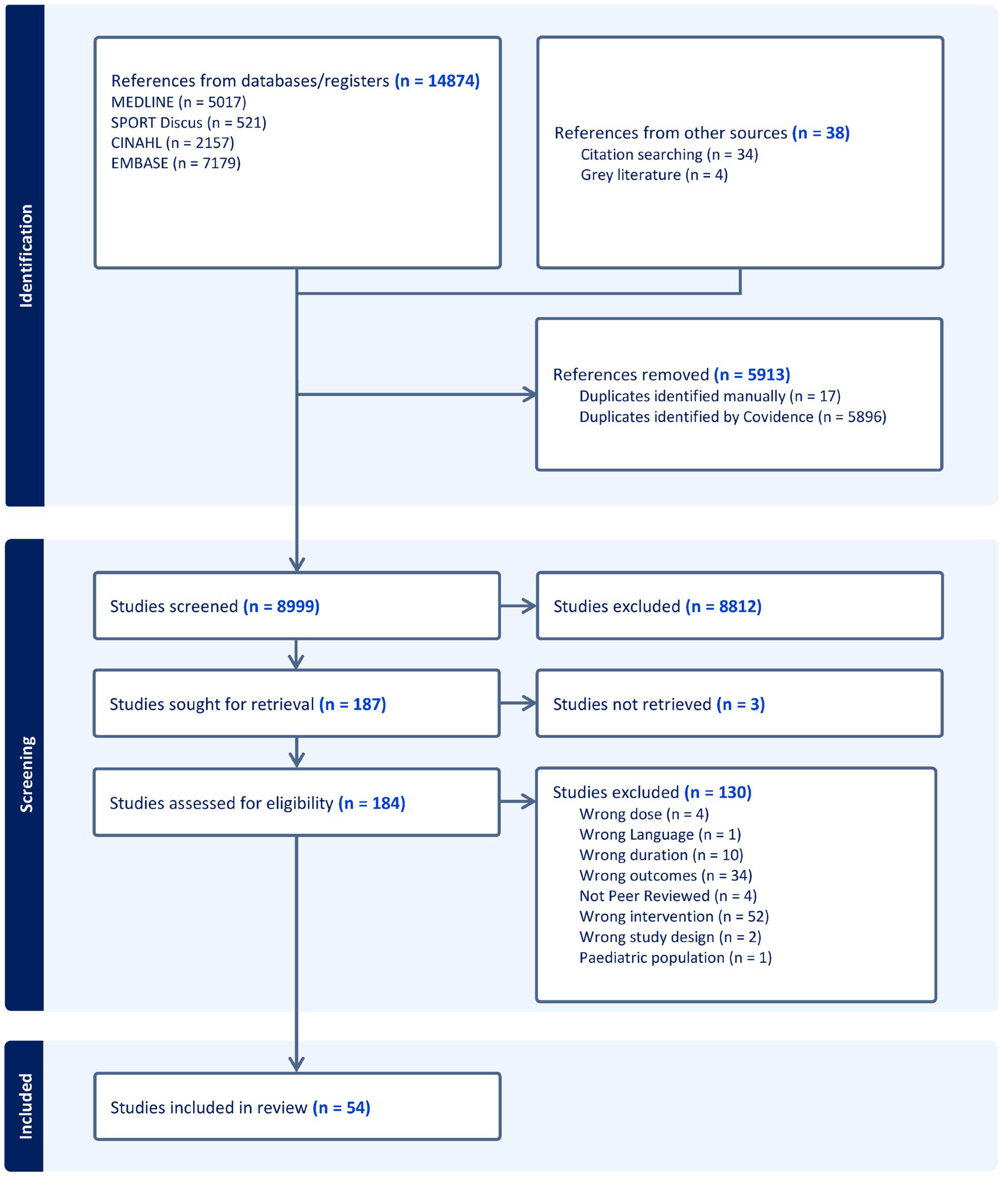
3.1. Study Selection
3.2. Study Characteristics
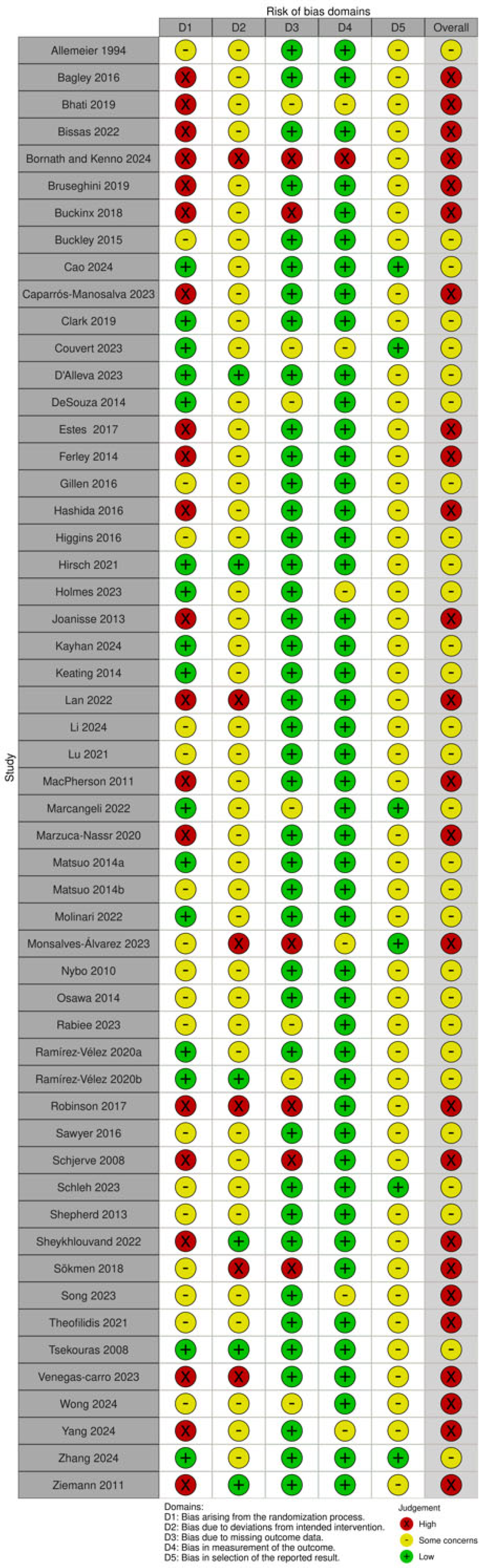
3.3. Risk of Bias
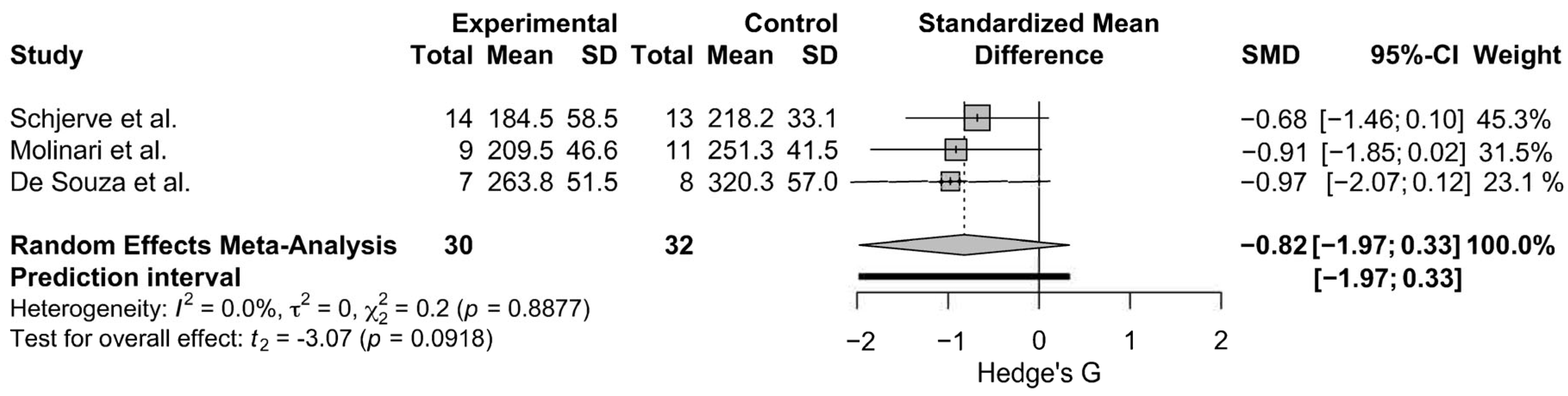
3.4. Meta-Analysis Results
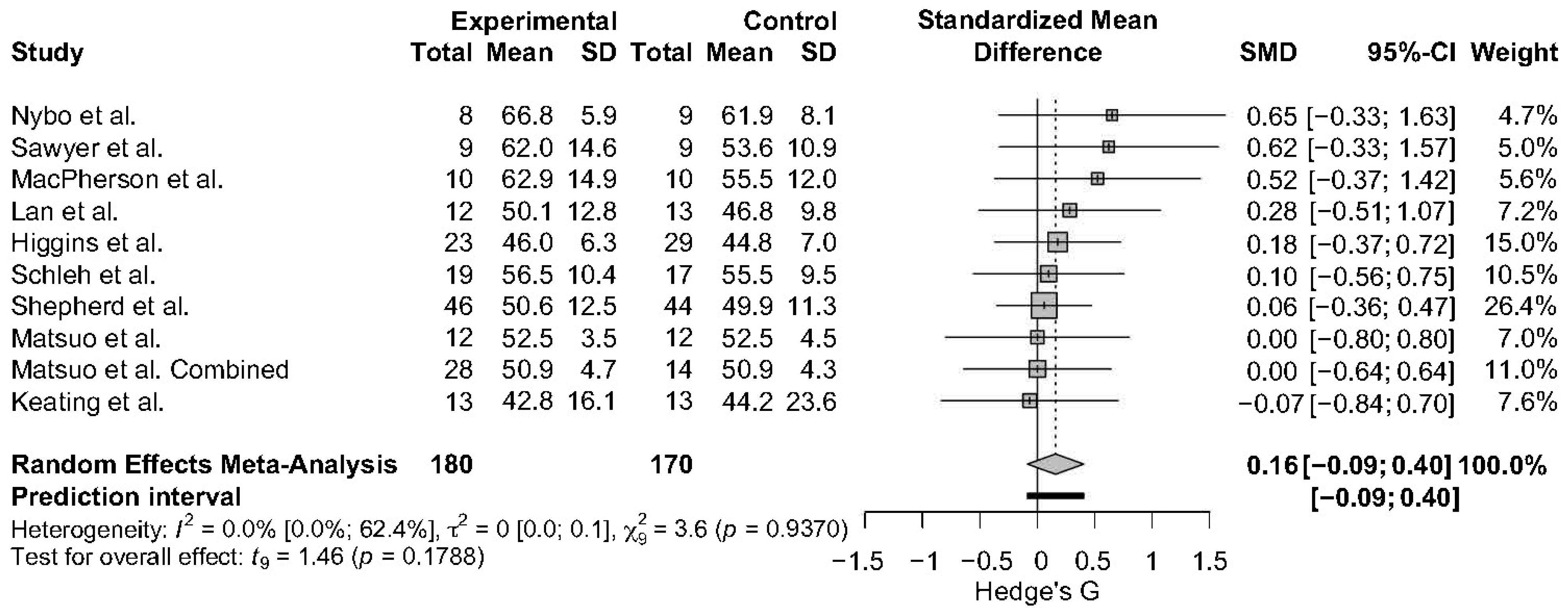
3.4.1. FFM
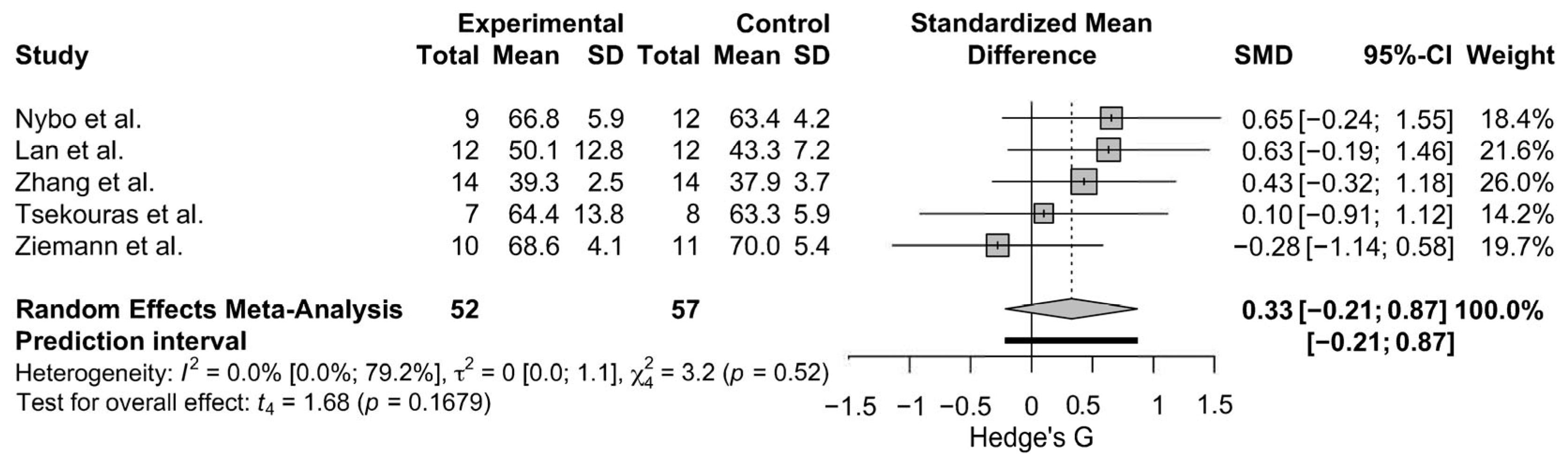
3.4.2. Leg Press 1-RM Strength
3.5. Weighted Effect Size and Percentage Change
3.5.1. Weighted Effect Size and Percentage Change for Muscle Hypertrophy
3.5.2. Weighted Effect Size and Percentage Change for Muscle Strength
4. Discussion
4.1. Hypertrophy
4.2. Strength
4.3. Muscle Endurance
4.4. Limitations
4.5. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.-M.; Nieman, D.C.; Swain, D.P.; American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef] [PubMed]
- Bennie, J.A.; De Cocker, K.; Teychenne, M.J.; Brown, W.J.; Biddle, S.J.H. The epidemiology of aerobic physical activity and muscle-strengthening activity guideline adherence among 383,928 U.S. adults. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 34. [Google Scholar] [CrossRef]
- Ebben, W.; Brudzynski, L. Motivations and barriers to exercise among college students. J. Exerc. Physiol. Online 2008, 11, 5. Available online: http://www.asep.org/asep/asep/EbbenJEPonlineOctober2008.pdf (accessed on 18 March 2024).
- MacInnis, M.J.; Gibala, M.J. Physiological adaptations to interval training and the role of exercise intensity: Training adaptations and the nature of the stimulus. J. Physiol. 2017, 595, 2915–2930. [Google Scholar] [CrossRef] [PubMed]
- Foster, C.; Farland, C.V.; Guidotti, F.; Harbin, M.; Roberts, B.; Schuette, J.; Tuuri, A.; Doberstein, S.T.; Porcari, J.P. The Effects of High Intensity Interval Training vs. Steady State Training on Aerobic and Anaerobic Capacity. J. Sports Sci. Med. 2015, 14, 747–755. [Google Scholar] [PubMed]
- Ziemann, E.; Grzywacz, T.; Łuszczyk, M.; Laskowski, R.; Olek, R.A.; Gibson, A.L. Aerobic and Anaerobic Changes with High-Intensity Interval Training in Active College-Aged Men. J. Strength Cond. Res. 2011, 25, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Wang, T.; Zhang, L.; Li, Q.; Bo, S. Comparison of the acute physiological and perceptual responses between resistance-type and cycling high-intensity interval training. Front. Physiol. 2022, 13, 986920. [Google Scholar] [CrossRef]
- Peake, J.M.; Tan, S.J.; Markworth, J.F.; Broadbent, J.A.; Skinner, T.L.; Cameron-Smith, D. Metabolic and hormonal responses to isoenergetic high-intensity interval exercise and continuous moderate-intensity exercise. Am. J. Physiol.-Endocrinol. Metab. 2014, 307, E539–E552. [Google Scholar] [CrossRef]
- Konopka, A.R.; Harber, M.P. Skeletal Muscle Hypertrophy after Aerobic Exercise Training. Exerc. Sport Sci. Rev. 2014, 42, 53–61. [Google Scholar] [CrossRef]
- Lasevicius, T.; Schoenfeld, B.J.; Silva-Batista, C.; Barros, T.D.S.; Aihara, A.Y.; Brendon, H.; Longo, A.R.; Tricoli, V.; Peres, B.D.A.; Teixeira, E.L. Muscle Failure Promotes Greater Muscle Hypertrophy in Low-Load but Not in High-Load Resistance Training. J. Strength Cond. Res. 2022, 36, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J.; Grgic, J.; Van Every, D.W.; Plotkin, D.L. Loading Recommendations for Muscle Strength, Hypertrophy, and Local Endurance: A Re-Examination of the Repetition Continuum. Sports 2021, 9, 32. [Google Scholar] [CrossRef]
- Schoenfeld, B.J. The Mechanisms of Muscle Hypertrophy and Their Application to Resistance Training. J. Strength Cond. Res. 2010, 24, 2857–2872. [Google Scholar] [CrossRef]
- Seiler, S.; Hetlelid, K.J. The impact of rest duration on work intensity and RPE during interval training. Med. Sci. Sports Exerc. 2005, 37, 1601–1607. [Google Scholar] [CrossRef] [PubMed]
- Vandewalle, H.; Pérès, G.; Monod, H. Standard Anaerobic Exercise Tests. Sports Med. 1987, 4, 268–289. [Google Scholar] [CrossRef] [PubMed]
- Callahan, M.J.; Parr, E.B.; Hawley, J.A.; Camera, D.M. Can High-Intensity Interval Training Promote Skeletal Muscle Anabolism? Sports Med. 2021, 51, 405–421. [Google Scholar] [CrossRef]
- Esbjörnsson, M.; Rundqvist, H.C.; Mascher, H.; Österlund, T.; Rooyackers, O.; Blomstrand, E.; Jansson, E. Sprint exercise enhances skeletal muscle p70S6k phosphorylation and more so in women than in men. Acta Physiol. 2012, 205, 411–422. [Google Scholar] [CrossRef]
- Rundqvist, H.C.; Montelius, A.; Osterlund, T.; Norman, B.; Esbjornsson, M.; Jansson, E. Acute sprint exercise transcriptome in human skeletal muscle. PLoS ONE 2019, 14, e0223024. [Google Scholar] [CrossRef]
- Bell, K.E.; Séguin, C.; Parise, G.; Baker, S.K.; Phillips, S.M. Day-to-Day Changes in Muscle Protein Synthesis in Recovery From Resistance, Aerobic, and High-Intensity Interval Exercise in Older Men. J. Gerontol. Ser. A 2015, 70, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Sultana, R.N.; Sabag, A.; Keating, S.E.; Johnson, N.A. The Effect of Low-Volume High-Intensity Interval Training on Body Composition and Cardiorespiratory Fitness: A Systematic Review and Meta-Analysis. Sports Med. 2019, 49, 1687–1721. [Google Scholar] [CrossRef]
- Erskine, R.M.; Fletcher, G.; Folland, J.P. The contribution of muscle hypertrophy to strength changes following resistance training. Eur. J. Appl. Physiol. 2014, 114, 1239–1249. [Google Scholar] [CrossRef]
- Seynnes, O.R.; de Boer, M.; Narici, M.V. Early skeletal muscle hypertrophy and architectural changes in response to high-intensity resistance training. J. Appl. Physiol. 2007, 102, 368–373. [Google Scholar] [CrossRef]
- Taber, C.B.; Vigotsky, A.; Nuckols, G.; Haun, C.T. Exercise-Induced Myofibrillar Hypertrophy is a Contributory Cause of Gains in Muscle Strength. Sports Med. 2019, 49, 993–997. [Google Scholar] [CrossRef]
- Schoenfeld, B.J.; Grgic, J.; Ogborn, D.; Krieger, J.W. Strength and Hypertrophy Adaptations Between Low- vs. High-Load Resistance Training: A Systematic Review and Meta-analysis. J. Strength Cond. Res. 2017, 31, 3508–3523. [Google Scholar] [CrossRef]
- Fliss, M.D.; Stevenson, J.; Mardan-Dezfouli, S.; Li, D.C.W.; Mitchell, C.J. Higher- and lower-load resistance exercise training induce load-specific local muscle endurance changes in young women: A randomised trial. Appl. Physiol. Nutr. Metab. 2022, 47, 1143–1159. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.; Kim, H.J.; Morton, R.W.; Harris, R.; Phillips, S.M.; Jeong, T.S.; Kim, C.K. Resistance Exercise–induced Changes in Muscle Phenotype Are Load Dependent. Med. Sci. Sports Exerc. 2019, 51, 2578–2585. [Google Scholar] [CrossRef]
- Mitchell, C.J.; Churchward-Venne, T.A.; West, D.W.D.; Burd, N.A.; Breen, L.; Baker, S.K.; Phillips, S.M. Resistance exercise load does not determine training-mediated hypertrophic gains in young men. J. Appl. Physiol. 2012, 113, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Bini, R.; Diefenthaler, F.; Carpes, F.; Mota, C.B. EXTERNAL WORK BILATERAL SYMMETRY DURING INCREMENTAL CYCLING EXERCISE. In Proceedings of the 25 International Symposium on Biomechanics in Sports, Ouro Preto, Brazil, 23–27 August 2007. [Google Scholar]
- Wirth, K.; Keiner, M.; Hartmann, H.; Sander, A.; Mickel, C. Effect of 8 weeks of free-weight and machine-based strength training on strength and power performance. J. Hum. Kinet. 2016, 53, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Hackett, D.A.; Ghayomzadeh, M.; Farrell, S.N.; Davies, T.B.; Sabag, A. Influence of total repetitions per set on local muscular endurance: A systematic review with meta-analysis and meta-regression. Sci. Sports 2022, 37, 405–420. [Google Scholar] [CrossRef]
- Hallal, P.C.; Andersen, L.B.; Bull, F.C.; Guthold, R.; Haskell, W.; Ekelund, U. Global physical activity levels: Surveillance progress, pitfalls, and prospects. Lancet 2012, 380, 247–257. [Google Scholar] [CrossRef]
- Pharr, J.R.; Terencio, M.A.M.; Bungum, T. Physical Activity Guidelines Compliance and Its Relationship With Preventative Health Behaviors and Risky Health Behaviors. J. Phys. Act. Health 2020, 17, 1003–1008. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Farrah, K.; Young, K.; Tunis, M.C.; Zhao, L. Risk of bias tools in systematic reviews of health interventions: An analysis of PROSPERO-registered protocols. Syst. Rev. 2019, 8, 280. [Google Scholar] [CrossRef] [PubMed]
- Schünemann, H.; Brożek, J.; Guyatt, G.; Oxman, A. (Eds.) GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations; Updated October 2013; The GRADE Working Group: Hamilton, ON, Canada, 2013; Available online: https://guidelinedevelopment.org/handbook (accessed on 10 April 2024).
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 2010, 1, 97–111. [Google Scholar] [CrossRef]
- Riley, R.D.; Higgins, J.P.T.; Deeks, J.J. Interpretation of random effects meta-analyses. BMJ 2011, 342, d549. Available online: https://www.bmj.com/content/342/bmj.d549 (accessed on 12 May 2025). [CrossRef]
- Shuster, J.J. Empirical vs. natural weighting in random effects meta-analysis. Stat. Med. 2010, 29, 1259–1265. [Google Scholar] [CrossRef]
- Durlak, J.A. How to Select, Calculate, and Interpret Effect Sizes. J. Pediatr. Psychol. 2009, 34, 917–928. [Google Scholar] [CrossRef]
- Brown, E.C.; Hew-Butler, T.; Marks, C.R.C.; Butcher, S.J.; Choi, M.D. The Impact of Different High-Intensity Interval Training Protocols on Body Composition and Physical Fitness in Healthy Young Adult Females. BioRes. Open Access 2018, 7, 177–185. [Google Scholar] [CrossRef]
- Hurst, C.; Weston, K.L.; Weston, M. The effect of 12 weeks of combined upper- and lower-body high-intensity interval training on muscular and cardiorespiratory fitness in older adults. Aging Clin. Exp. Res. 2019, 31, 661–671. [Google Scholar] [CrossRef]
- Islam, H.; Siemens, T.L.; Matusiak, J.B.L.; Sawula, L.; Bonafiglia, J.T.; Preobrazenski, N.; Jung, M.E.; Gurd, B.J. Cardiorespiratory fitness and muscular endurance responses immediately and 2 months after a whole-body Tabata or vigorous-intensity continuous training intervention. Appl. Physiol. Nutr. Metab. 2020, 45, 650–658. [Google Scholar] [CrossRef]
- Blue, M.N.M.; Smith-Ryan, A.E.; Trexler, E.T.; Hirsch, K.R. The effects of high intensity interval training on muscle size and quality in overweight and obese adults. J. Sci. Med. Sport 2018, 21, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Bhati, P.; Bansal, V.; Moiz, J.A. Comparison of different volumes of high intensity interval training on cardiac autonomic function in sedentary young women. Int. J. Adolesc. Med. Health 2019, 31, 20170073. [Google Scholar] [CrossRef]
- Bruseghini, P.; Capelli, C.; Calabria, E.; Rossi, A.P.; Tam, E. Effects of High-Intensity Interval Training and Isoinertial Training on Leg Extensors Muscle Function, Structure, and Intermuscular Adipose Tissue in Older Adults. Front. Physiol. 2019, 10, 1260. [Google Scholar] [CrossRef] [PubMed]
- Buckinx, F.; Gouspillou, G.; Carvalho, L.; Marcangeli, V.; El Hajj Boutros, G.; Dulac, M.; Noirez, P.; Morais, J.; Gaudreau, P.; Aubertin-Leheudre, M. Effect of High-Intensity Interval Training Combined with L-Citrulline Supplementation on Functional Capacities and Muscle Function in Dynapenic-Obese Older Adults. J. Clin. Med. 2018, 7, 561. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; De La Rosa, A.B.; DeRevere, J.L.; Astorino, T.A. Effects of various interval training regimes on changes in maximal oxygen uptake, body composition, and muscular strength in sedentary women with obesity. Eur. J. Appl. Physiol. 2019, 119, 879–888. [Google Scholar] [CrossRef]
- Couvert, A.; Goumy, L.; Maillard, F.; Esbrat, A.; Lanchais, K.; Saugrain, C.; Verdier, C.; Doré, E.; Chevarin, C.; Adjtoutah, D.; et al. Effects of a Cycling versus Running HIIT Program on Fat Mass Loss and Gut Microbiota Composition in Men with Overweight/Obesity. Med. Sci. Sports Exerc. 2024, 56, 839–850. [Google Scholar] [CrossRef]
- D’Alleva, M.; Vaccari, F.; Graniero, F.; Giovanelli, N.; Floreani, M.; Fiori, F.; Marinoni, M.; Parpinel, M.; Lazzer, S. Effects of 12-week combined training versus high intensity interval training on cardiorespiratory fitness, body composition and fat metabolism in obese male adults. J. Exerc. Sci. Fit. 2023, 21, 193–201. [Google Scholar] [CrossRef]
- Gillen, J.B.; Martin, B.J.; MacInnis, M.J.; Skelly, L.E.; Tarnopolsky, M.A.; Gibala, M.J. Twelve Weeks of Sprint Interval Training Improves Indices of Cardiometabolic Health Similar to Traditional Endurance Training despite a Five-Fold Lower Exercise Volume and Time Commitment. PLoS ONE 2016, 11, e0154075. [Google Scholar] [CrossRef]
- Higgins, M.F.; James, R.S.; Price, M.J. The effects of sodium bicarbonate (NaHCO3) ingestion on high intensity cycling capacity. J. Sports Sci. 2013, 31, 972–981. [Google Scholar] [CrossRef]
- Hirsch, K.R.; Greenwalt, C.E.; Saylor, H.E.; Gould, L.M.; Harrison, C.H.; Brewer, G.J.; Blue, M.N.M.; Ferrando, A.A.; Huffman, K.M.; Mayer-Davis, E.J.; et al. High-intensity interval training and essential amino acid supplementation: Effects on muscle characteristics and whole-body protein turnover. Physiol. Rep. 2021, 9, e14655. [Google Scholar] [CrossRef]
- Holmes, A.J.; Stratton, M.T.; Bailly, A.R.; Gottschall, J.S.; Feito, Y.; Ha, P.L.; Lavigne, A.; Persaud, K.; Gagnon, H.L.; Krueger, A.; et al. Effects of plyometric- and cycle-based high-intensity interval training on body composition, aerobic capacity, and muscle function in young females: A field-based group fitness assessment. Appl. Physiol. Nutr. Metab. 2023, 48, 932–945. [Google Scholar] [CrossRef]
- Keating, S.E.; Machan, E.A.; O’Connor, H.T.; Gerofi, J.A.; Sainsbury, A.; Caterson, I.D.; Johnson, N.A. Continuous Exercise but Not High Intensity Interval Training Improves Fat Distribution in Overweight Adults. J. Obes. 2014, 2014, 834865. [Google Scholar] [CrossRef]
- Lan, C.; Liu, Y.; Wang, Y. Effects of different exercise programs on cardiorespiratory fitness and body composition in college students. J. Exerc. Sci. Fit. 2022, 20, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Seo, J.-W.; Bae, J.-H.; Jiang, S.; Sung, Y.; Jamrasi, P.; Ahn, S.Y.; Han, S.; Kim, S.; Kim, C.; et al. Effects of High-Intensity Interval Walking on Cognitive and Physical Functions in Older Adults: A Randomized Pilot Study. Cureus 2024, 16, e68165. [Google Scholar] [CrossRef]
- Lu, Y.; Wiltshire, H.D.; Baker, J.S.; Wang, Q. The Effects of Running Compared with Functional High-Intensity Interval Training on Body Composition and Aerobic Fitness in Female University Students. Int. J. Environ. Res. Public Health 2021, 18, 11312. [Google Scholar] [CrossRef]
- Macpherson, R.E.K.; Hazell, T.J.; Olver, T.D.; Paterson, D.H.; Lemon, P.W.R. Run Sprint Interval Training Improves Aerobic Performance but Not Maximal Cardiac Output. Med. Sci. Sports Exerc. 2011, 43, 115. [Google Scholar] [CrossRef]
- Marcangeli, V.; Youssef, L.; Dulac, M.; Carvalho, L.P.; Hajj-Boutros, G.; Reynaud, O.; Guegan, B.; Buckinx, F.; Gaudreau, P.; Morais, J.A.; et al. Impact of high-intensity interval training with or without L-citrulline on physical performance, skeletal muscle, and adipose tissue in obese older adults. J. Cachexia Sarcopenia Muscle 2022, 13, 1526–1540. [Google Scholar] [CrossRef] [PubMed]
- Marzuca-Nassr, G.N.; Artigas-Arias, M.; Olea, M.A.; SanMartín-Calísto, Y.; Huard, N.; Durán-Vejar, F.; Beltrán-Fuentes, F.; Muñoz-Fernández, A.; Alegría-Molina, A.; Sapunar, J.; et al. High-intensity interval training on body composition, functional capacity and biochemical markers in healthy young versus older people. Exp. Gerontol. 2020, 141, 111096. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, T.; Saotome, K.; Seino, S.; Eto, M.; Shimojo, N.; Matsushita, A.; Iemitsu, M.; Ohshima, H.; Tanaka, K.; Mukai, C. Low-volume, high-intensity, aerobic interval exercise for sedentary adults: VO2max, cardiac mass, and heart rate recovery. Eur. J. Appl. Physiol. 2014, 114, 1963–1972. [Google Scholar] [CrossRef]
- Matsuo, T.; Saotome, K.; Seino, S.; Shimojo, N.; Matsushita, A.; Iemitsu, M.; Ohshima, H.; Tanaka, K.; Mukai, C. Effects of a Low-Volume Aerobic-Type Interval Exercise on VO2max and Cardiac Mass. Med. Sci. Sports Exerc. 2014, 46, 42. [Google Scholar] [CrossRef]
- Monsalves-Álvarez, M.; Jiménez, T.; Bunout, D.; Barrera, G.; Hirsch, S.; Sepúlveda-Guzman, C.; Silva, C.; Rodriguez, J.M.; Troncoso, R.; De La Maza, M.P. High-intensity interval training prevents muscle mass loss in overweight Chilean young adults during a hypocaloric-Mediterranean diet: A randomized trial. Front. Nutr. 2023, 10, 1181436. [Google Scholar] [CrossRef]
- Nybo, L.; Sundstrup, E.; Jakobsen, M.D.; Mohr, M.; Hornstrup, T.; Simonsen, L.; Bülow, J.; Randers, M.B.; Nielsen, J.J.; Aagaard, P.; et al. High-Intensity Training versus Traditional Exercise Interventions for Promoting Health. Med. Sci. Sports Exerc. 2010, 42, 1951–1958. [Google Scholar] [CrossRef]
- Osawa, Y.; Tabata, S.; Katsukawa, F.; Ishida, H.; Oguma, Y.; Kawai, T.; Itoh, H.; Okuda, S.; Matsumoto, H.; Azuma, K. Effects of 16-week high-intensity interval training using upper and lower body ergometers on aerobic fitness and morphological changes in healthy men: A preliminary study. Open Access J. Sports Med. 2014, 5, 257–265. [Google Scholar] [CrossRef]
- Rabiee, M.; Daryanoosh, F.; Salesi, M.; Tahmasebi, R.; Koushkie, M. The Effect of Eight Weeks of Mediterranean Diet and High-Intensity Interval Training on Body Composition in Obese and Overweight Premenopausal Women. Int. J. Nutr. Sci. 2023, 8, 117–124. [Google Scholar] [CrossRef]
- Robinson, M.M.; Dasari, S.; Konopka, A.R.; Johnson, M.L.; Manjunatha, S.; Esponda, R.R.; Carter, R.E.; Lanza, I.R.; Nair, K.S. Enhanced Protein Translation Underlies Improved Metabolic and Physical Adaptations to Different Exercise Training Modes in Young and Old Humans. Cell Metab. 2017, 25, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Vélez, R.; Tordecilla-Sanders, A.; Téllez-T, L.A.; Camelo-Prieto, D.; Hernández-Quiñonez, P.A.; Correa-Bautista, J.E.; Garcia-Hermoso, A.; Ramírez-Campillo, R.; Izquierdo, M. Effect of Moderate- Versus High-Intensity Interval Exercise Training on Heart Rate Variability Parameters in Inactive Latin-American Adults: A Randomized Clinical Trial. J. Strength Cond. Res. 2020, 34, 3403–3415. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, B.J.; Tucker, W.J.; Bhammar, D.M.; Ryder, J.R.; Sweazea, K.L.; Gaesser, G.A. Effects of high-intensity interval training and moderate-intensity continuous training on endothelial function and cardiometabolic risk markers in obese adults. J. Appl. Physiol. 2016, 121, 279–288. [Google Scholar] [CrossRef]
- Schleh, M.W.; Ahn, C.; Ryan, B.J.; Chugh, O.K.; Luker, A.T.; Luker, K.E.; Gillen, J.B.; Ludzki, A.C.; Van Pelt, D.W.; Pitchford, L.M.; et al. Both moderate- and high-intensity exercise training increase intramyocellular lipid droplet abundance and modify myocellular distribution in adults with obesity. Am. J. Physiol.-Endocrinol. Metab. 2023, 325, E466–E479. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, S.O.; Cocks, M.; Tipton, K.D.; Ranasinghe, A.M.; Barker, T.A.; Burniston, J.G.; Wagenmakers, A.J.M.; Shaw, C.S. Sprint interval and traditional endurance training increase net intramuscular triglyceride breakdown and expression of perilipin 2 and 5: Perilipin expression and IMTG metabolism. J. Physiol. 2013, 591, 657–675. [Google Scholar] [CrossRef]
- Theofilidis, G.; Bogdanis, G.C.; Stavropoulos-Kalinoglou, A.; Krase, A.A.; Tsatalas, T.; Shum, G.; Sakkas, G.K.; Koutedakis, Y.; Karatzaferi, C. The effects of training with high-speed interval running on muscle performance are modulated by slope. Physiol. Rep. 2021, 9, e14656. [Google Scholar] [CrossRef]
- Tsekouras, Y.E.; Magkos, F.; Kellas, Y.; Basioukas, K.N.; Kavouras, S.A.; Sidossis, L.S. High-intensity interval aerobic training reduces hepatic very low-density lipoprotein-triglyceride secretion rate in men. Am. J. Physiol.-Endocrinol. Metab. 2008, 295, E851–E858. [Google Scholar] [CrossRef]
- Zhang, K.; Guo, B.; Yang, M.; Jia, Y.; Zhang, K.; Wang, L. The assessment of sports performance by grip pressure using flexible piezoresistive pressure sensors in seven sports events. Sci. Rep. 2024, 14, 31750. [Google Scholar] [CrossRef]
- Allemeier, C.A.; Fry, A.C.; Johnson, P.; Hikida, R.S.; Hagerman, F.C.; Staron, R.S. Effects of sprint cycle training on human skeletal muscle. J. Appl. Physiol. 1994, 77, 2385–2390. [Google Scholar] [CrossRef] [PubMed]
- Bagley, L.; Slevin, M.; Bradburn, S.; Liu, D.; Murgatroyd, C.; Morrissey, G.; Carroll, M.; Piasecki, M.; Gilmore, W.S.; McPhee, J.S. Sex differences in the effects of 12 weeks sprint interval training on body fat mass and the rates of fatty acid oxidation and VO2max during exercise. BMJ Open Sport Exerc. Med. 2016, 2, e000056. [Google Scholar] [CrossRef] [PubMed]
- De Souza, E.O.; Tricoli, V.; Aoki, M.S.; Roschel, H.; Brum, P.C.; Bacurau, A.V.N.; Silva-Batista, C.; Wilson, J.M.; Neves, M.; Soares, A.G.; et al. Effects of Concurrent Strength and Endurance Training on Genes Related to Myostatin Signaling Pathway and Muscle Fiber Responses. J. Strength Cond. Res. 2014, 28, 3215–3223. [Google Scholar] [CrossRef]
- Estes, R.R.; Malinowski, A.; Piacentini, M.; Thrush, D.; Salley, E.; Losey, C.; Hayes, E. The Effect of High Intensity Interval Run Training on Cross-sectional Area of the Vastus Lateralis in Untrained College Students. Int. J. Exerc. Sci. 2017, 10, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Hashida, R.; Takano, Y.; Matsuse, H.; Kudo, M.; Bekki, M.; Omoto, M.; Nago, T.; Kawaguchi, T.; Torimura, T.; Shiba, N. Electrical Stimulation of the Antagonist Muscle During Cycling Exercise Interval Training Improves Oxygen Uptake and Muscle Strength. J. Strength Cond. Res. 2021, 35, 111–117. [Google Scholar] [CrossRef]
- Higgins, S.; Fedewa, M.V.; Hathaway, E.D.; Schmidt, M.D.; Evans, E.M. Sprint interval and moderate-intensity cycling training differentially affect adiposity and aerobic capacity in overweight young-adult women. Appl. Physiol. Nutr. Metab. 2016, 41, 1177–1183. [Google Scholar] [CrossRef]
- Joanisse, S.; Gillen, J.B.; Bellamy, L.M.; McKay, B.R.; Tarnopolsky, M.A.; Gibala, M.J.; Parise, G. Evidence for the contribution of muscle stem cells to nonhypertrophic skeletal muscle remodeling in humans. FASEB J. 2013, 27, 4596–4605. [Google Scholar] [CrossRef]
- Ramírez-Vélez, R.; Izquierdo, M.; Castro-Astudillo, K.; Medrano-Mena, C.; Monroy-Díaz, A.L.; Castellanos-Vega, R.D.P.; Triana-Reina, H.R.; Correa-Rodríguez, M. Weight Loss after 12 Weeks of Exercise and/or Nutritional Guidance Is Not Obligatory for Induced Changes in Local Fat/Lean Mass Indexes in Adults with Excess of Adiposity. Nutrients 2020, 12, 2231. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Mei, T.; Duan, J.; Yan, X.; McNaughton, L.; He, Z. Genome-wide Association Study of Exercise-induced Skeletal Muscle Hypertrophy and the Construction of Predictive Model. Physiol. Genom. 2024, 56, 578–589. [Google Scholar] [CrossRef]
- Bissas, A.; Paradisis, G.P.; Nicholson, G.; Walker, J.; Hanley, B.; Havenetidis, K.; Cooke, C.B. Development and Maintenance of Sprint Training Adaptations: An Uphill-Downhill Study. J. Strength Cond. Res. 2022, 36, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Bornath, D.P.D.; Kenno, K.A. Physiological Responses to Increasing Battling Rope Weight During Two 3-Week High-Intensity Interval Training Programs. J. Strength Cond. Res. 2022, 36, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Buckley, S.; Knapp, K.; Lackie, A.; Lewry, C.; Horvey, K.; Benko, C.; Trinh, J.; Butcher, S. Multimodal high-intensity interval training increases muscle function and metabolic performance in females. Appl. Physiol. Nutr. Metab. 2015, 40, 1157–1162. [Google Scholar] [CrossRef]
- Cao, M.; Yang, B.; Tang, Y.; Wang, C.; Yin, L. Effects of low-volume functional and running high-intensity interval training on physical fitness in young adults with overweight/obesity. Front. Physiol. 2024, 15, 1325403. [Google Scholar] [CrossRef] [PubMed]
- Caparrós-Manosalva, C.; Garrido-Muñoz, N.; Alvear-Constanzo, B.; Sanzana-Laurié, S.; Artigas-Arias, M.; Alegría-Molina, A.; Vidal-Seguel, N.; Espinoza-Araneda, J.; Huard, N.; Pagnussat, A.S.; et al. Effects of high-intensity interval training on lean mass, strength, and power of the lower limbs in healthy old and young people. Front. Physiol. 2023, 14, 1223069. [Google Scholar] [CrossRef]
- Ferley, D.D.; Osborn, R.W.; Vukovich, M.D. The Effects of Incline and Level-Grade High-Intensity Interval Treadmill Training on Running Economy and Muscle Power in Well-Trained Distance Runners. J. Strength Cond. Res. 2014, 28, 1298–1309. [Google Scholar] [CrossRef]
- Kayhan, R.F.; Bayrakdaroğlu, S.; Ceylan, H.İ.; Eken, Ö.; Bayrakdaroğlu, Y.; Badicu, G.; Al-Mhanna, S.B.; Enoiu, R.-S.; Ardigò1, L.P. Effects of different rest intervals in high intensity interval training programs on VO2max, body composition, and isokinetic strength and power. J. Mens Health 2024, 20, 1–11. [Google Scholar]
- Molinari, T.; Molinari, T.; Rabello, R.; Rodrigues, R. Effects of 8 weeks of high-intensity interval training or resistance training on muscle strength, muscle power and cardiorespiratory responses in trained young men. Sport Sci. Health 2022, 18, 887–896. [Google Scholar] [CrossRef]
- Schjerve, I.E.; Tyldum, G.A.; Tjønna, A.E.; Stølen, T.; Loennechen, J.P.; Hansen, H.E.M.; Haram, P.M.; Heinrich, G.; Bye, A.; Najjar, S.M.; et al. Both aerobic endurance and strength training programmes improve cardiovascular health in obese adults. Clin. Sci. 2008, 115, 283–293. [Google Scholar] [CrossRef]
- Sheykhlouvand, M.; Arazi, H.; Astorino, T.A.; Suzuki, K. Effects of a New Form of Resistance-Type High-Intensity Interval Training on Cardiac Structure, Hemodynamics, and Physiological and Performance Adaptations in Well-Trained Kayak Sprint Athletes. Front. Physiol. 2022, 13, 850768. [Google Scholar] [CrossRef]
- Sökmen, B.; Witchey, R.L.; Adams, G.M.; Beam, W.C. Effects of Sprint Interval Training With Active Recovery vs. Endurance Training on Aerobic and Anaerobic Power, Muscular Strength, and Sprint Ability. J. Strength Cond. Res. 2018, 32, 624–631. [Google Scholar] [CrossRef]
- Song, T.; Jilikeha, J.; Deng, Y. Physiological and Biochemical Adaptations to a Sport-Specific Sprint Interval Training in Male Basketball Athletes. J. Sports Sci. Med. 2023, 22, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Venegas-Carro, M.; Herring, J.T.; Riehle, S.; Kramer, A. Jumping vs. running: Effects of exercise modality on aerobic capacity and neuromuscular performance after a six-week high-intensity interval training. PLoS ONE 2023, 18, e0281737. [Google Scholar] [CrossRef]
- Wong, P.Y.; Soh, S.M.M.; Chu, W.-J.M.; Lim, M.X.C.; Jones, L.E.; Selvaraj, S.; Chow, K.M.S.; Choo, H.W.D.; Aziz, A.R. A single all-out bout of 30-s sprint-cycle performed on 5 consecutive days per week over 6 weeks does not enhance cardiovascular fitness, maximal strength, and clinical health markers in physically active young adults. Eur. J. Appl. Physiol. 2024, 124, 1861–1874. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, C.; Hu, M.; Fang, Y.; Shi, Y.; Tse, A.C.-Y.; Lo, S.-K.; Wong, S.H.-S.; Sun, F. The effect of different high-intensity interval training protocols on cardiometabolic and inflammatory markers in sedentary young women: A randomized controlled trial. J. Sports Sci. 2024, 42, 751–762. [Google Scholar] [CrossRef]
- Nybo, L.; Pedersen, K.; Christensen, B.; Aagaard, P.; Brandt, N.; Kiens, B. Impact of carbohydrate supplementation during endurance training on glycogen storage and performance. Acta Physiol. 2009, 197, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Bagley, L.; Al-Shanti, N.; Bradburn, S.; Baig, O.; Slevin, M.; McPhee, J.S. Sex Comparison of Knee Extensor Size, Strength, and Fatigue Adaptation to Sprint Interval Training. J. Strength Cond. Res. 2021, 35, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Joan Dawson, M.; Gadian, D.G.; Wilkie, D.R. Muscular fatigue investigated by phosphorus nuclear magnetic resonance. Nature 1978, 274, 861–866. [Google Scholar] [CrossRef]
- Green, H.J. Mechanisms of muscle fatigue in intense exercise. J. Sports Sci. 1997, 15, 247–256. [Google Scholar] [CrossRef]
- Engstrom, C.M.; Loeb, G.E.; Reid, J.G.; Forrest, W.J.; Avruch, L. Morphometry of the human thigh muscles. A comparison between anatomical sections and computer tomographic and magnetic resonance images. J. Anat. 1991, 176, 139–156. [Google Scholar]
- Mitsiopoulos, N.; Baumgartner, R.N.; Heymsfield, S.B.; Lyons, W.; Gallagher, D.; Ross, R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J. Appl. Physiol. 1998, 85, 115–122. [Google Scholar] [CrossRef]
- Mechelli, F.; Arendt-Nielsen, L.; Stokes, M.; Agyapong-Badu, S. Validity of Ultrasound Imaging Versus Magnetic Resonance Imaging for Measuring Anterior Thigh Muscle, Subcutaneous Fat, and Fascia Thickness. Methods Protoc. 2019, 2, 58. [Google Scholar] [CrossRef]
- Tavoian, D.; Ampomah, K.; Amano, S.; Law, T.D.; Clark, B.C. Changes in DXA-derived lean mass and MRI-derived cross-sectional area of the thigh are modestly associated. Sci. Rep. 2019, 9, 10028. [Google Scholar] [CrossRef]
- Bolanowski, M.; Nilsson, B.E. Assessment of human body composition using dual-energy x-ray absorptiometry and bioelectrical impedance analysis. Med. Sci. Monit. 2001, 7, 1029–1033. [Google Scholar] [PubMed]
- Pietiläinen, K.H.; Kaye, S.; Karmi, A.; Suojanen, L.; Rissanen, A.; Virtanen, K.A. Agreement of bioelectrical impedance with dual-energy X-ray absorptiometry and MRI to estimate changes in body fat, skeletal muscle and visceral fat during a 12-month weight loss intervention. Br. J. Nutr. 2013, 109, 1910–1916. [Google Scholar] [CrossRef] [PubMed]
- Burkhart, T.A.; Arthurs, K.L.; Andrews, D.M. Manual segmentation of DXA scan images results in reliable upper and lower extremity soft and rigid tissue mass estimates. J. Biomech. 2009, 42, 1138–1142. [Google Scholar] [CrossRef]
- Nordez, A.; Jolivet, E.; Südhoff, I.; Bonneau, D.; de Guise, J.A.; Skalli, W. Comparison of methods to assess quadriceps muscle volume using magnetic resonance imaging. J. Magn. Reson. Imaging 2009, 30, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Saeterbakken, A.H.; Stien, N.; Paulsen, G.; Behm, D.G.; Andersen, V.; Solstad, T.E.J.; Prieske, O. Task Specificity of Dynamic Resistance Training and Its Transferability to Non-trained Isometric Muscle Strength: A Systematic Review with Meta-analysis. Sports Med. 2025, 55, 1651–1676. [Google Scholar] [CrossRef]
- Schoenfeld, B.J.; Wilson, J.M.; Lowery, R.P.; Krieger, J.W. Muscular adaptations in low- versus high-load resistance training: A meta-analysis. Eur. J. Sport Sci. 2016, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pallares, J.G.; Barranco-Gil, D.; Rodríguez-Rielves, V.; De Pablos, R.; Buendía-Romero, Á.; Martínez-Cava, A.; Franco-López, F.; Sánchez-Redondo, I.R.; Iriberri, J.; Revuelta, C.; et al. Cyclists do not need to incorporate off-bike resistance training to increase strength, muscle-tendon structure, and pedaling performance: Exploring a high-intensity on-bike method. Biol. Sport 2025, 42, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Salzgeber, A.; Porcari, J.P.; Howard, C.; Arney, B.E.; Kovacs, A.; Gillette, C.; Foster, C. Muscle Activation During Several Battle Rope Exercises. Master’s Thesis, Wisconsin-La Crosse University, La Crosse, WI, USA, 2019. [Google Scholar]
| Study | Sample Size | Training Status/Age | Group Name | Protocol | Modality | Duration (Weeks) | Hypertrophy Outcome | Method(s) of Assessment |
|---|---|---|---|---|---|---|---|---|
| Bhati 2019 [45] | 17 | Sedentary, Young adults | HIIT (LV) | 1 × (4 min at 85–95% of HR max, 3 min at 70% HR max) | Running | 6 | FFM ↑ | BIA |
| Bhati 2019 [45] | 15 | Sedentary, Younger Adults | HIIT (HV) | 4 × (4 min at 85–95% of HR max, 3 min at 70% HR max) | Running | 6 | FF︎M ↔︎ | BIA |
| Bruseghini 2019 [46] | 12 | Recreational, Older Adults | HIIT | 7 × (2 min at 85–95% VO2max, 2 min rest) | Cycling | 8 | FFM ↔︎ | DXA |
| Buckinx 2018 [47] | 30 | Sedentary Obese, Older Adults | HIIT | 10 × (30 s at 80–85% HR max, 90 s at 65% HR max) | Elliptical | 12 | FFM ↑ | DXA |
| Clark 2019 [48] | 8 | Sedentary Obese, Adults | HIIT (Per) | 6–10 × (20 s–2 min at 65–110% peak power, 1–2 min rest) | Cycling | 6 | FFM ↔︎ | ADP |
| Clark 2019 [48] | 9 | Sedentary Obese, Adults | HIIT (Trad) | 10 × (1 min at 70–85% peak power, 60 s rest) | Cycling | 6 | FFM ↑ | ADP |
| Couvert 2024 [49] | 8 | TS Unknown, Obese/Overweight, Adults | HIIT | 9 × (45 s at 80–85% HR max, 90 s active recovery) | Running | 12 | FFM ↔︎ | DXA |
| Couvert 2024 [49] | 8 | TS Unknown, Obese/Overweight, Adults | HIIT | 10 × (45 s at 80–85% HR max, 90 s active recovery) | Cycling | 12 | FFM ↔︎ | DXA |
| D’Alleva 2023 [50] | 16 | Sedentary, Obese, Adults | HIIT | 5–7 × (2 min at 95% VO2peak, 1 min at 50% VO2peak) | Running | 12 | FFM ↔︎ | BIA |
| Gillen 2016 [51] | 8 | Sedentary Obese, Adults | HIIT (Fed) | 10 × (1 min at 90% HR max, 1 min at 50 W) | Cycling | 6 | FFM ↔︎ | DXA |
| Gillen 2016 [51] | 8 | Sedentary Obese, Adults | HIIT (Fasted) | 10 × (1 min at 90% HR max, 1 min at 50 W) | Cycling | 6 | FFM ↔︎ | DXA |
| Higgins 2016 [52] | 23 | Sedentary Obese, Younger Adults | SIT | 4–7 × (30 s at maximal effort, 4 min of active rest) | Cycling | 6 | FFM ↔︎ | DXA |
| Hirsch 2021 [53] | 19 | Untrained Obese, Adults | HIIT | 6–10 × (1 min at 90% watt-max, 60 s rest) | Cycling | 8 | FFM ↑ | DXA |
| Holmes 2023 [54] | 15 | Recreational, Younger Adults | HIIT | 30 min (20–80 s at unknown intensity, 10–60 s rest) | Cycling | 8 | FFM ↑ | DXA |
| Keating 2014 [55] | 13 | Sedentary, Adults | HIIT | 4–6 × (30–60 s at 120% VO2peak, 2–3 min at 30 W) | Cycling | 12 | FFM ↔︎ | DXA |
| Lan 2022 [56] | 12 | Sedentary, Younger Adults | HIIT | 4 × (4 min at 85–95% HR max, 3 min at 64–76% HR max) | Running | 8 | FFM ↔︎, SMM ↔︎ | BIA |
| Li 2024 [57] | 13 | Untrained, Older Adults | HIIT | 5 × (3 min at 85% HR max, 3 min at 55% HR max) | Walking | 8 | SMM ↑ | BIA |
| Lu 2021 [58] | 10 | Untrained, Younger Adults | SIT | 4 × (30 s at max effort, 30 s rest | Running | 12 | FFM ↑ | BIA |
| MacPherson 2011 [59] | 10 | Recreational, Younger Adults | SIT | 4–6 × (30 s at max effort, 4 min active rest) | Running | 6 | FFM ↑ | ADP |
| Marcangeli 2022 [60] | 45 | Sedentary Obese, Older Adults | HIIT | 10 × (30 s at 80–85% HR max, 90 s at 65% HR max) | Elliptical | 12 | FFM ↑ | DXA |
| Marzuca-Nassr 2020 [61] | 10 | Sedentary, Younger Adults | HIIT | 10 × (1 min at 90% HR max, 2 min rest) | Cycling | 12 | FFM ↔︎ | DXA |
| Marzuca-Nassr 2020 [61] | 10 | Sedentary, Older Adults | HIIT | 10 × (1 min at 90% HR max, 2 min rest) | Cycling | 12 | FFM ↔︎ | DXA |
| Matsuo 2014a [62] | 12 | Sedentary, Adults | HIIT | 3 × (3 min at 80–85% VO2max, 2 min at 50% VO2max) | Cycling | 8 | FFM ↔︎ | DXA |
| Matsuo 2014b [63] | 14 | Sedentary, Adults | SIT | 7 × (30 s at 120% VO2max, 15 s rest) | Cycling | 8 | FFM ↑ | DXA |
| Matsuo 2014b [63] | 14 | Sedentary, Adults | HIIT | 3 × (3 min 80–90% VO2max, 2 min at 50% VO2max) | Cycling | 8 | FFM ↑ | DXA |
| Monsalves-Álvarez [64] | 11 | TS Unknown, Overweight/Obese Adults | HIIT | 10 × (1 min at 85–90% HR max, 1 min at 50 W) | Cycling | 12 | FFM ↔︎ | DXA |
| Nybo 2010 [65] | 8 | Untrained, Adults | HIIT | 5 × (2 min at 95% HR max, 1 min rest) | Running | 12 | FFM ↔︎ | DXA |
| Osawa 2014 [66] | 7 | Untrained, Adults | HIIT (Leg) | 8–12 × (1 min at >90% watt-peak, 60 s active rest) | Cycling | 16 | FFM ↔︎ | DXA |
| Osawa 2014 [66] | 5 | Untrained, Adults | HIIT (Arm/Leg) | 8–12 × (60 s at >90% watt-peak, 60 s active rest) (half arm, half leg) | Cycling/Arm Cycling | 16 | FFM ↔︎ | DXA |
| Rabbiee 2023 [67] | 9 | Sedentary, Obese/Overweight, Adults | HIIT | 4–6 × (1 min at 90–95% HR max, 1 min rest) | Cycling | 8 | SMM ↔︎ | BIA |
| Robinson 2017 [68] | 14 | Untrained, Younger Adults | HIIT (Young) | 4 × (4 min at >90% of VO2max, 3 min at 0 W) | Cycling | 12 | FFM ↑ | DXA |
| Robinson 2017 [68] | 9 | Untrained, Older Adults | HIIT (Old) | 4 × (4 min at >90% of VO2max, 3 min at 0 W) | Cycling | 12 | FFM ↑ | DXA |
| Ramirez-Velez 2020a [69] | 14 | Sedentary, Adults | HIIT | 4 × (4 min at 85–95% HR max, 4 min at 65% HR max) week 3–12 max) week 3–12 | Run/Walk with Incline | 12 | FFM ↑ | DXA |
| Sawyer 2016 [70] | 9 | TS Unknown Obese, Adults | HIIT | 10 × (1 min at 90–95% of HR max, 1 min at 25–50 W) | Cycling | 8 | FFM ↔︎ | DXA |
| Schleh 2023 [71] | 19 | Sedentary, Obese, Adults | HIIT | 10 × (1 min at 90% HR max, 1 min at 65% HR max) | Cycling/Rowing/ Running/ Elliptical | 12 | FFM ↔︎ | DXA |
| Shepherd 2013 [72] | 42 | Sedentary, Adults | HIIT | 4–12 × (15–60 s at >90% HR max, 45–120 s active rest) | Cycling | 10 | FFM ↔︎ | BIA |
| Theofilidis 2021 [73] | 7 | Recreational, Adults | HIIT (Up) | 10 × (30 s at 90% MAS +10% grade, 60 s rest) | Running | 8 | FFM ↔︎ | BIA |
| Theofilidis 2021 [73] | 7 | Recreational, Adults | HIIT (Down) | 10 × (30 s at 90% MAS −10% grade, 60 s rest) | Running | 8 | FFM ↔︎ | BIA |
| Tsekouras 2008 [74] | 7 | Sedentary, Younger Adults | HIIT | 4 × (4 min at 90% VO2peak, 4 min at 60% VO2peak) | Running | 8 | FFM ↔︎ | DXA |
| Zhang 2024 [75] | 14 | Sedentary, Younger Adults | HIIT | 2–3 × (12–15 × 30 s at max effort, 10 s rest) | Cycling | 8 | FFM ↔︎ | BIA |
| Ziemann 2011 [6] | 10 | Recreational, Younger Adults | HIIT | 6 × (90 s at 80% VO2max, 3 min rest) | Cycling | 6 | FF︎M ↔︎ | BIA |
| Study | Sample Size | Training Status/Age | Group Name | Protocol | Modality | Duration (Weeks) | Hypertrophy Outcome | Method(s) of Assessment |
|---|---|---|---|---|---|---|---|---|
| Allemeier 1994 [76] | 11 | Untrained, Young Adults | SIT | 3 × (30 s Wingate, 20 min rest) | Cycling | 6 | Type 1 CSA ↔︎, Type 2a CSA ↔︎, Type 2× CSA ↔︎ | Histology |
| Bagley 2016 [77] | 15 | Recreational, Adults | SIT (Female) | 4 × (20 s at 175–200% VO2peak, 2 min rest) | Cycling | 12 | LLM ↔︎, Thigh Lean Mass ↔︎, Quad CSA ↑ | DXA, MRI |
| Bagley 2016 [77] | 16 | Recreational, Adults | SIT (Male) | 4 × (20 s at 175–200% VO2peak, 2 min rest) | Cycling | 12 | LLM ↔︎,Thigh Lean Mass ↔︎, Quad CSA ↑ | DXA, MRI |
| Bruseghini 2019 [46] | 12 | Recreational, Older Adults | HIIT | 7 × (2 min at 85–95% VO2max, 2 min rest) | Cycling | 8 | Quad CSA ↑, Quad Volume ↑ | MRI |
| De Souza 2014 [78] | 8 | Recreational, Adults | HIIT | 15–20 × (60 s at VO2peak, rest time/intensity not reported) | Running | 8 | Thigh CSA ↔︎ Type 1 CSA ↔︎, Type 2a CSA ↔︎, Type 2× CSA ↔︎ | MRI, Histology |
| Estes 2017 [79] | 12 | Recreational, Younger Adults | HIIT | 4 × (4 min at 90–95% HR max, 3 min rest) | Running | 10 | VL CSA ↑ | Ultrasound |
| Gillen 2016 [51] | 8 | Sedentary Obese, Adults | HIIT (Fed) | 10 × (1 min at 90% HR max, 1 min at 50 W) | Cycling | 6 | LLM ↑, Gynoid LM ↑ | DXA |
| Gillen 2016 [51] | 8 | Sedentary Obese, Adults | HIIT (Fasted) | 10 × (1 min at 90% HR max, 1 min at 50 W) | Cycling | 6 | LLM ↑, Gynoid LM ↑ | DXA |
| Hashida 2016 [80] | 15 | Recreational, Younger Adults | HIIT | 5 × (2 min at 60–90% VO2peak, 2 min at 40% VO2peak) | Cycling | 6 | RF Muscle Thickness ↔︎ | Ultrasound |
| Higgins 2016 [81] | 23 | Sedentary Obese, Younger Adults | SIT | 4–7 × (30 s at maximal effort, 4 min of active rest) | Cycling | 6 | LLM ↑ | DXA |
| Hirsch 2021 [53] | 19 | Untrained Obese, Adults | HIIT | 6–10 × (1 min at 90%-watt-max, 60 s rest) | Cycling | 8 | Thigh LM ↑, VL CSA ↑, VL Volume ↑ | DXA, Ultrasound |
| Holmes 2023 [54] | 15 | Recreational, Younger Adults | HIIT | 30 min (20–80 s at unknown intensity, 10–60 s rest) | Cycling | 8 | LLM ↔︎, ALM ↔︎, VL CSA ↔︎ | DXA, Ultrasound |
| Joanisse 2013 [82] | 15 | Sedentary, Adults | HIIT | 10 × (1 min at 90% HR max, 1 min at 50 W or passive rest) | Cycling | 6 | Type 1 CSA ↔︎, Type 2 CSA ↔︎, Hybrid CSA ↔︎ | Histology |
| Marcangeli 2022 [60] | 45 | Sedentary Obese, Older Adults | HIIT | 10 × (30 s at 80–85% HR max, 90 s at 65% HR max) | Elliptical | 12 | LLM ↑, ALM ↔︎ | DXA |
| Marzuca-Nassr 2020 [61] | 10 | Sedentary, Younger Adults | HIIT | 10 × (1 min at 90% HR max, 2 min rest) | Cycling | 12 | LLM ↑ | DXA |
| Marzuca-Nassr 2020 [67] | 10 | Sedentary, Older Adults | HIIT | 10 × (1 min at 90% HR max, 2 min rest) | Cycling | 12 | LLM ↔︎ | DXA |
| Monsalves-Álvarez 2023 [64] | 11 | TS Unknown, Overweight/Obese Adults | HIIT | 10 × (1 min at 85–90% HR max, 1 min at 50 W) | Cycling | 12 | RF CSA ↔︎ | Ultrasound |
| Nybo 2010 [65] | 8 | Untrained, Adults | HIIT | 5 × (2 min at 95% HR max, 1 min rest) | Running | 12 | LLM ↔︎ | DXA |
| Osawa 2014 [66] | 7 | Untrained, Adults | HIIT (Leg) | 8–12 × (1 min at >90% watt-peak, 60 s active rest) | Cycling | 16 | Quad CSA ↑, Hamstring CSA ↔︎, LBLM ↔︎, UBLM, ↔︎ Psoas CSA ↔︎, Alab CSA ↔︎, Spinal CSA ↔︎ | DXA, MRI |
| Osawa 2014 [66] | 5 | Untrained, Adults | HIIT (Arm/Leg) | 8–12 × (60 s at >90% watt-peak, 60 s active rest) (half arm, half leg) | Cycling/Arm Cycling | 16 | Quad CSA ↑, Hamstring CSA LBLM ↔︎, UBLM ↔︎, Psoas CSA ↑, Alab CSA ↔︎, Spinal CSA ↔︎ | DXA, MRI |
| Ramirez-Velez 2020b [83] | 11 | Sedentary, Adults | HIIT | 4 × (4 min at 85–95% HR max, 4 min at 65% HR max) week 3–12 | Running | 12 | LLM ↔︎, ALM ↔︎, Trunk LM ↔︎ | BIA |
| Theofilidis 2021 [73] | 7 | Recreational, Adults | HIIT (Uphill) | 10 × (30 s at 90% MAS +10% grade, 60 s rest) | Hill Running | 8 | VL Thickness↓, VL Length ↓ | BIA, Ultrasound |
| Theofilidis 2021 [73] | 7 | Recreational, Adults | HIIT (Downhill) | 10 × (30 s at 90% MAS −10% grade, 60 s rest) | Running | 8 | VL Thickness↓, VL Length ↓ | BIA, Ultrasound |
| Yang 2024 [84] | 261 | Sedentary, Younger Adults | HIIT | 4 × (4 min at 80–90% VO2max, 3 min recovery at 50–55% VO2max) week 5–12 | Running | 12 | RF Muscle Thickness ↑ | Ultrasound |
| Author | Sample Size | Training Status/Age | Group Name | Protocol | Modality | Duration (Weeks) | Strength Outcome |
|---|---|---|---|---|---|---|---|
| Bagley 2016 [77] | 15 | Recreational, Adults | SIT (Female) | 4 × (20 s at 175–200% VO2peak, 2 min rest) | Cycling | 12 | ISOM Knee Extension (90°) ↔︎, ISOK Knee Extension (60°/s) ↔︎, ISOK Knee Extension (120°/s) ↔︎, ISOK Knee Extension (180°/s) ↔︎, ISOK Knee Extension (240°/s) |
| Bagley 2016 [77] | 16 | Recreational, Adults | SIT (Male) | 4 × (20 s at 175–200% VO2peak, 2 min rest) | Cycling | 12 | ISOM Knee Extension (90°) ↔︎, ISOK Knee Extension (60°/s) ↔︎, ISOK Knee Extension (120°/s) ↔︎, ISOK Knee Extension (180°/s) ↔︎, ISOK Knee Extension (240°/s) ↔︎ |
| Bhati 2019 [45] | 17 | Sedentary, Younger Adults | HIIT (LV) | 1 × (4 min at 85–95% of HR max, 3 min at 70% HR max) | Running | 6 | ISOM Knee Extension (90°) ↔︎ |
| Bhati 2019 [45] | 15 | Sedentary, Younger Adults | HIIT (HV) | 4 × (4 min at 85–95% HR max, 3 min at 70% HR max) | Running | 6 | ISOM Knee Extension (90°) ↔︎ |
| Bissas 2022 [85] | 14 | Recreational, Younger Adults | SIT (Up/Down) | 6 × (80 m at max effort, 4–6 min rest) | Running | 6 | ISOM Knee Extension (107°) ↑, ISOM Knee Flexion (107°) ↔︎ |
| Bissas 2022 [85] | 7 | Recreational, Younger Adults | SIT | 6 × (80 m at max effort, 4–6 min rest) | Running | 6 | ISOM Knee Extension (107°) ↔︎, ISOM Knee Flexion (107°) ↔︎ |
| Bornath and Kenno 2022 [86] | 15 | Recreational, Younger Adults | SIT (Female) | 10 × (30 s at max effort, 60 s rest) | Battle Ropes | 6 | ISOM Shoulder Flexion (90°) ↑, ISOM Shoulder Extension (90°) ↑ |
| Bornath and Kenno 2022 [86] | 18 | Recreational, Younger Adults | SIT (Male) | 10 × (30 s at max effort, 60 s rest) | Battle Ropes | 6 | ISOM Shoulder Flexion (90°) ↑, ISOM Shoulder Extension (90°) ↑ |
| Bruseghini 2019 [46] | 12 | Recreational, Older Adults | HIIT | 7 × (2 min at 85–95% VO2max, 2 min rest) | Cycling | 8 | ISOM Knee Extension (90°) ↔︎, ISOM Knee Extension (60°) ↑, Eccentric ISOK Knee Extension (60°/s) ↔︎, Eccentric ISOK Knee Extension (120°/s) ↔︎, ISOK Knee Extension (60°/s) ↔︎, ISOK Knee Extension (120°/s) ↔︎ |
| Buckinx 2018 [47] | 30 | Sedentary Obese, Older Adults | SIT | 10 × (30 s at 80–85% HR max, 90 s at 65% HR max) | Elliptical | 12 | ISOM Knee Extension (135°) ↔︎ |
| Buckley 2015 [87] | 14 | Recreational, Younger Adults | HIIT | 6 × (1 min at RPE of 9–10/10, 3 min rest) | Rowing | 6 | Squat 1-RM ↔︎, Deadlift 1-RM ↔︎ |
| Cao 2024 [88] | 12 | Sedentary, Younger Adults | SIT | 4 × (4 × 30 s at 100–120% MAS, 30 s rest at 50% MAS) | Running | 12 | Back Extension Force ↑, Hand grip Strength ↑ |
| Clark 2019 [48] | 8 | Sedentary Obese, Adults | HIIT (Per) | 6–10 × (20 s–2 min at 65–110% peak power, 1–2 min rest) | Cycling | 6 | ISOK Knee Extension (60°/s) ↔︎, ISOK Knee Flexion (60°/s) ↔︎ |
| Clark 2019 [48] | 9 | Sedentary Obese, Adults | HIIT (Trad) | 10 × (1 min at 70–85% peak power, 60 s rest) | Cycling | 6 | ISOK Knee Extension (60°/s) ↔︎, ISOK Knee Flexion (60°/s) ↔︎ |
| Caparrós-Manosalva 2023 [89] | 9 | Untrained, Younger Adults | HIIT(Young) | 10 × (1 min at 90% HR max, 2 min rest) | Cycling | 12 | ISOM Knee Extension (90°) ↑ |
| Caparrós-Manosalva 2023 [89] | 9 | Untrained, Older Adults | HIIT (Old) | 10 × (1 min at 90% HR max, 2 min rest) | Cycling | 12 | ISOM Knee Extension (90°) ↑ |
| De Souza 2014 [78] | 8 | Recreational, Younger Adults | HIIT | 15–20 × (60 s at VO2peak, rest time/intensity not reported) | Running | 8 | Leg Press 1RM ↔︎ |
| Ferley 2014 [90] | 12 | Endurance Trained, Younger Adults | SIT (Flat) | 4–6 × (60% T max at V max, time to 65% HR max) | Running | 6 | ISOK Knee Flexion (90°/s) ↔︎, ISOK Knee Flexion (180°/s) ↑, ISOK Knee Flexion (300°/s) ↑ |
| Ferley 2014 [90] | 12 | Endurance Trained, Younger Adults | SIT (Up) | 10–14 × (30 s at V max, time to 65% HR max) | Running | 6 | ISOK Knee Flexion (90°/s) ↔︎, ISOK Knee Flexion (180°/s) ↑, ISOK Knee Flexion (300°/s) ↑ |
| Hashida 2021 [80] | 15 | Recreational, Younger Adults | HIIT | 5 × (2 min at 60–90% VO2peak, 2 min 40% VO2peak) | Cycling | 6 | ISOK Knee Extension (60°/s) ↔︎ |
| Holmes 2023 [54] | 15 | Recreational, Younger Adults | HIIT | 30 min (20–80 s at unknown intensity, 10–60 s rest) | Cycling | 8 | ISOK Knee Extension (60°/s) ↔︎, PF Extension (60°/s) ↔︎ |
| Kayhan 2024 [91] | 9 | Recreational, Younger Adults | HIIT (Short rest) | 2 × 300 m, 2 × 300 m, 2 × 400 m, 2 × 300, 2 × 200, all at 85% HRR, rest until 45% HRR | Running | 8 | ISOK Knee Extension (60°/s) ↔︎, ISOK Knee Flexion (60°/s) ↔︎, ISOK Knee Extension (240°/s) ↔︎, ISOK Knee Flexion (240°/s) ↔︎ |
| Kayhan 2024 [91] | 10 | Recreational, Younger Adults | HIIT (Long rest) | 2 × 300 m, 2 × 300 m, 2 × 400 m, 2 × 300, 2 × 200, all at 85% HRR, rest until 35% HRR | Running | 8 | ISOK Knee Extension (60°/s) ↔︎, ISOK Knee Flexion (60°/s) ↔︎, ISOK Knee Extension (240°/s) ↔︎, ISOK Knee Flexion (240°/s) ↔︎ |
| Li 2024 [57] | 13 | Untrained, Older Adults | HIIT | 5 × (3 min at 85% HR max, 3 min at 55% HR max) | Walking | 8 | Hand Grip Strength ↓ |
| Monsalves-Álvarez 2023 [64] | 11 | TS Unknown, Overweight/Obese Adults | HIIT | 10 × (1 min at 85–90% HR max, 1 min at 50 W) | Cycling | 12 | Hand Grip Strength ↔︎, Quadriceps Strength ↔︎ |
| Molinari 2022 [92] | 9 | Resistance/Endurance Trained, Younger Adults | HIIT | 10 × (60 s at 85–95% HR max, 3 min walk) | Running | 8 | Leg Press 1-RM ↑, Knee Extension 1-RM ↔︎ |
| Robinson 2017 [68] | 14 | Untrained, Younger Adults | HIIT (Young) | 4 × (4 min at >90% of VO2max, 3 min at 0 W) | Cycling | 12 | Leg Press 1-RM ↔︎ |
| Robinson 2017 [68] | 9 | Untrained, Older Adults | HIIT (Old) | 4 × (4 min at >90% of VO2max, 3 min at 0 W) | Cycling | 12 | Leg Press 1-RM ↔︎ |
| Schjerve 2008 [93] | 14 | TS Unknown, Obese, Adults | HIIT | 4 × (4 min at HRmax, 3 min at 50–60% of HR max) | Running | 12 | Leg Press 1-RM ↔︎ |
| Sheykhlouvand 2022 [94] | 8 | Endurance Trained, Younger Adults | HIIT | 6 × (unknown duration at 100% VO2peak velocity, 1:1 work to rest ratio) | Kayaking | 8 | 1-RM One Arm Cable Row ↔︎ |
| Sökmen 2018 [95] | 20 | Recreational, Younger Adults | SIT | 40 min (200 m sprint, 200 m walk) | Running | 10 | ISOK Knee Extension (60°/s) ↔︎, ISOK Knee Flexion (60°/s) ↔︎, ISOK Knee Extension (300°/s) ↑, ISOK Knee Flexion (300°/s) ↑ |
| Song 2023 [96] | 10 | Trained, Younger Adults | SIT | 3 × (7–10 × 15 s at max effort, 15 s rest) | Running | 6 | Leg Press 1-RM ↔︎ |
| Theofilidis 2021 [73] | 7 | Recreational, Adults | SIT(Up) | 10 × (30 s at 90% MAS +10% grade, 60 s rest) | Running | 8 | ISOM Knee Extension (65°) ↔︎, ISOM Knee Flexion (30°) ↔︎, ISOK Knee Extension (60°/s) ↔︎ |
| Theofilidis 2021 [73] | 7 | Recreational, Adults | SIT (Down) | 10 × (30 s at 90% MAS −10% grade, 60 s rest) | Running | 8 | ISOM Knee Extension (65°) ↔︎, ISOM Knee Flexion (30°) ↔︎, ISOK Knee Extension (60°/s) ↔︎ |
| Venegas-Carro 2023 [97] | 15 | Recreational, Younger Adults | SIT | 4–8 × (20–30 s max effort, 10–40 s rest) | Running | 6 | ISOM Knee Extension (90°) ↔︎, ISOM PF ↔︎ |
| Wong 2024 [98] | 11 | Recreational, Younger Adults | SIT | 1 × (30 s at max effort) | Cycling | 6 | ISOK Knee Extension (30°/s) ↔︎, ISOK Knee Flexion (30°/s) ↔︎, ISOK Knee Extension (300°/s) ↔︎, ISOK Knee Flexion (300°/s) ↔︎, |
| Zhang 2024 [99] | 14 | Sedentary, Younger Adults | HIIT | 2–3 × (12–15 × 30 s at max effort, 10 s rest) | Cycling | 8 | Grip Strength ↔︎ |
| Author | Sample Size | Training Status/Age | Group Name | Protocol | Modality | Duration (Weeks) | ME Outcome |
|---|---|---|---|---|---|---|---|
| Bagley 2016 [77] | 15 | Recreational, Adults | SIT (Female) | 4 × (20 s at 175–200% VO2peak, 2 min rest) | Cycling | 12 | Fatigue index—60 reps at max intensity (Knee Extension ISOK 120°/s) ↑ |
| Bagley 2016 [77] | 16 | Recreational, Adults | SIT (Male) | 4 × (20 s at 175–200% VO2peak, 2 min rest) | Cycling | 12 | Fatigue index—60 reps at max intensity (Knee Extension ISOK 120°/s) ↑ |
| Bornath and Kenno 2022 [86] | 15 | Recreational, Younger Adults | SIT (Female) | 10 × (30 s at max effort, 60 s rest) | Battle Ropes | 6 | Sit-ups (reps completed) ↑, Push-ups (reps completed) ↑ |
| Bornath and Kenno 2022 [86] | 18 | Recreational, Younger Adults | SIT (Male) | 10 × (30 s at max effort, 60 s rest) | Battle Ropes | 6 | Sit-ups (reps completed) ↑, Push-ups (reps completed) ↑ |
| Buckley 2015 [87] | 15 | Recreational, Younger Adults | HIIT | 6 × (1 min at RPE 9–10/10, 3 min rest) | Rowing | 6 | 70% pre-training squat 1-RM (reps completed) ↔︎ |
| Cao 2024 [88] | 12 | Sedentary, Younger Adults | SIT | 4 × (4 × 30 s at 100–120% MAS, 30 s rest at 50% MAS) | Running | 12 | Push-ups (reps completed) ↑ |
| Theofilidis 2021 [73] | 7 | Recreational, Adults | HIIT (Up) | 10 × (30 s at 90% MAS +10% grade, 60 s rest) | Running | 8 | Work till >50% MVC not maintained (Knee Extension ISOK 60°/s) ↑ |
| Theofilidis 2021 [73] | 7 | Recreational, Adults | HIIT (Down) | 10 × (30 s at 90% MAS −10% grade, 60 s rest) | Running | 8 | Work till >50% MVC not maintained (Knee Extension ISOK 60°/s) ↑ |
| Outcome | Weighted ES | 95% CI | Weighted %∆ |
|---|---|---|---|
| FFM (N = 463) | 0.06 | −0.03, 0.15 | 1.17 ± 1.64% |
| LLM (N = 159) | 0.04 | 0.02, 0.07 | 0.61 ± 2.36% |
| Quadriceps CSA (N = 71) | 0.36 | 0.34, 0.37 | 4.72 ± 1.35% |
| Outcome | Weighted ES | ES 95% CI | Weighted %∆ |
|---|---|---|---|
| Leg Press 1-RM (N = 41) | 0.16 | 0.13, 0.19 | 3.45 ± 2.19% |
| ISOK 60 (N = 163) | 0.01 | −0.02, 0.04 | 0.35 ± 4.88% |
| ISOM 90 (N = 108) | 0.19 | 0.15, 0.22 | 4.94 ± 5.82% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiens, L.; Losciale, J.M.; Fliss, M.D.; Abercrombie, M.J.; Darabi, D.; Li, J.; Barclay, R.; Mitchell, C.J. Does High-Intensity Interval Training Increase Muscle Strength, Muscle Mass, and Muscle Endurance? A Systematic Review and Meta-Analysis. Sports 2025, 13, 293. https://doi.org/10.3390/sports13090293
Wiens L, Losciale JM, Fliss MD, Abercrombie MJ, Darabi D, Li J, Barclay R, Mitchell CJ. Does High-Intensity Interval Training Increase Muscle Strength, Muscle Mass, and Muscle Endurance? A Systematic Review and Meta-Analysis. Sports. 2025; 13(9):293. https://doi.org/10.3390/sports13090293
Chicago/Turabian StyleWiens, Lucas, Justin M. Losciale, Matthew D. Fliss, Max J. Abercrombie, Darius Darabi, Jedd Li, Rowan Barclay, and Cameron J. Mitchell. 2025. "Does High-Intensity Interval Training Increase Muscle Strength, Muscle Mass, and Muscle Endurance? A Systematic Review and Meta-Analysis" Sports 13, no. 9: 293. https://doi.org/10.3390/sports13090293
APA StyleWiens, L., Losciale, J. M., Fliss, M. D., Abercrombie, M. J., Darabi, D., Li, J., Barclay, R., & Mitchell, C. J. (2025). Does High-Intensity Interval Training Increase Muscle Strength, Muscle Mass, and Muscle Endurance? A Systematic Review and Meta-Analysis. Sports, 13(9), 293. https://doi.org/10.3390/sports13090293







