The Women’s Tennis Association (WTA) Multidisciplinary Education and Treatment Protocol for the Female Athlete Triad (1996–2022)
Abstract
1. Introduction
1.1. History of the Female Athlete Triad
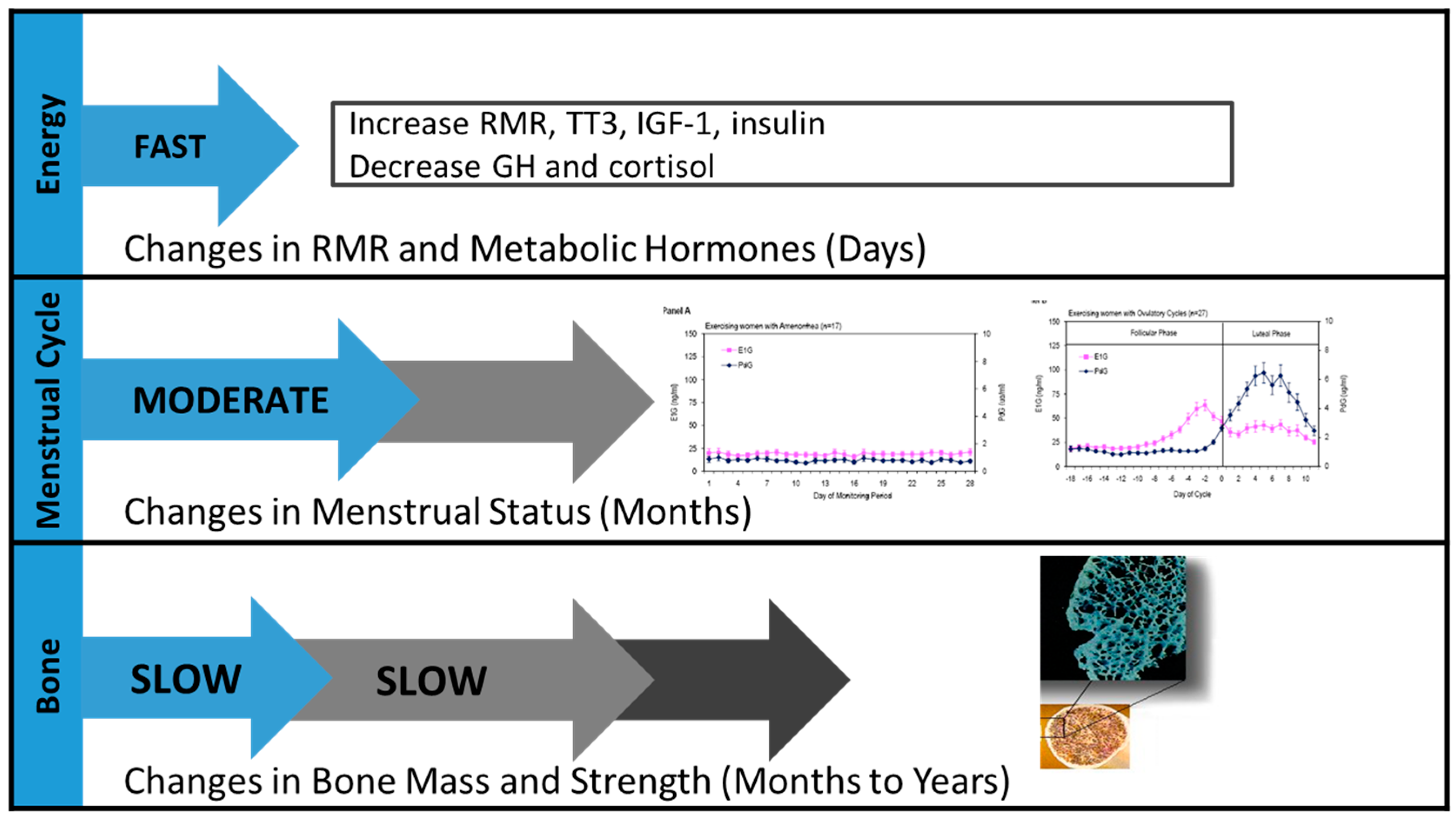
1.2. The Basic Physiology Underlying the Triad
1.3. The Triad in Tennis Athletes
1.4. Development of a WTA Triad Protocol
2. Triad Prevention and Education
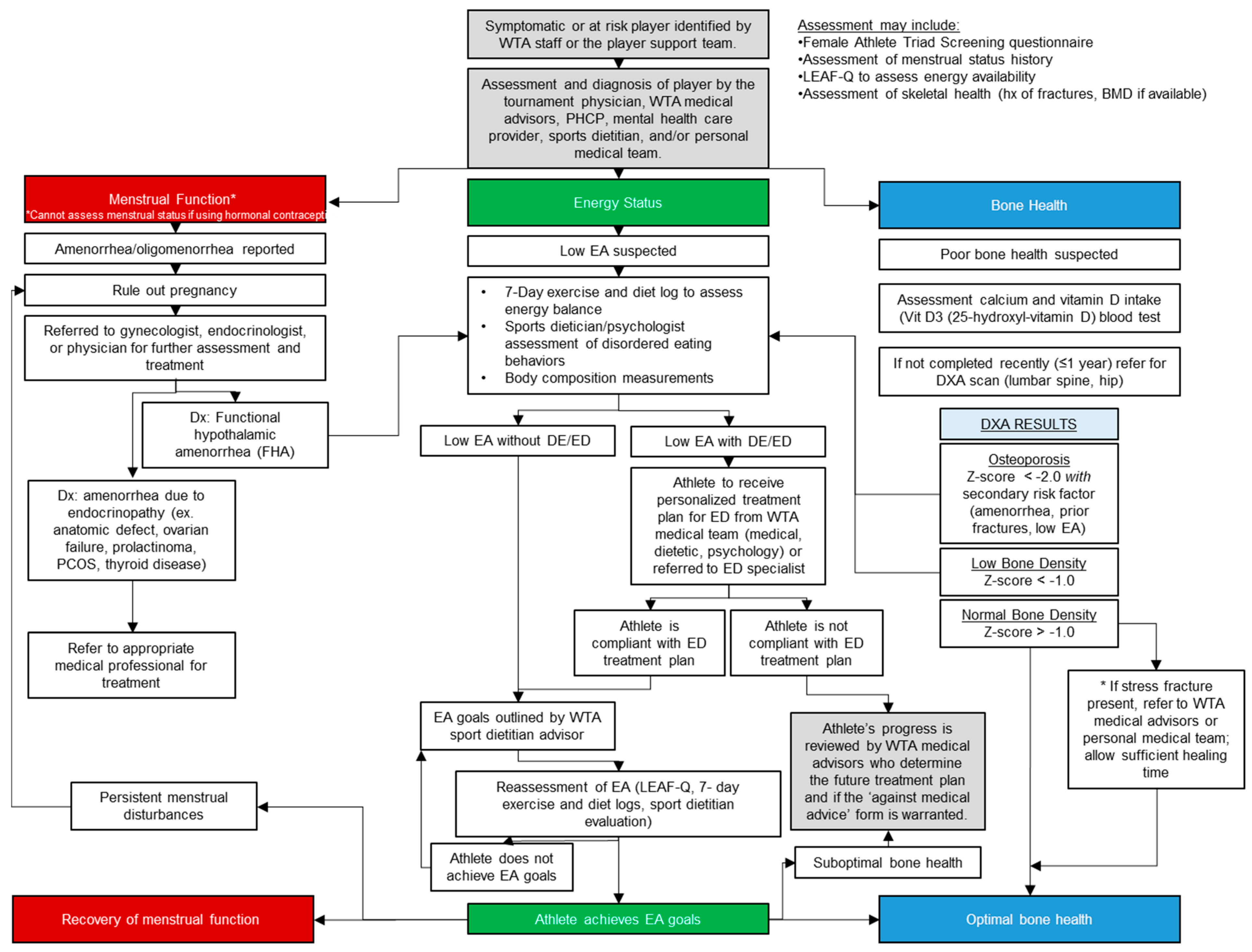
3. Screening and Diagnosis of the Triad in Tennis Athletes
3.1. Screening
3.2. Diagnosis
3.2.1. Energy Deficiency/Low Energy Availability
3.2.2. Menstrual Disturbances
3.2.3. Low Bone Mineral Density
4. Treatment and Management
- Regular periods can be an indicator (“barometer”) of good health and adequate nutrition.
- Estrogen and progesterone are important hormones that regulate the female reproductive system. These hormones also help build and maintain strong bones.
- Menstrual disorders may contribute to difficulty conceiving or maintaining a pregnancy, but you could still get pregnant even if you are not getting a period. If that athlete is sexually active, contraception is advised.
- Not having a period could mean the athlete has an underlying health condition that needs to be investigated.
- Consequences of long-term amenorrhea, like low BMD, may not be completely reversible; thus, early diagnosis and intervention are critical.
5. Future Directions and Protocol Modifications
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclaimer
References
- De Souza, M.J.; Nattiv, A.; Joy, E.; Misra, M.; Williams, N.I.; Mallinson, R.J.; Gibbs, J.C.; Olmsted, M.; Goolsby, M.; Matheson, G.; et al. 2014 female athlete triad coalition consensus statement on treatment and return to play of the female athlete triad: 1st international conference held in San Francisco, California, May 2012 and 2nd international conference held in Indianapolis, Indiana, May 2013. Br. J. Sports Med. 2014, 48, 289. [Google Scholar] [CrossRef]
- Nattiv, A.; Loucks, A.B.; Manore, M.M.; Sanborn, C.F.; Sundgot-Borgen, J.; Warren, M.P. American college of sports medicine position stand. The female athlete triad. Med. Sci. Sports Exerc. 2007, 39, 1867–1882. [Google Scholar] [CrossRef] [PubMed]
- Yeager, K.K.; Agostini, R.; Nattiv, A.; Drinkwater, B. The female athlete triad: Disordered eating, amenorrhea, osteoporosis. Med. Sci. Sports Exerc. 1993, 25, 775–777. [Google Scholar] [CrossRef]
- Otis, C.L.; Drinkwater, B.; Johnson, M.; Loucks, A.; Wilmore, J. American college of sports medicine position stand. The female athlete triad. Med. Sci. Sports Exerc. 1997, 29, i–ix. [Google Scholar] [CrossRef] [PubMed]
- Loucks, A.B.; Verdun, M.; Heath, E.M. Low energy availability, not stress of exercise, alters lh pulsatility in exercising women. J. Appl. Physiol. 1998, 84, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Williams, N.I.; Helmreich, D.L.; Parfitt, D.B.; Caston-Balderrama, A.; Cameron, J.L. Evidence for a causal role of low energy availability in the induction of menstrual cycle disturbances during strenuous exercise training. J. Clin. Endocrinol. Metab. 2001, 86, 5184–5193. [Google Scholar] [CrossRef]
- Bullen, B.A.; Skrinar, G.S.; Beitins, I.Z.; von Mering, G.; Turnbull, B.A.; McArthur, J.W. Induction of menstrual disorders by strenuous exercise in untrained women. N. Engl. J. Med. 1985, 312, 1349–1353. [Google Scholar] [CrossRef]
- Williams, N.I.; Caston-Balderrama, A.L.; Helmreich, D.L.; Parfitt, D.B.; Nosbisch, C.; Cameron, J.L. Longitudinal changes in reproductive hormones and menstrual cyclicity in cynomolgus monkeys during strenuous exercise training: Abrupt transition to exercise-induced amenorrhea. Endocrinology 2001, 142, 2381–2389. [Google Scholar] [CrossRef]
- Williams, N.I.; Berga, S.L.; Cameron, J.L. Synergism between psychosocial and metabolic stressors: Impact on reproductive function in cynomolgus monkeys. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E270–E276. [Google Scholar] [CrossRef]
- Gibbs, J.C.; Williams, N.I.; De Souza, M.J. Prevalence of individual and combined components of the female athlete triad. Med. Sci. Sports Exerc. 2013, 45, 985–996. [Google Scholar] [CrossRef]
- Sundgot-Borgen, J.; Torstveit, M.K. Prevalence of eating disorders in elite athletes is higher than in the general population. Clin. J. Sport Med. 2004, 14, 25–32. [Google Scholar] [CrossRef]
- Torstveit, M.K.; Sundgot-Borgen, J. Participation in leanness sports but not training volume is associated with menstrual dysfunction: A national survey of 1276 elite athletes and controls. Br. J. Sports Med. 2005, 39, 141–147. [Google Scholar] [CrossRef]
- Sophia, B.; Kelly, P.; Ogan, D.; Larson, A. Self reported history of eating disorders, training, weight control methods, and body satisfaction in elite female runners competing at the 2020 U.S. Olympic marathon trials. Int. J. Exerc. Sci. 2022, 15, 721–732. [Google Scholar] [PubMed]
- Martinsen, M.; Sundgot-Borgen, J. Higher prevalence of eating disorders among adolescent elite athletes than controls. Med. Sci. Sports Exerc. 2013, 45, 1188–1197. [Google Scholar] [CrossRef]
- Mountjoy, M.; Sundgot-Borgen, J.; Burke, L.; Carter, S.; Constantini, N.; Lebrun, C.; Meyer, N.; Sherman, R.; Steffen, K.; Budgett, R.; et al. The IOC consensus statement: Beyond the female athlete triad—Relative energy deficiency in sport (RED-S). Br. J. Sports Med. 2014, 48, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Mountjoy, M.; Sundgot-Borgen, J.; Burke, L.; Ackerman, K.E.; Blauwet, C.; Constantini, N.; Lebrun, C.; Lundy, B.; Melin, A.; Meyer, N.; et al. International olympic committee (IOC) consensus statement on relative energy deficiency in sport (RED-S): 2018 update. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 316–331. [Google Scholar] [CrossRef]
- De Souza, M.J.; Williams, N.I.; Nattiv, A.; Joy, E.; Misra, M.; Loucks, A.B.; Matheson, G.; Olmsted, M.P.; Barrack, M.; Mallinson, R.J.; et al. Misunderstanding the female athlete triad: Refuting the ioc consensus statement on relative energy deficiency in sport (RED-S). Br. J. Sports Med. 2014, 48, 1461–1465. [Google Scholar] [CrossRef] [PubMed]
- Williams, N.I.; Koltun, K.J.; Strock, N.C.A.; De Souza, M.J. Perspectives for progress—Female athlete triad and relative energy deficiency in sport: A focus on scientific rigor. Exerc. Sport Sci. Rev. 2019, 47, 197–205. [Google Scholar] [CrossRef]
- Jeukendrup, A.E.; Areta, J.L.; Van Genechten, L.; Langan-Evans, C.; Pedlar, C.R.; Rodas, G.; Sale, C.; Walsh, N.P. Does relative energy deficiency in sport (REDs) syndrome exist? Sports Med. 2024, 54, 2793–2816. [Google Scholar] [CrossRef]
- Wade, G.N.; Schneider, J.E. Metabolic fuels and reproduction in female mammals. Neurosci. Biobehav. Rev. 1992, 16, 235–272. [Google Scholar] [CrossRef]
- Committee on Adolescent Health Care. Committee opinion No. 651 summary: Menstruation in girls and adolescents: Using the menstrual cycle as a vital sign. Obstet. Gynecol. 2015, 126, 1328. [Google Scholar] [CrossRef] [PubMed]
- De Souza, M.J.; Miller, B.E.; Loucks, A.B.; Luciano, A.A.; Pescatello, L.S.; Campbell, C.G.; Lasley, B.L. High frequency of luteal phase deficiency and anovulation in recreational women runners: Blunted elevation in follicle-stimulating hormone observed during luteal-follicular transition. J. Clin. Endocrinol. Metab. 1998, 83, 4220–4232. [Google Scholar] [CrossRef] [PubMed]
- Koltun, K.J.; De Souza, M.J.; Scheid, J.L.; Williams, N.I. Energy availability is associated with luteinizing hormone pulse frequency and induction of luteal phase defects. J. Clin. Endocrinol. Metab. 2020, 105, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Beitins, I.Z.; McArthur, J.W.; Turnbull, B.A.; Skrinar, G.S.; Bullen, B.A. Exercise induces two types of human luteal dysfunction: Confirmation by urinary free progesterone. J. Clin. Endocrinol. Metab. 1991, 72, 1350–1358. [Google Scholar] [CrossRef]
- De Souza, M.J.; Lee, D.K.; VanHeest, J.L.; Scheid, J.L.; West, S.L.; Williams, N.I. Severity of energy-related menstrual disturbances increases in proportion to indices of energy conservation in exercising women. Fertil. Steril. 2007, 88, 971–975. [Google Scholar] [CrossRef]
- Ihle, R.; Loucks, A.B. Dose-response relationships between energy availability and bone turnover in young exercising women. J. Bone Miner. Res. 2004, 19, 1231–1240. [Google Scholar] [CrossRef]
- Southmayd, E.A.; Williams, N.I.; Mallinson, R.J.; De Souza, M.J. Energy deficiency suppresses bone turnover in exercising women with menstrual disturbances. J. Clin. Endocrinol. Metab. 2019, 104, 3131–3145. [Google Scholar] [CrossRef]
- Papageorgiou, M.; Elliott-Sale, K.J.; Parsons, A.; Tang, J.C.Y.; Greeves, J.P.; Fraser, W.D.; Sale, C. Effects of reduced energy availability on bone metabolism in women and men. Bone 2017, 105, 191–199. [Google Scholar] [CrossRef]
- Papageorgiou, M.; Martin, D.; Colgan, H.; Cooper, S.; Greeves, J.P.; Tang, J.C.Y.; Fraser, W.D.; Elliott-Sale, K.J.; Sale, C. Bone metabolic responses to low energy availability achieved by diet or exercise in active eumenorrheic women. Bone 2018, 114, 181–188. [Google Scholar] [CrossRef]
- Weitzmann, M.N.; Pacifici, R. Estrogen deficiency and bone loss: An inflammatory tale. J. Clin. Investig. 2006, 116, 1186–1194. [Google Scholar] [CrossRef]
- De Souza, M.J.; West, S.L.; Jamal, S.A.; Hawker, G.A.; Gundberg, C.M.; Williams, N.I. The presence of both an energy deficiency and estrogen deficiency exacerbate alterations of bone metabolism in exercising women. Bone 2008, 43, 140–148. [Google Scholar] [CrossRef]
- Southmayd, E.A.; Mallinson, R.J.; Williams, N.I.; Mallinson, D.J.; De Souza, M.J. Unique effects of energy versus estrogen deficiency on multiple components of bone strength in exercising women. Osteoporos. Int. 2017, 28, 1365–1376. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.B. Weight concern, body image, and abnormal eating in college women tennis players and their coaches. Int. J. Sport. Nutr. Exerc. Metab. 2000, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.; Foltz, S. Attitudes toward weight and eating in young women tennis players, their parents, and their coaches. Eat. Disord. 1999, 7, 191–205. [Google Scholar] [CrossRef]
- Cignarelli, J. The skinny on athletic stardom: Disordered eating and body dissatisfaction in elite female tennis players. J. Med. Sci. Tennis 2019, 24, 6–10. [Google Scholar]
- Coelho, G.M.; de Farias, M.L.; de Mendonca, L.M.; de Mello, D.B.; Lanzillotti, H.S.; Ribeiro, B.G.; Soares Ede, A. The prevalence of disordered eating and possible health consequences in adolescent female tennis players from Rio de Janeiro, Brazil. Appetite 2013, 64, 39–47. [Google Scholar] [CrossRef]
- Filaire, E.; Massart, A.; Hua, J.; Le Scanff, C. Dietary intake, eating behaviors, and diurnal patterns of salivary cortisol and alpha-amylase secretion among professional young adult female tennis players. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 233–242. [Google Scholar] [CrossRef]
- Barrack, M.T.; Gibbs, J.C.; De Souza, M.J.; Williams, N.I.; Nichols, J.F.; Rauh, M.J.; Nattiv, A. Higher incidence of bone stress injuries with increasing female athlete triad-related risk factors: A prospective multisite study of exercising girls and women. Am. J. Sports Med. 2014, 42, 949–958. [Google Scholar] [CrossRef]
- Vanheest, J.L.; Rodgers, C.D.; Mahoney, C.E.; De Souza, M.J. Ovarian suppression impairs sport performance in junior elite female swimmers. Med. Sci. Sports Exerc. 2014, 46, 156–166. [Google Scholar] [CrossRef]
- Otis, C.L. A Review of the Age Eligibility Commission Report; Fall Edition; USTA Sport Science Newsletter: Orlando, FL, USA, 1994; pp. 1–3. [Google Scholar]
- Marx, R.G.; Saint-Phard, D.; Callahan, L.R.; Chu, J.; Hannafin, J.A. Stress fracture sites related to underlying bone health in athletic females. Clin. J. Sport Med. 2001, 11, 73–76. [Google Scholar] [CrossRef]
- International Society for Clinical Densitometry (ISCD). 2023 ISCD Official Positions—Adults. Available online: https://iscd.org/wp-content/uploads/2024/03/2023-ISCD-Adult-Positions.pdf (accessed on 29 January 2025).
- Gordon, C.M.; Ackerman, K.E.; Berga, S.L.; Kaplan, J.R.; Mastorakos, G.; Misra, M.; Murad, M.H.; Santoro, N.F.; Warren, M.P. Functional hypothalamic amenorrhea: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2017, 102, 1413–1439. [Google Scholar] [CrossRef]
- Klein, D.A.; Paradise, S.L.; Reeder, R.M. Amenorrhea: A systematic approach to diagnosis and management. Am. Fam. Physician 2019, 100, 39–48. [Google Scholar] [PubMed]
- Stoyel, H.; Delderfield, R.; Shanmuganathan-Felton, V.; Stoyel, A.; Serpell, L. A qualitative exploration of sport and social pressures on elite athletes in relation to disordered eating. Front. Psychol. 2021, 12, 633490. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.; Page, L. Managing athletes with eating issues: The WTA tour approach. J. Med. Sci. Tennis 2010, 15, 6–11. [Google Scholar]
- Taylor, W.C. The preparticipation physical: The WTA experience and findings. In Tennis Medicine; Di Giacomo, G., Ellenbecker, T., Kibler, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Wells, K.R.; Jeacocke, N.A.; Appaneal, R.; Smith, H.D.; Vlahovich, N.; Burke, L.M.; Hughes, D. The Australian institute of sport (AIS) and national eating disorders collaboration (NEDC) position statement on disordered eating in high performance sport. Br. J. Sports Med. 2020, 54, 1247–1258. [Google Scholar] [CrossRef]
- A Healthy Innagural Season for the Hologic-WTA Partnership. Available online: https://www.wtatennis.com/news/2983829/a-healthy-inaugural-season-for-the-hologic-wta-partnership (accessed on 14 June 2025).
- Myerson, M.; Gutin, B.; Warren, M.P.; May, M.T.; Contento, I.; Lee, M.; Pi-Sunyer, F.X.; Pierson, R.N., Jr.; Brooks-Gunn, J. Resting metabolic rate and energy balance in amenorrheic and eumenorrheic runners. Med. Sci. Sports Exerc. 1991, 23, 15–22. [Google Scholar] [CrossRef]
- Strock, N.C.A.; Koltun, K.J.; Southmayd, E.A.; Williams, N.I.; De Souza, M.J. Indices of resting metabolic rate accurately reflect energy deficiency in exercising women. Int. J. Sport Nutr. Exerc. Metab. 2020, 30, 14–24. [Google Scholar] [CrossRef]
- Loucks, A.B.; Heath, E.M. Induction of low-T3 syndrome in exercising women occurs at a threshold of energy availability. Am. J. Physiol. 1994, 266, R817–R823. [Google Scholar] [CrossRef]
- Koehler, K.; De Souza, M.J.; Williams, N.I. Less-than-expected weight loss in normal-weight women undergoing caloric restriction and exercise is accompanied by preservation of fat-free mass and metabolic adaptations. Eur. J. Clin. Nutr. 2017, 71, 365–371. [Google Scholar] [CrossRef]
- Burke, L.M.; Lundy, B.; Fahrenholtz, I.L.; Melin, A.K. Pitfalls of conducting and interpreting estimates of energy availability in free-living athletes. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 350–363. [Google Scholar] [CrossRef]
- De Souza, M.J.; Koltun, K.J.; Strock, N.C.A.; Williams, N.I. Rethinking the concept of an energy availability threshold and its role in the female athlete triad. Curr. Opin. Physiol. 2019, 10, 35–42. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R., Jr.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 compendium of physical activities: A second update of codes and met values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Whitt, M.C.; Irwin, M.L.; Swartz, A.M.; Strath, S.J.; O’Brien, W.L.; Bassett, D.R., Jr.; Schmitz, K.H.; Emplaincourt, P.O.; et al. Compendium of physical activities: An update of activity codes and met intensities. Med. Sci. Sports Exerc. 2000, 32, S498–S504. [Google Scholar] [CrossRef] [PubMed]
- Rose, G.L.; Farley, M.J.; Ward, L.C.; Slater, G.J.; Skinner, T.L.; Keating, S.E.; Schaumberg, M.A. Accuracy of body composition measurement techniques across the age span. Appl. Physiol. Nutr. Metab. 2022, 47, 482–494. [Google Scholar] [CrossRef] [PubMed]
- The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [Google Scholar] [CrossRef]
- Bergstrom, I.; Crisby, M.; Engstrom, A.M.; Holcke, M.; Fored, M.; Jakobsson Kruse, P.; Of Sandberg, A.M. Women with anorexia nervosa should not be treated with estrogen or birth control pills in a bone-sparing effect. Acta Obstet. Gynecol. Scand. 2013, 92, 877–880. [Google Scholar] [CrossRef]
- Yakar, S.; Rosen, C.J.; Beamer, W.G.; Ackert-Bicknell, C.L.; Wu, Y.; Liu, J.L.; Ooi, G.T.; Setser, J.; Frystyk, J.; Boisclair, Y.R.; et al. Circulating levels of IGF-1 directly regulate bone growth and density. J. Clin. Investig. 2002, 110, 771–781. [Google Scholar] [CrossRef]
- Ackerman, K.E.; Singhal, V.; Baskaran, C.; Slattery, M.; Campoverde Reyes, K.J.; Toth, A.; Eddy, K.T.; Bouxsein, M.L.; Lee, H.; Klibanski, A.; et al. Oestrogen replacement improves bone mineral density in oligo-amenorrhoeic athletes: A randomised clinical trial. Br. J. Sports Med. 2019, 53, 229–236. [Google Scholar] [CrossRef]
- Ackerman, K.E.; Singhal, V.; Slattery, M.; Eddy, K.T.; Bouxsein, M.L.; Lee, H.; Klibanski, A.; Misra, M. Effects of estrogen replacement on bone geometry and microarchitecture in adolescent and young adult oligoamenorrheic athletes: A randomized trial. J. Bone Miner. Res. 2020, 35, 248–260. [Google Scholar] [CrossRef]
- International Society for Clinical Densitometry (ISCD). 2019 ISCD Official Positions—Adult; International Society for Clinical Densitometry (ISCD): Middletown, CT, USA, 2019. [Google Scholar]
- International Society for Clinical Densitomtry (ISCD). 2019 ISCD Official Positions-Pediatrics. Available online: https://iscd.org/wp-content/uploads/2024/03/2019-ISCD-Pediatric-Postions.pdf (accessed on 29 January 2025).
- Goel, H.; Binkley, N.; Boggild, M.; Chan, W.P.; Leslie, W.D.; McCloskey, E.; Morgan, S.L.; Silva, B.C.; Cheung, A.M. Clinical use of trabecular bone score: The 2023 ISCD official positions. J. Clin. Densitom. 2024, 27, 101452. [Google Scholar] [CrossRef]
- Goel, H.; Binkley, N.; Hans, D.; Leslie, W.D. Bone density and trabecular bone score to predict fractures in adults aged 20–39 years: A registry-based study. Osteoporos. Int. 2023, 34, 1085–1091. [Google Scholar] [CrossRef]
- Tenforde, A.S.; Carlson, J.L.; Sainani, K.L.; Chang, A.O.; Kim, J.H.; Diaz, R.; Golden, N.H.; Fredericson, M. Lower trabecular bone score and spine bone mineral density are associated with bone stress injuries and triad risk factors in collegiate athletes. PM&R 2021, 13, 945–953. [Google Scholar] [CrossRef]
- De Souza, M.J.; Mallinson, R.J.; Strock, N.C.A.; Koltun, K.J.; Olmstead, M.; Ricker, E.A.; Scheid, J.L.; Allaway, H.C.; Mallinson, D.J.; Kuruppumullage Don, P.; et al. Randomized controlled trial of the effects of increased energy intake on menstrual recovery in exercising women with menstrual disturbances: The “refuel” study. Hum. Reprod. 2021, 36, 2285–2297. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.C.; Cheung, M.Y.; Barrack, M.T.; Nattiv, A. Restoration of menses with nonpharmacologic therapy in college athletes with menstrual disturbances: A 5-year retrospective study. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Drinkwater, B.L.; Nilson, K.; Ott, S.; Chesnut, C.H., 3rd. Bone mineral density after resumption of menses in amenorrheic athletes. JAMA 1986, 256, 380–382. [Google Scholar] [CrossRef]
- De Souza, M.J.; Ricker, E.A.; Mallinson, R.J.; Allaway, H.C.M.; Koltun, K.J.; Strock, N.C.A.; Gibbs, J.C.; Kuruppumullage Don, P.; Williams, N.I. Bone mineral density in response to increased energy intake in exercising women with oligomenorrhea/amenorrhea: The refuel randomized controlled trial. Am. J. Clin. Nutr. 2022, 115, 1457–1472. [Google Scholar] [CrossRef]
- Cialdella-Kam, L.; Guebels, C.P.; Maddalozzo, G.F.; Manore, M.M. Dietary intervention restored menses in female athletes with exercise-associated menstrual dysfunction with limited impact on bone and muscle health. Nutrients 2014, 6, 3018–3039. [Google Scholar] [CrossRef]
- Ravelli, M.N.; Schoeller, D.A. Traditional self-reported dietary instruments are prone to inaccuracies and new approaches are needed. Front. Nutr. 2020, 7, 90. [Google Scholar] [CrossRef]
- Hills, A.P.; Mokhtar, N.; Byrne, N.M. Assessment of physical activity and energy expenditure: An overview of objective measures. Front. Nutr. 2014, 1, 5. [Google Scholar] [CrossRef]
- Mallinson, R.J.; Williams, N.I.; Ricker, E.A.; Allaway, H.C.M.; De Souza, M.J. Multiple eumenorrheic cycles are necessary to observe a significant increase in estrogen exposure and ovulation in exercising females with functional hypothalamic oligo/amenorrhea: Insights from the REFUEL Study. PM&R 2024. submitted. [Google Scholar]
- Tenforde, A.S.; Carlson, J.L.; Chang, A.; Sainani, K.L.; Shultz, R.; Kim, J.H.; Cutti, P.; Golden, N.H.; Fredericson, M. Association of the female athlete triad risk assessment stratification to the development of bone stress injuries in collegiate athletes. Am. J. Sports Med. 2017, 45, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Tenforde, A.S.; Katz, N.B.; Sainani, K.L.; Carlson, J.L.; Golden, N.H.; Fredericson, M. Female athlete triad risk factors are more strongly associated with trabecular-rich versus cortical-rich bone stress injuries in collegiate athletes. Orthop. J. Sports Med. 2022, 10, 23259671221123588. [Google Scholar] [CrossRef] [PubMed]
- Tenforde, A.S.; Parziale, A.L.; Popp, K.L.; Ackerman, K.E. Low bone mineral density in male athletes is associated with bone stress injuries at anatomic sites with greater trabecular composition. Am. J. Sports Med. 2018, 46, 30–36. [Google Scholar] [CrossRef]
- Nattiv, A.; Kennedy, G.; Barrack, M.T.; Abdelkerim, A.; Goolsby, M.A.; Arends, J.C.; Seeger, L.L. Correlation of mri grading of bone stress injuries with clinical risk factors and return to play: A 5-year prospective study in collegiate track and field athletes. Am. J. Sports Med. 2013, 41, 1930–1941. [Google Scholar] [CrossRef]
- Ackerman, K.E.; Putman, M.; Guereca, G.; Taylor, A.P.; Pierce, L.; Herzog, D.B.; Klibanski, A.; Bouxsein, M.; Misra, M. Cortical microstructure and estimated bone strength in young amenorrheic athletes, eumenorrheic athletes and non-athletes. Bone 2012, 51, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, K.E.; Nazem, T.; Chapko, D.; Russell, M.; Mendes, N.; Taylor, A.P.; Bouxsein, M.L.; Misra, M. Bone microarchitecture is impaired in adolescent amenorrheic athletes compared with eumenorrheic athletes and nonathletic controls. J. Clin. Endocrinol. Metab. 2011, 96, 3123–3133. [Google Scholar] [CrossRef]
- Female athlete issues for the team physician: A consensus statement-2017 update. Med. Sci. Sports Exerc. 2018, 50, 1113–1122. [CrossRef]
- Herring, S.A.; Kibler, W.B.; Putukian, M. The team physician and the return-to-play decision: A consensus statement-2012 update. Med. Sci. Sports Exerc. 2012, 44, 2446–2448. [Google Scholar] [CrossRef]
- National Athletic Trainers’ Association. Appropriate Medical Care Standards for Secondary School-Aged Athletes. 2019. Available online: https://www.nata.org/sites/default/files/nata_appropriate_medical_care_standards.pdf (accessed on 29 January 2025).
- Association of Summer Olympic International Federations. Health Care Guidelines for International Federation Events. 2020. Available online: https://www.asoif.com/sites/default/files/documents/main/health_care_guidelines_for_international_federation_events.pdf (accessed on 29 January 2025).
| Low Energy Availability | Menstrual Disturbances | Low Bone Mineral Density |
|---|---|---|
| Fatigue | Long and irregular menstrual cycles of 36–90 days, defined as oligomenorrhea | Family history of osteoporosis |
| Irritability and mood changes | Complete absence of menstrual cycles, defined as amenorrhea | Stress fracture and/or other secondary clinical risk factors for fracture, previous fractures [41] |
| Difficulty handling travel or time zone changes | Difficulty conceiving or maintaining pregnancy | Nutrition: low calcium intake and/or low vitamin D status, restrictive calories or recent weight loss, restrictive diets (veganism, vegetarianism, etc.), history of eating disorders |
| Poor recovery after training | Initiation of hormonal contraceptive use in response to irregular/absent menstrual cycles | Medication: corticosteroid medications for 3+ months [42] |
| Difficulty healing from minor injuries | Primary amenorrhea (no menses by age 15 years) [21,43,44] | Hormonal deficiencies: amenorrhea (primary and secondary), low testosterone, hypoestrogenism (estrogen is important in building and maintaining bone mass) |
| History of recent dieting or comments about needing to lose weight | Medical conditions that can reduce bone mass: lupus, cirrhosis, alcoholism, atherosclerosis, thyroid disease, hyperparathyroidism, celiac disease | |
| Restrictive eating patterns, food faddism | ||
| Preoccupation with weight | ||
| Unable to get into “high gear” during training or a match | ||
| Lack of energy or fading in long matches | ||
| Failing to improve performance despite ongoing training | ||
| Changes in menstrual cycle | ||
| Frequent illness |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricker, E.A.; Koltun, K.J.; Otis, C.L.; Peavler, A.S.; De Souza, M.J. The Women’s Tennis Association (WTA) Multidisciplinary Education and Treatment Protocol for the Female Athlete Triad (1996–2022). Sports 2025, 13, 205. https://doi.org/10.3390/sports13070205
Ricker EA, Koltun KJ, Otis CL, Peavler AS, De Souza MJ. The Women’s Tennis Association (WTA) Multidisciplinary Education and Treatment Protocol for the Female Athlete Triad (1996–2022). Sports. 2025; 13(7):205. https://doi.org/10.3390/sports13070205
Chicago/Turabian StyleRicker, Emily A., Kristen J. Koltun, Carol L. Otis, Anna S. Peavler, and Mary Jane De Souza. 2025. "The Women’s Tennis Association (WTA) Multidisciplinary Education and Treatment Protocol for the Female Athlete Triad (1996–2022)" Sports 13, no. 7: 205. https://doi.org/10.3390/sports13070205
APA StyleRicker, E. A., Koltun, K. J., Otis, C. L., Peavler, A. S., & De Souza, M. J. (2025). The Women’s Tennis Association (WTA) Multidisciplinary Education and Treatment Protocol for the Female Athlete Triad (1996–2022). Sports, 13(7), 205. https://doi.org/10.3390/sports13070205










