Evaluating the Effects of Exercise on Inflammation Markers in Musculoskeletal Pain: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Search Strategy
2.3. Inclusion Criteria
2.4. Data Extraction
2.5. Risk of Bias Assessment and Summary of Evidence
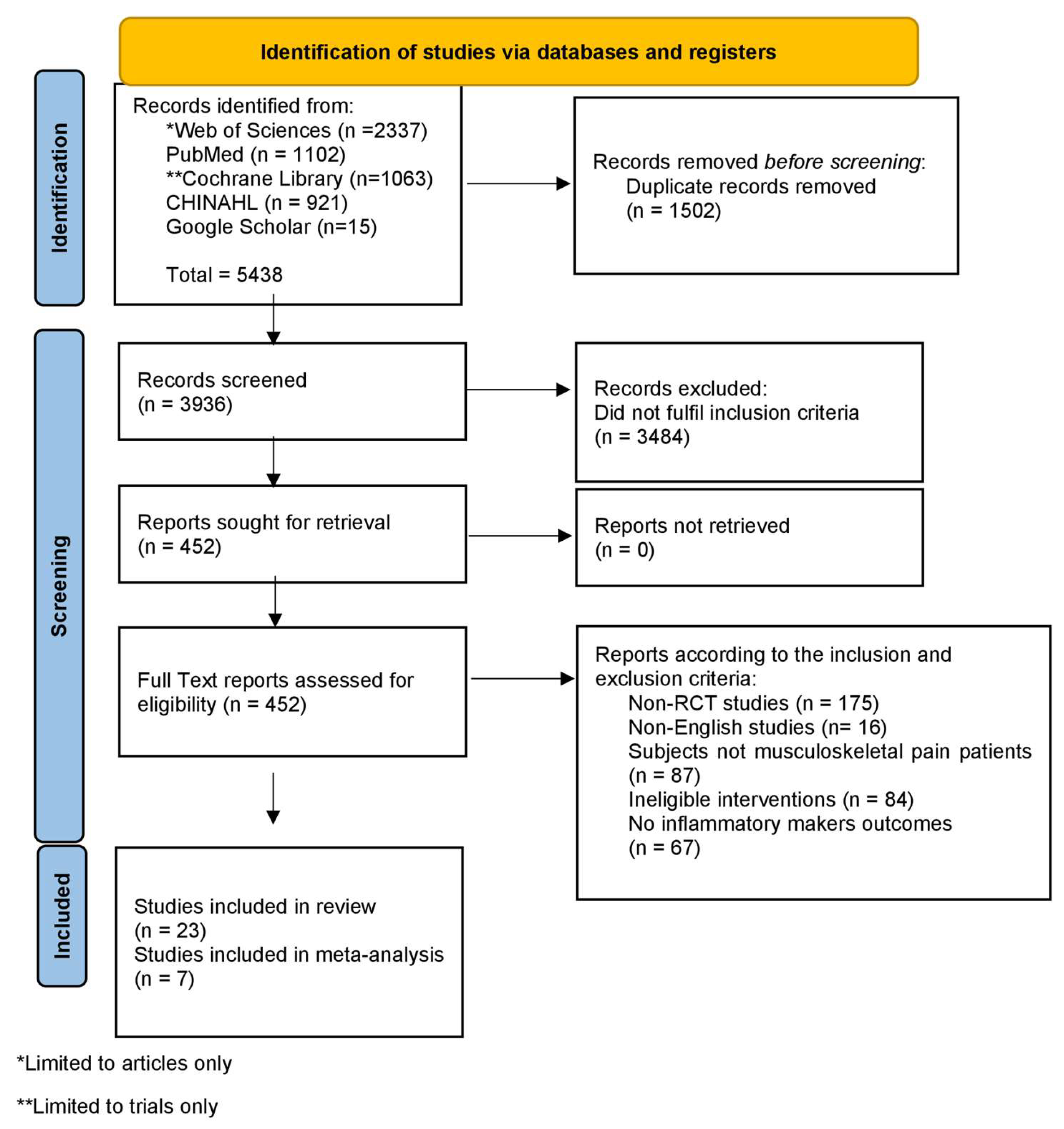
3. Results
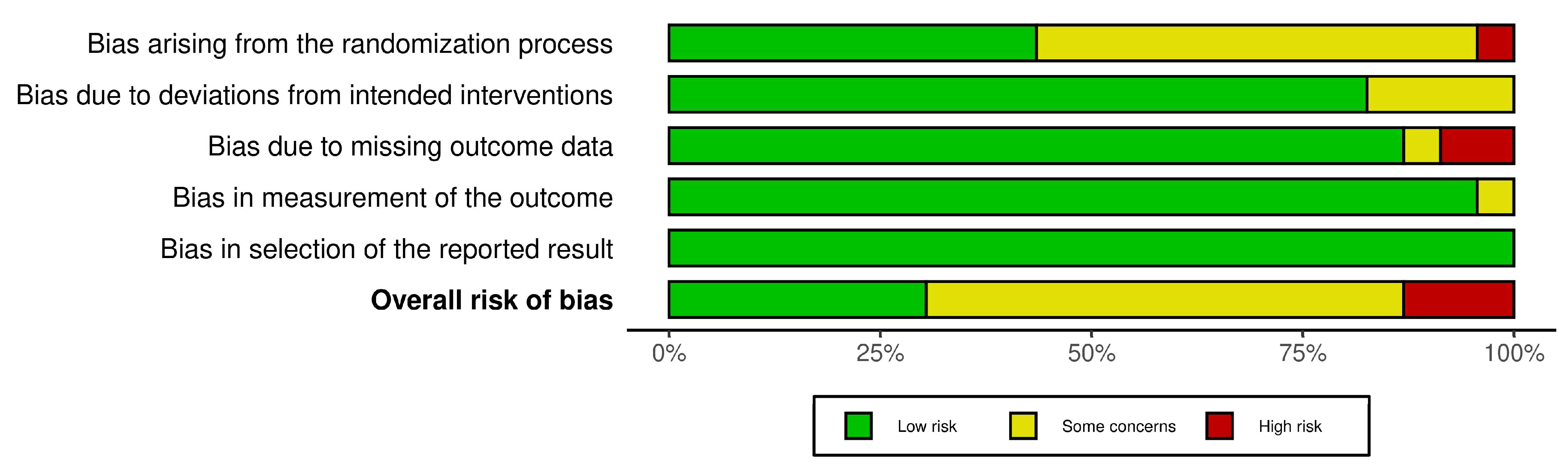
3.1. Risk of Bias Assessment
3.2. Meta-Analysis
3.3. GRADE Recommendations
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A. Search Strings and Databases
- Medline (via PubMed)
- CINAHL (via EBSCOhost)
- Web of Science (WOS)
- Cochrane Library
References
- Chen, X.; Coombes, B.K.; Sjøgaard, G.; Jun, D.; O’Leary, S.; Johnston, V. Workplace-Based Interventions for Neck Pain in Office Workers: Systematic Review and Meta-Analysis. Phys. Ther. 2018, 98, 40–62. [Google Scholar] [CrossRef]
- Moreira-Silva, I.; Teixeira, P.M.; Santos, R.; Abreu, S.; Moreira, C.; Mota, J. The Effects of Workplace Physical Activity Programs on Musculoskeletal Pain. Workplace Health Saf. 2016, 64, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, E.V.; Gomes, A.R.S.; Tanhoffer, A.I.P.; Leite, N. Effects of Exercise on Pain of Musculoskeletal Disorders: A Systematic Review. Acta Ortopédica Bras. 2014, 22, 334–338. [Google Scholar] [CrossRef]
- Tan, L.; Cicuttini, F.M.; Fairley, J.; Romero, L.; Estee, M.; Hussain, S.M.; Urquhart, D.M. Does Aerobic Exercise Effect Pain Sensitisation in Individuals with Musculoskeletal Pain? A Systematic Review. BMC Musculoskelet. Disord. 2022, 23, 113. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K. Anti-inflammatory Effects of Exercise: Role in Diabetes and Cardiovascular Disease. Eur. J. Clin. Investig. 2017, 47, 600–611. [Google Scholar] [CrossRef]
- da Luz Scheffer, D.; Latini, A. Exercise-Induced Immune System Response: Anti-Inflammatory Status on Peripheral and Central Organs. Biochim. Biophys. Acta-Mol. Basis Dis. 2020, 1866, 165823. [Google Scholar] [CrossRef]
- Docherty, S.; Harley, R.; McAuley, J.J.; Crowe, L.A.N.; Pedret, C.; Kirwan, P.D.; Siebert, S.; Millar, N.L. The Effect of Exercise on Cytokines: Implications for Musculoskeletal Health: A Narrative Review. BMC Sports Sci. Med. Rehabil. 2022, 14, 5. [Google Scholar] [CrossRef]
- Canlı, K.; Billens, A.; Van Oosterwijck, J.; Meeus, M.; De Meulemeester, K. Systemic Cytokine Level Differences in Patients with Chronic Musculoskeletal Spinal Pain Compared to Healthy Controls and Its Association with Pain Severity: A Systematic Review. Pain Med. 2022, 23, 1947–1964. [Google Scholar] [CrossRef]
- Jrad, A.I.S.; Trad, M.; Bzeih, W.; El Hasbani, G.; Uthman, I. Role of Pro-Inflammatory Interleukins in Osteoarthritis: A Narrative Review. Connect. Tissue Res. 2023, 64, 238–247. [Google Scholar] [CrossRef]
- Balchin, C.; Tan, A.L.; Golding, J.; Bissell, L.-A.; Wilson, O.J.; McKenna, J.; Stavropoulos-Kalinoglou, A. Acute Effects of Exercise on Pain Symptoms, Clinical Inflammatory Markers and Inflammatory Cytokines in People with Rheumatoid Arthritis: A Systematic Literature Review. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1–16. [Google Scholar] [CrossRef]
- Spel, L.; Martinon, F. Inflammasomes Contributing to Inflammation in Arthritis. Immunol. Rev. 2020, 294, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Beringer, A.; Miossec, P. Systemic Effects of IL-17 in Inflammatory Arthritis. Nat. Rev. Rheumatol. 2019, 15, 491–501. [Google Scholar] [CrossRef]
- Ferrara, V.; Toti, A.; Ghelardini, C.; Mannelli, L.C. Interferon-Gamma and Neuropathy: Balance Between Pain and Neuroprotection. Neural Regen. Res. 2022, 17, 2700. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; O’Garra, A. IL-10 Family Cytokines IL-10 and IL-22: From Basic Science to Clinical Translation. Immunity 2019, 50, 871–891. [Google Scholar] [CrossRef] [PubMed]
- Waters, R.S.; Perry, J.S.A.; Han, S.; Bielekova, B.; Gedeon, T. The Effects of Interleukin-2 on Immune Response Regulation. Math. Med. Biol. A J. IMA 2018, 35, 79–119. [Google Scholar] [CrossRef]
- Metsios, G.S.; Moe, R.H.; Kitas, G.D. Exercise and Inflammation. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101504. [Google Scholar] [CrossRef]
- Suzuki, K. Chronic Inflammation as an Immunological Abnormality and Effectiveness of Exercise. Biomolecules 2019, 9, 223. [Google Scholar] [CrossRef]
- Smith, B.E.; Hendrick, P.; Bateman, M.; Holden, S.; Littlewood, C.; Smith, T.O.; Logan, P. Musculoskeletal Pain and Exercise—Challenging Existing Paradigms and Introducing New. Br. J. Sports Med. 2019, 53, 907–912. [Google Scholar] [CrossRef]
- Rosa Neto, J.C.; Lira, F.S.; Oyama, L.M.; Zanchi, N.E.; Yamashita, A.S.; Batista, M.L.; Oller do Nascimento, C.M.; Seelaender, M. Exhaustive Exercise Causes an Anti-Inflammatory Effect in Skeletal Muscle and a pro-Inflammatory Effect in Adipose Tissue in Rats. Eur. J. Appl. Physiol. 2009, 106, 697. [Google Scholar] [CrossRef]
- Cerqueira, É.; Marinho, D.A.; Neiva, H.P.; Lourenço, O. Inflammatory Effects of High and Moderate Intensity Exercise—A Systematic Review. Front. Physiol. 2020, 10, 1550. [Google Scholar] [CrossRef]
- Pedersen, B.K. Muscle as a Secretory Organ. In Comprehensive Physiology; Wiley: Hoboken, NJ, USA, 2013; pp. 1337–1362. [Google Scholar] [CrossRef]
- Alizaei Yousefabadi, H.; Niyazi, A.; Alaee, S.; Fathi, M.; Mohammad Rahimi, G.R. Anti-Inflammatory Effects of Exercise on Metabolic Syndrome Patients: A Systematic Review and Meta-Analysis. Biol. Res. Nurs. 2021, 23, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Khalafi, M.; Malandish, A.; Rosenkranz, S.K. The Impact of Exercise Training on Inflammatory Markers in Postmenopausal Women: A Systemic Review and Meta-Analysis. Exp. Gerontol. 2021, 150, 111398. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions Version 6.0; Cochrane: London, UK, 2019. [Google Scholar]
- Higgins, J.P.T.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A.C. Chapter 8: Assessing Risk of Bias in a Randomized Trial [Last Updated October 2019]. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.5; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V., Eds.; Cochrane: London, UK, 2024. [Google Scholar]
- Prasad, M. Introduction to the GRADE Tool for Rating Certainty in Evidence and Recommendations. Clin. Epidemiol. Glob. Health 2024, 25, 101484. [Google Scholar] [CrossRef]
- Melikoglu, M.A.; Karatay, S.; Senel, K.; Akcay, F. Association between Dynamic Exercise Therapy and IGF-1 and IGFBP-3 Concentrations in the Patients with Rheumatoid Arthritis. Rheumatol. Int. 2006, 26, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Bjersing, J.L.; Dehlin, M.; Erlandsson, M.; Bokarewa, M.I.; Mannerkorpi, K. Changes in Pain and Insulin-like Growth Factor 1 in Fibromyalgia during Exercise: The Involvement of Cerebrospinal Inflammatory Factors and Neuropeptides. Arthritis Res. Ther. 2012, 14, R162. [Google Scholar] [CrossRef]
- Simão, A.P.; Avelar, N.C.; Tossige-Gomes, R.; Neves, C.D.; Mendonça, V.A.; Miranda, A.S.; Teixeira, M.M.; Teixeira, A.L.; Andrade, A.P.; Coimbra, C.C.; et al. Functional Performance and Inflammatory Cytokines after Squat Exercises and Whole-Body Vibration in Elderly Individuals with Knee Osteoarthritis. Arch. Phys. Med. Rehabil. 2012, 93, 1692–1700. [Google Scholar] [CrossRef]
- Sjøgaard, G.; Zebis, M.K.; Kiilerich, K.; Saltin, B.; Pilegaard, H. Exercise Training and Work Task Induced Metabolic and Stress-Related MRNA and Protein Responses in Myalgic Muscles. Biomed Res. Int. 2013, 2013, 984523. [Google Scholar] [CrossRef]
- ZHANG, S.-L.; LIU, H.-Q.; Xu, X.-Z.; ZHI, J.; GENG, J.-J.; CHEN, J. Effects of Exercise Therapy on Knee Joint Function and Synovial Fluid Cytokine Levels in Patients with Knee Osteoarthritis. Mol. Med. Rep. 2013, 7, 183–186. [Google Scholar] [CrossRef]
- Delaney, C.L.; Miller, M.D.; Allan, R.B.; Spark, J.I. The Impact of Different Supervised Exercise Regimens on Endothelial Function in Patients with Intermittent Claudication. Vascular 2015, 23, 561–569. [Google Scholar] [CrossRef]
- Samut, G.; Dinçer, F.; Özdemir, O. The Effect of Isokinetic and Aerobic Exercises on Serum Interleukin-6 and Tumor Necrosis Factor Alpha Levels, Pain, and Functional Activity in Patients with Knee Osteoarthritis. Mod. Rheumatol. 2015, 25, 919–924. [Google Scholar] [CrossRef]
- Sandstad, J.; Stensvold, D.; Hoff, M.; Nes, B.M.; Arbo, I.; Bye, A. The Effects of High Intensity Interval Training in Women with Rheumatic Disease: A Pilot Study. Eur. J. Appl. Physiol. 2015, 115, 2081–2089. [Google Scholar] [CrossRef] [PubMed]
- Ernberg, M.; Christidis, N.; Ghafouri, B.; Bileviciute-Ljungar, I.; Löfgren, M.; Bjersing, J.; Palstam, A.; Larsson, A.; Mannerkorpi, K.; Gerdle, B.; et al. Plasma Cytokine Levels in Fibromyalgia and Their Response to 15 Weeks of Progressive Resistance Exercise or Relaxation Therapy. Mediat. Inflamm. 2018, 2018, 3985154. [Google Scholar] [CrossRef]
- Lau, Y.N.; Ng, J.; Lee, S.Y.; Li, L.C.; Kwan, C.M.; Fan, S.M.; Leung, B.P.L.; Lo, C.N. A Brief Report on the Clinical Trial on Neural Mobilization Exercise for Joint Pain in Patients with Rheumatoid Arthritis. Z. Rheumatol. 2019, 78, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Nadi, M.; Bambaeichi, E.; Marandi, S.M. Comparison of the Effect of Two Therapeutic Exercises on the Inflammatory and Physiological Conditions and Complications of Diabetic Neuropathy in Female Patients. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 1493–1501. [Google Scholar] [CrossRef]
- Nambi, G.; Abdelbasset, W.; Alrawaili, S.; Elnegamy, T.; Abodonya, A.; Saleh, A.; Verma, A. Effects of Isokinetic Knee Muscle Training on Bone Morphogenetic Proteins and Inflammatory Biomarkers in Post-Traumatic Osteoarthritis after Anterior Cruciate Ligament Injury: A Randomized Trial. J. Rehabil. Med. 2020, 52, jrm00098. [Google Scholar] [CrossRef]
- Nambi, G.; Abdelbasset, W.K.; Alsubaie, S.F.; Moawd, S.A.; Verma, A.; Saleh, A.K.; Ataalla, N.N. Isokinetic Training—Its Radiographic and Inflammatory Effects on Chronic Low Back Pain: A Randomized Controlled Trial. Medicine 2020, 99, E23555. [Google Scholar] [CrossRef] [PubMed]
- Nambi, G.; Abdelbasset, W.K.; Elsayed, S.H.; Khalil, M.A.; Alrawaili, S.M.; Alsubaie, S.F. Comparative Effects of Virtual Reality Training and Sensory Motor Training on Bone Morphogenic Proteins and Inflammatory Biomarkers in Post-Traumatic Osteoarthritis. Sci. Rep. 2020, 10, 15864. [Google Scholar] [CrossRef]
- Oğuz, R.; Belviranlı, M.; Okudan, N. Effects of Exercise Training Alone and in Combination With Kinesio Taping on Pain, Functionality, and Biomarkers Related to the Cartilage Metabolism in Knee Osteoarthritis. Cartilage 2021, 13 (Suppl. S1), 1791S–1800S. [Google Scholar] [CrossRef]
- Park, S.; Min, S.; Park, S.H.; Yoo, J.; Jee, Y.S. Influence of Isometric Exercise Combined With Electromyostimulation on Inflammatory Cytokine Levels, Muscle Strength, and Knee Joint Function in Elderly Women With Early Knee Osteoarthritis. Front. Physiol. 2021, 12, 1–15. [Google Scholar] [CrossRef]
- Du, J.; Liang, C.; Guo, C. The Efficacy of Selected Tai Chi Movements and Hand Exercise for People with Rheumatoid Arthritis. Arch. Budo 2022, 18, 175–182. [Google Scholar]
- Nambi, G.; Alghadier, M.; Kashoo, F.Z.; Aldhafian, O.R.; Nwihadh, N.A.; Saleh, A.K.; Omar, M.A.; Hassan, T.G.T.; Ibrahim, M.N.A.; El Behairy, H.F.; et al. Effects of Virtual Reality Exercises versus Isokinetic Exercises in Comparison with Conventional Exercises on the Imaging Findings and Inflammatory Biomarker Changes in Soccer Players with Non-Specific Low Back Pain: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2022, 20, 524. [Google Scholar] [CrossRef]
- Roberts, M.J.; Hamrouni, M.; Linsley, V.; Moorthy, A.; Bishop, N.C. Exercise as an Anti-Inflammatory Therapy in Axial Spondyloarthritis Therapeutic Intervention (EXTASI) Study: A Randomized Controlled Trial. Rheumatol. Adv. Pract. 2024, 8, rkae062. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, M.; Gangestad, S.W. Rethinking IL-6 and CRP: Why They Are More than Inflammatory Biomarkers, and Why It Matters. Brain. Behav. Immun. 2018, 70, 61–75. [Google Scholar] [CrossRef]
- Jungen, M.J.; ter Meulen, B.C.; van Osch, T.; Weinstein, H.C.; Ostelo, R.W.J.G. Inflammatory Biomarkers in Patients with Sciatica: A Systematic Review. BMC Musculoskelet. Disord. 2019, 20, 156. [Google Scholar] [CrossRef]
- Oghumu, S.N.; Okafor, U.A.C.; Salu, O.B.; Tella, B.A.; Nicholas Oghumu, S.; Okafor, U.A.C.; Salu, O.B.; Tella, B.A.; Oghumu, S.N.; Okafor, U.A.C.; et al. Effects of Lumbar Stabilization and Graded Activity Exercises on Selected Biochemical Mediators and Clinical Outcomes in Patients With Non-Specific Chronic Low Back Pain. Iran. Rehabil. J. 2023, 21, 663–694. [Google Scholar] [CrossRef]
- Minobes-Molina, E.; Nogués, M.R.; Giralt, M.; Casajuana, C.; de Souza, D.L.B.; Jerez-Roig, J.; Romeu, M. Effectiveness of Specific Stabilization Exercise Compared with Traditional Trunk Exercise in Women with Non-Specific Low Back Pain: A Pilot Randomized Controlled Trial. PeerJ 2020, 8, e10304. [Google Scholar] [CrossRef]
- Uzunel, E.; Kronhed, A.-C.G.; Alin, C.K.; Ahmed, A.S.; Wändell, P.; Salminen, H. The Effect of Group Training or Spinal Orthosis on Quality of Life and Potential Plasma Markers of Pain in Older Women With Osteoporosis. A Randomized Controlled Trial. Arch. Rehabil. Res. Clin. Transl. 2023, 5, 100297. [Google Scholar] [CrossRef]
- Nambi, G.; Abdelbasset, W.K.; Alrawaili, S.M.; Alsubaie, S.F.; Abodonya, A.M.; Saleh, A.K. Virtual Reality or Isokinetic Training; Its Effect on Pain, Kinesiophobia and Serum Stress Hormones in Chronic Low Back Pain: A Randomized Controlled Trial. Technol. Healh Care 2021, 29, 155–166. [Google Scholar] [CrossRef]



| Authors | Study Design | Country of Study | Main Diagnosis | Sample Size | Age and Gender Ratio | Inclusion Criteria | Exclusion Criteria |
|---|---|---|---|---|---|---|---|
| Melikoglu et al., 2006 | RCT | Turkey | RA | 40 | Dynamic exercise group: 46.4 ± 8.3 years old ROM exercise group: 50.3 ± 9.7 years old Control group: 43.2 ± 6.9 years old All females | Diagnosed with RA according to the 1987 revised ACR criteria. Female participants only. Not clinically active disease, defined by morning stiffness duration > 30 min, six or more tender joints, three or more swollen joints, or ESR > 28 mm/h. Stable dosage of disease-modifying drugs in the last 3 months. Functional status class I–II as defined by the ACR. | Use of oral contraceptive drugs. Comorbid medical conditions known to affect IGF status. Inability to tolerate walking on treadmill. Sedentary healthy controls were selected according to a questionnaire quantifying physical activity undertaken during a normal week. |
| Bjersing et al., 2012 | RCT | Sweden | FM | 49 | 20 to 60 years old All females | Interested in exercising outdoors twice a week for 15 weeks. Able to perform a bicycle test at 50 watts. | Non-Swedish speakers/readers. Severe somatic or psychiatric diseases. Ongoing or planned physical therapy, including exercise. Inability to adhere to scheduled exercise sessions. |
| Simão et al., 2012 | RCT | Brazil | KOA | 32 | Whole-body vibration group: 72.0 ± 7.4 years old; 2 males and 10 females Squat exercise group: 69.0 ± 3.7 years old; 1 male and 9 females Control group: 71.0 ± 5.3 years old; 1 male and 10 females | Patients aged 60 years or older with a diagnosis of KOA based on the ACR clinical and radiographic criteria. | Recent knee trauma, use of any locomotion device, physiotherapy or other rehabilitation in the last 3 months, absence of clinical and cognitive conditions for physical activities, glucocorticoid use for at least 2 months, orthopedic disease, neurological, respiratory, or acute cardiac issues, vestibular disorders, immunosuppression or immunodeficiency, lack of sphincter control, or cognitive deficits. |
| Sjøgaard et al., 2013 | RCT | Denmark | Trapezius myalgia | 28 | Specific strength training group: 44.0 ± 9.8 years old General fitness training group: 44.0 ± 9.8 years old Healthy control group: 44.0 ± 9.1 years old All females | Females with trapezius myalgia employed in jobs with repetitive and monotonous work tasks, diagnosed based on pain or discomfort in the neck/shoulder region for more than 30 days in the last year, and clinical signs of palpable tenderness and tightness in the trapezius muscle. | Serious conditions such as previous trauma or injuries, life-threatening diseases, cardiovascular diseases, or arthritis in the neck and shoulder. |
| Zhang et al., 2013 | RCT | China | KOA | 100 | Treatment group: 53.0 ± 5.6 years old; 20 males and 30 females Control group: 52.3 ± 7.0 years old; 18 males and 32 females | Diagnosed with KOA based on American Rheumatism Association criteria. Age 40–70 years. Disease classification of I-III by X-ray. Not receiving other treatment methods or medication. | Significant stenosis of the joint space or bone bridge connection between the joints (bony ankylosis). Disease classification IV by X-ray. Primary disease of the knee affecting joint structures. Active gastrointestinal diseases, cardiovascular, cerebrovascular, liver, kidney, or hematopoietic system diseases. Mental illness, pregnancy, lactation, and known allergy to diclofenac. |
| Delaney et al., 2015 | RCT | Australia | Intermittent claudication | 35 | Experimental group: 73.4 ± 9.1 years old; 12 males and 5 females Control group: 69.4 ± 9.6 years old; 13 males and 4 females. | Clinical history consistent with intermittent claudication. ABPI < 0.9. Radiographic evidence of infra-inguinal disease without aorto-iliac disease | Diagnosis of critical limb ischemia. Recent (<12 months) peripheral vascular intervention (surgery or endovascular). Pre-existing cardio-respiratory conditions limiting exercise capacity. |
| Samut et al., 2015 | RCT | Turkey | KOA | 42 | Isokinetic exercise: 62.5 ± 7.7 years old Aerobic exercise: 57.6 ± 5.8 years old Control group: 60.9 ± 8.9 years old All females | Postmenopausal women and men aged over 50 years. Diagnosed with KOA according to the ACR criteria. Kellgren–Lawrence grade 2–3 knee OA. Sedentary lifestyle (less than 60 min of moderate- to high-intensity activity per week). | Cooperation problems, depression, cognitive impairment, neurologic impairment/disease, orthopedic problems, or inflammatory arthritis. Regular exercise habits, physical therapy or intra-articular injection in the last three months, or cardiovascular problems. End-stage disease, immune-suppressive drug usage, infection or inflammatory condition, pregnancy, or malignant disease. |
| Sandstad et al., 2015 | Randomized crossover design study | Norway | RA | 18 | High-intensity interval training group: 32.4 ± 8.3 years old Control group: 33.4 ± 8.5 years old All females | Women aged 20–50 years with RA or adult-onset JIA, stable disease with no changes in medication for the last 2 months. | Regular exercise training > 2 times a week before the study, participation in <80% of exercise sessions, heart disease, lung disease, pregnancy, lactation, active arthritis despite medication, or new synthetic or biologic DMARDs. |
| Ernberg et al., 2018 | Randomized controlled multicenter trial | Sweden | FM | 125 | Progressive resistance exercise group: 51.2 ± 9.4 years old Relaxation therapy group: 51.2 ± 9.4 years old Healthy controls: 48.2 ± 11.4 years old All females | Women with FM diagnosed according to the ACR-1990 classification criteria. Working age (20–65 years). | High BP (>160/90 mmHg), osteoarthritis in the hip or knee, other severe somatic or psychiatric disorders, primary causes of pain other than FM, high alcohol consumption (audit > 6), participation in a rehabilitation program within the past year, regular resistance exercise or relaxation therapy inability to understand or speak Swedish, and inability to refrain from analgesics, NSAID, or hypnotics for 48 h prior to examinations. |
| Lau et al., 2019 | RCT | Hong Kong | RA | 21 | Neural mobilization group: 57.5 ± 7.1 years old Control group: 57.5 ± 7.1 years old All females | Patients fulfilling the ACR 1987 revised criteria for rheumatoid arthritis. Non-smokers. | Not explicitly listed in the visible text but generally includes those who cannot follow the exercise protocol or have other contraindications to exercise. |
| Nadi et al., 2019 | Quasi-experimental study | Iran | Diabetic neuropathy | 45 | Exercise for peripheral neuropathy group: 55.46 ± 2.47 years old; Resistance group: 56.13 ± 3.39 years old Control group: 54.80 ± 3.29 years old All females | Female sex, age range 50–60 years, minimum diabetes history of 7 years, HbA1c ≥ 6.5%, mild-to-moderate diabetic neuropathy, and fasting blood glucose ≥ 156 mg/dL. | Serious diseases (autoimmune, liver, renal, thyroid, cerebrovascular injury, fractures, tumor), voluntary withdrawal, irregular meeting attendance, failure to complete tests, weight change > 3 kg during intervention, other diabetes complications, medication change, anti-inflammatory drug use, and blood glucose imbalance before training. |
| Minobes-Molina et al., 2020 | RCT | Spain | Non-specific LBP | 39 | Traditional trunk exercise group: 50.9 ± 11.0 years old Specific stabilization exercise group: 50.1 ± 9.8 years old All females | Females aged between 18 and 70 years. Diagnosed with non-specific LBP (fewer than 6 weeks of pain duration) by a specialist doctor. Imaging used to rule out other spinal disorders. Not under pharmacological treatment for pain. | Diagnosed with other spinal disorders. Any serious co-morbidities (e.g., cancer, severe lung pathology). Inability to perform exercises. Presence of cognitive impairment. Having followed a specific training program with a physiotherapist in the previous three months. Having been treated with analgesic infiltration in the previous 6 weeks. Failure to follow their 20-treatment schedule exactly. |
| Nambi, Abdelbasset, Elsayed, et al., 2020 | RCT | Saudi Arabia | PTOA following ACL injury | 60 | Virtual reality training group: 22.8 ± 1.3 years old Sensory motor training group: 22.6 ± 1.4 years old Control group: 21.9 ± 1.3 years old All males | Male football players, age 18–25 years. Chronic (≥3 months) PTOA following ACL injury. Pain rating 4–8 on VAS. | Other orthopedic, neural, systemic, or psychological conditions, awaiting surgery, previous treatment or physical training, and unwillingness to participate. |
| Nambi et al., 2020 | RCT | Saudi Arabia | Chronic (≥3 months) OA after ACL injury | 60 | Isokinetic training group: 22.3 ± 1.2 years old Sensory motor training group: 22.4 ± 1.5 years old Control group: 22.9 ± 1.7 years old All males | University football players. Age between 18 and 25 years. Male players. Chronic (≥3 months) OA after ACL injury. Pain intensity between 4 and 8 on VAS | Severe musculoskeletal, neural, somatic, or psychiatric conditions. Awaiting surgery. Alcohol or drug abuse. Involvement in other weight and balance training programs. Other soft-tissue injuries, fracture of the lower limbs and pelvic bone, or deformities. |
| Nambi, Abdelbasset, Alsubaie, et al., 2020 | RCT | Saudi Arabia | Chronic LBP | 60 | Isokinetic training group: 22.1 ± 1.8 years old Core stabilization training group: 22.3 ± 1.7 years old Control group: 21.9 ± 1.8 years old All males | University male football players aged 18–25 years. Chronic (≥3 months) LBP. Pain intensity between 4 and 8 on VAS. Diagnosed with LBP by an orthopedic surgeon and referred for physical therapy. | Severe musculoskeletal, neural, somatic, and psychiatric conditions. Other soft tissue injuries, fractures of the lower limbs and pelvic bone, or deformities. Awaiting spine surgery. Alcohol or drug abuse. Involvement in other weight and balance training programs. |
| Oğuz et al., 2021 | RCT | Turkey | KOA | 22 | Exercise training group: 51.0 ± 3.7 years old Exercise training + kinesio taping group: 48.2 ± 7.6 years old All females | Female patients, aged 38–60 years, diagnosed with KOA according to the ACR criteria, and classified as Kellgren–Lawrence index II and III. | RA, severe organ failure, previous joint replacement, osteoporosis, and diagnosis of any disease that could limit performance. |
| Park et al., 2021 | RCT | South Korea | KOA | 75 | Isometric exercise + electromyostimulation group: 65.7 ± 3.2 years old Isometric exercise group: 66.9 ± 4.6 years old Control group: 68.0 ± 4.2 years old All females | Females aged over 60 years. Diagnosed with early KOA (Grade I or II) based on bilateral radiographic examination. Irregular exercise habits for the previous six months. No medications, including steroids or intra-articular injections within the last three months. | Deformities of the knee, hip, or back. Central or peripheral nervous system involvement. Use of pacemakers or internal metallic materials. History of impairment of a major organ system or psychological disorders. |
| Du et al., 2022 | RCT | China | RA | 20 | Experimental group: 43.8 ± 3.57 years old; 8 females and 2 males Control group: 52.4 ± 3.35 years old; 8 females and 2 males | Age between 30 and 65 years. Diagnosed with RA using the ACR/EULAR 2010 classification criteria. Low-to-moderate Disease Activity Score in 28 joints (2.6 < DAS28 < 5.1). Stable medical treatment (no alternative medical treatments during study period). No participation in structured exercise for the preceding 3 months. | Heart or kidney disease and other pre-existing medical conditions that might prevent participation in the exercise program. |
| Jayabalan et al., 2022 | Two-phase crossover sequential design | US | KOA | 10 | KOA participants: 67.5 ± 5.3 years old; 7 males and 3 females Control participants: 62.2 ± 3.0 years old; 3 males and 2 females | Participants with symptomatic unilateral medial KOA meeting ACR clinical criteria. Regular golfers (playing at least 1–2 times per month). | Previous total knee arthroplasty or other lower limb joint pain (hips or ankles) or LBP. Severe cardiovascular disease, uncontrolled hypertension, or stroke. Participants who typically require pain medications during a round of golf. |
| Nambi et al., 2023 | RCT | Saudi Arabia | CNLBP | 60 | Virtual reality exercise group: 23.2 ± 1.6 years old Isokinetic exercise group: 22.9 ± 1.7 years old Conventional exercise group: 22.8 ± 1.8 years old All males | Male soccer players aged 18–25 years. CNLBP for three or more months. Pain score ranging from 4 to 8 on 10 cm VAS. | Lumbar stenosis, lumbar radiculopathy, lumbar spondylolisthesis, or spinal injuries. Serious pathologies of the thoracolumbar spine. Associated low-back muscle and tendon injuries or fracture of the pelvic bone and lower extremity bones. Spine dysfunctions, awaiting spine surgery. Participants taking steroids, medications, or analgesics. Serious pathologies of the thoracolumbar spine. Participating in other resistance training and physical training programs. |
| Oghumu et al., 2023 | RCT | Nigeria | CNLBP | 54 | Lumbar stabilization exercise group: 49.5 ± 7.8 years old; 11 males and 15 females Graded activity exercise group: 49.5 ± 7.8 years old; 11 males and 15 females Control group: 46.5 ± 6.1 years old; 15 males and 12 females | Patients with CNLBP referred for physiotherapy, aged 18–60 years, with or without radiating symptoms for at least three months. | Spinal inflammatory disease, history of spinal fracture/dislocation, motor/sensory deficit, pregnancy, systemic or medical conditions like diabetes, hematological disorders, acute/chronic liver diseases, autoimmune diseases, use of pain medications within the last two days, unwillingness to stop analgesics, history of psychotropic medications, open wounds, and history of smoking/drinking in the last six months. |
| Uzunel et al., 2023 | RCT | Sweden | Back pain and self-reported osteoporosis | 113 | Exercise group: 77.7 ± 8.6 years old Spinal orthosis group: 78.0 ± 8.7 years old Control group: 72.9 ± 7.9 years old All females | Women aged 60–93 years. Self-reported osteoporosis. Experiencing back pain with or without vertebral fractures. | Inability to follow the research protocol. Insufficient Swedish language skills. Diagnosed with spinal stenosis. |
| Roberts et al., 2024 | RCT | UK | axSpA | 20 | Exercise group: 47.1 ± 11.4 years old; 5 males and 5 females Control group: 43.6 ± 10.1 years old; 7 males and 3 females | Diagnosed with axSpA, on stable NSAID dose, no heart disease or anemia, not pregnant/planning pregnancy, able to meet study demands, and low habitual physical activity. | History of heart disease, high physical activity level (≥1 h moderate activity per day), or inability to walk. |
| Intervention Group | Comparison 1 | Comparison 2 | Outcome Measures | Follow-Up Period | |||
|---|---|---|---|---|---|---|---|
| Authors | Inflammatory Biomarkers | Non-Inflammatory Biomarkers | Other Outcomes | ||||
| Melikoglu et al., 2006 | Dynamic exercise program on a treadmill, maintaining heart rate at 60% of age-predicted maximum heart rate. Five sessions per week for two weeks. Duration of each session: 20 min. | ROM exercises for all joint movements of the upper and lower extremities, performed actively at a low pace. Exercise frequency: Five sessions per week for two weeks. Duration of each session: 20 min. | Nil | CRP, ESR | IGF-1, IGFBP-3 | VAS, morning stiffness duration, HAQ, RAI | 15 days |
| Bjersing et al., 2012 | Engaged in a moderate- to high-intensity Nordic walking program, conducted twice a week for 40 to 45 min over 15 weeks. | Engaged in supervised low-intensity walking, conducted twice a week for 40 to 45 min over 15 weeks. | Nil | IL-6, IL-8, SP, NPY, MMP-3 | IGF-1 | Pain score, 6MWT | 15 weeks, 30 weeks |
| Simão et al., 2012 | Squat Exercises + Whole-Body Vibration: 12 weeks, 3 times per week. Squats were performed on a vibratory platform with a 10–60° knee flexion range, maintaining 3 s of isometric contraction at 60° flexion. | Squat Group: Participants performed the same squat exercises as the platform group but without vibration, for the same duration and frequency. | Control Group: Participants did not receive any training and were instructed not to change their lifestyle during the study. | sTNFR1, sTNFR2 | NA | WOMAC, Berg Balance Scale, gait speed test, 6MWT | 12 weeks |
| Sjøgaard et al., 2013 | Specific Strength Training: 10 weeks, 3 times per week, 20 min per session. Performed 5 different dumbbell exercises targeting the neck and shoulder muscles (1-arm row, shoulder abduction, shoulder elevation, reverse flies, upright row). Progression from 12 repetitions maximum (RM) (~70% of max) to 8 RM (~80% of max). | General Fitness Training: 10 weeks, 3 times per week, 20 min per session of cycling on a Monark ergometer at 50–70% of VO2max, with progressive intensity increase. | Reference Group: Participants attended weekly health counseling sessions (~1 hr per week) on workplace ergonomics, diet, general health, relaxation, and stress management, but did not perform any structured physical exercise. | IL-6, TNF-α | IGF-1, HSP72, HSc70 | Pain intensity, muscle metabolic adaptations (Glut4, GS, PDH-E1α, CytC, HKII), functional capacity | 10 weeks |
| Zhang et al., 2013 | Exercise Therapy: 4 weeks, 4 days per week. Sessions included knee joint flexion and extension, followed by isometric quadriceps contractions at 0° and 90° knee flexion. Each contraction lasted 10 s, followed by 10 s relaxation, repeated 10 times per session, with 5 sets per angle and 1 min rest between sets. Performed in a sitting position to avoid weight-bearing stress. Participants also received oral diclofenac sodium (75 mg, twice daily). | Control Group: Participants received only oral diclofenac sodium (75 mg, twice daily) without exercise intervention. | Nil | TNF-α, hs-CRP, MMP-3 | NA | Knee function index score, pain intensity, synovial fluid cytokine levels | 4 weeks |
| Delaney et al., 2015 | Treadmill-based SET, participants walked until claudication pain became unbearable, rested until pain resolved, repeated cycle for the session duration | Combination-based SET, three sets of 8–12 repetitions of hamstring curls, seated calf press, leg press, knee extension, and hip abduction/adduction, followed by walking on the treadmill until onset of claudication pain, rest until pain resolved, cycle repeated for session duration. | Nil | NO, ADMA | NA | PFWD, FMD, RHI, QoL | 12 weeks |
| Samut et al., 2015 | Isokinetic Exercise: 6 weeks, 3 days per week. Training included concentric flexion and extension exercises at angular velocities of 60°/s, 90°/s, 120°/s, and 180°/s using a Biodex Isokinetic System. One set of contraction in the first session was increased by 1 set per session until reaching 6 sets. Rest periods: 20 s between sets, 2 min between legs. | Aerobic Exercise: 6 weeks, 3 days per week on a treadmill. Training intensity was set at 65–70% of age-related heart rate for the first 4 weeks, then increased to 70–75% for the last 2 weeks. Sessions included a 5 min warm-up and 5 min cool-down. | No Intervention: Participants were informed about knee osteoarthritis and given recommendations, but no exercise intervention was provided. | IL-6, TNF-α, CRP | NA | VAS, WOMAC, functional capacity (6MWT), muscle strength (30 s sit-to-stand test) | 6 weeks |
| Sandstad et al., 2015 | High-Intensity Interval Training: 10 weeks, 2 times per week, performed on spinning bikes. Each session consisted of 4 × 4 min high-intensity intervals at 85–95% of HRmax, interspersed with 3 min recovery periods at ~70% of HRmax. Each session started with a 10 min warm-up and lasted a total of 35 min. | Participants continued their usual lifestyle without structured exercise. | Nil | CRP, PTX3 | IGF-1, COMP | VAS, DAS28, body composition (BMI, body fat percentage, muscle mass), cardiovascular fitness (VO2max, 1 min heart rate recovery), functional disability (MHAQ) | 10 weeks |
| Ernberg et al., 2018 | 15 weeks, twice per week, supervised by trained physical therapists, 10 min of bicycling warm-up, 50 min strength training focusing on lower extremities, initial loads at 40% MVC progressing to 70–80% MVC. | 15 weeks, twice per week, supervised by experienced physiotherapists, autogenic training including relaxation and autosuggestion, followed by stretching exercises, approximately 25 min per session. | Nil | IL-2, IL-6, IL-8, IL-17A, TNF-α, IP-10, Eotaxin | NA | Pain, 6MWT, PPT, FIQ, PDI, SF-36 | 15 weeks |
| Lau et al., 2019 | Participants performed neural mobilization exercises targeting the median nerve, musculocutaneous nerve, femoral nerve, saphenous nerve, and the entire nervous system. The exercises were performed 10 times per set, 2 sets per day for 4–8 weeks. | Participants performed gentle joint mobilization exercises for the fingers, wrist, elbow, shoulder, spine, and lower limbs without touching the end range of joints and stressing the nervous system. Exercises were performed 10 times per set, 2 sets per day for 4–8 weeks. | Nil | ESR | NA | RAID score, pain, coping (self-efficacy) | 4–8 weeks |
| Nadi et al., 2019 | Resistance Group: 12 weeks, three sessions per week, exercises with 30% repetition maximum including leg press, arm curls, military press, push-ups, squats, knee extensions, heel raises, back extensions, knee sit-ups, and upright rowing. | EPN Group: 12 weeks, three sessions per week, 12 lower extremity movements including hamstring stretching, knee swirling, gradual stretching of the sciatic nerve, stretching of the leg muscles, and ankle range movements exercises. | Control Group: Continued with their daily activities without any specific intervention. | TNF-α, IL-10, CRP | FBG, HbA1c | Pain, tingling, static and dynamic balance (DEMMI), MNSI | 12 weeks |
| Minobes-Molina et al., 2020 | Traditional Trunk Exercise Program (TTEP): 20 sessions, 3–5 times per week, first 5 sessions with infrared lamp and TENS, followed by 15 sessions with 10 exercises focusing on global abdominal and back muscles. | Specific Stabilization Exercise Program (SSEP): 20 sessions, 3–5 times per week, first 5 sessions with infrared lamp and TENS, followed by 15 sessions with 10 exercises focusing on transversus abdominis, multifidus, and internal oblique muscles. | Nil | IL-6, TNF-α | NA | VAS, disability (RMDQ) | 1 month |
| Nambi, Abdelbasset, Elsayed, et al., 2020 | Virtual Reality Training: 4 weeks, 20 min per session, two sessions per day, 5 days per week. Training involved the use of the Pro-Kin system PK 252 N focusing on knee muscle improvement. Exercises included virtual tasks such as shooting balls by moving the knee joint in different directions. | Sensory Motor Training: 4 weeks, 5 repetitions per set for 3 sets, 3 min rest between sets, 5 days per week. Exercises included static standing on hard and foam plates, single-leg standing with closed eyes, semi knee bending, dynamic forward kicking, T-band kicking, toe jumping, heel jumping, and bilateral and unilateral squatting. | Control Group: Supervised conventional exercise program for knee muscles, including quadriceps, hamstrings, glutei, and calf muscles. Exercises performed 10–15 repetitions per set for 3 sets, 1 min rest between sets, 5 days per week, with stretching exercises for each muscle group. | CRP, TNF-α, IL-2, IL-4, IL-6 | NA | VAS, WOMAC score | 4 weeks, 8 weeks, 3 months |
| Nambi et al., 2020 | Isokinetic Training Group: 4 weeks, exercises performed at angular speeds of 60, 90, and 120 degrees/s, with 15 repetitions per set for 3 sets, 5 days per week. Training involved using an isokinetic dynamometer to perform knee flexion and extension exercises. | Sensory Motor Training Group: 4 weeks, exercises performed in three stages (static, dynamic, and functional), with 5 repetitions per set for 3 sets, 5 days per week. Exercises included static standing on firm and soft surfaces, single-leg standing with eyes closed, knee bends, forward stepping thrusts, T-band kicks, toe skipping, heel skipping, and squats. | Control Group: Home-based exercises including stretching and strengthening exercises for quadriceps, hamstrings, glutei, and calf muscles. Exercises performed 10–15 repetitions/day, 5 days a week, for 4 weeks. | CRP, TNF-α, IL-2, IL-4, IL-6 | NA | VAS, WOMAC score | 4 weeks, 8 weeks, 6 months |
| Nambi, Abdelbasset, Alsubaie, et al., 2020 | Isokinetic Training: 4 weeks, 5 days/week. Training on an isokinetic dynamometer (Biodex, New York, NY, USA) in a standing position with trunk flexion/extension at 60°, 90°, and 120°/s, 15 reps per set, 3 sets per session. Participants were stabilized using Velcro straps to prevent compensatory movements. | Core Stabilization Training: 4 weeks, 5 days/week. Exercises performed on Swiss ball (supine bridge, sit-up, arms-legs cross lifting, side bridge). Participants performed 3 sets of 10 reps, holding positions for 10 s with 3 s rest between. | Conventional Exercise: 4 weeks, 5 days/week. Focused on isometric and isotonic core exercises for abdominal and back muscles, performed at 10–15 reps/set for 3 sets. Stretching (3 reps, 10 sec) for hamstrings, hip flexors, and lumbar extensors. | CRP, TNF-α, IL-2, IL-4, IL-6, IL-8 | NA | VAS, CSA of paraspinal muscles (Psoas major, Quadratus lumborum, Multifidus, Erector spinae) | 4 weeks |
| Oğuz et al., 2021 | Isokinetic Training Group: 4 weeks, performed at angular speeds of 60, 90, and 120 degrees/s, with 15 repetitions per set for 3 sets, 5 days per week. Training involved using an isokinetic dynamometer to perform trunk flexion and extension exercises. | Graded Activity Exercise Group: Focused on increasing activity tolerance through individualized sub-maximal exercises with cognitive behavioral principles. The intervention was also conducted over 10 weeks, with sessions twice a week. Exercises included isometric strengthening, bicycle ergometry, and cognitive behavioral therapy. | The control group received no intervention and served as a baseline for comparisons. | MMP-1, MMP-3 | COMP | VAS, WOMAC, functional status | 6 weeks |
| Park et al., 2021 | Isometric Exercise + Whole-Body Electromyostimulation (WB-EMS): 8 weeks, 3 days per week, 30 min per session. Exercises included abdomen crunches, bridge, leg raises, side planks, back extension, front planks, front lunges, and squats. Performed in an alternation of a 6 s contraction with a 4 s break. The WB-EMS suit was used with stimulation settings (85 Hz frequency, 350 µs impulse width). | Isometric exercise: performed the same isometric exercises while wearing a WB-EMS suit without electrical stimulation provided. | Control group did not perform exercises but participated in meditation and light stretching for 30 min while wearing the WB-EMS suit without stimulation. | IL-6, TNF-α, CRP, Resistin | NA | VAS, KOOS, muscle strength, body composition (fat mass, fat percentage, skeletal muscle mass) | 8 weeks |
| Du et al., 2022 | A 12-week training program with 50 min tai chi movements and 20 min hand exercises. One session per week with a professional instructor and daily practice at home. | Participants maintained their usual lifestyle without any special exercise intervention. | Nil | CRP, ESR, RF | NA | DAS28, TJC, SJC, morning stiffness, HAQ, SDS, SAS | 12 weeks |
| Jayabalan et al., 2022 | A 12-week exercise program consisting of two sessions per week. Each session included 40 min of progressive resistance training to improve muscle strength, joint mobility, and overall physical function. | The control group did not participate in the exercise program and continued their usual activities without any additional intervention. | Nil | TNF-α, IL-1β, IL-6, MMP-3, MMP-13 | COMP | Pain, step count, RPE, heart rate, MVPA duration | Same day post intervention measurement |
| Nambi et al., 2023 | Virtual Reality Exercise: 4 weeks, 30 min/session, 5 days/week. Training with the Pro-Kin Techno Body system using a car race game, where participants moved their trunk in different directions to control the game. Resistance increased by modifying road steepness, terrain, and number of opposing cars. | Isokinetic Exercise: 4 weeks, 30 min/session, 5 days/week. Training with Humac Norm (Stoughton, MA, USA) isokinetic dynamometer. Participants performed trunk flexion and extension at 45°, 60°, and 90°/s while standing upright, with Velcro stabilization to isolate trunk movements. | Conventional Exercise: 4 weeks, 30 min/session, 5 days/week. Performed routine core and balance exercises targeting abdominal, and back muscles. Included 10–15 repetitions per set with 3 sets per session and stretching exercises for the lower limb muscles. | CRP, TNF-α, IL-2, IL-4, IL-6 | NA | VAS, CSA of paraspinal muscles (Psoas major, Quadratus lumborum, Multifidus, Erector spinae) | 4 weeks |
| Oghumu et al., 2023 | Lumbar Stabilization Exercise: 10 weeks, 2 sessions per week. Core-focused exercises targeting transversus abdominis, multifidus, pelvic floor, and diaphragm. | Graded Activity Exercise: 10 weeks, 2 sessions per week. Based on time-contingency and cognitive–behavioral principles. Activities included isometric strength training (quadriceps, hamstrings, glutes, back extensors), cycling ergometry, and proprioceptive training. Pain tolerance was reinforced with behavioral modification strategies. | Health control group with no intervention | IL-1A, IL-6, IL-18R1, IL-18RAP, COX-2 | NA | VAS, disability (RMDQ), catastrophizing, diverting attention, cognitive coping, pain reinterpretation | 10 weeks |
| Uzunel et al., 2023 | Exercise Group: 6 months, 1 h supervised group exercise per week, led by a physiotherapist. The program included gym machines, resistance bands, balance plate, and Bobath ball exercises focusing on back extensor and shoulder muscle strength, leg muscle strength, balance, and posture. Participants also performed a home exercise program at least 4 times per week. | Spinal Orthosis Group: Wore the activating Spinomed spinal orthosis for 10 min per day initially, increasing gradually to at least 2 h per day. The orthosis provided feedback to activate back extensor muscles. | Control Group: Participants were instructed to continue with their usual lifestyle without structured exercise or orthosis use. | IL-6 | NA | VAS, QUALEFFO-41, SF-36, spinal orthosis effects, functional mobility | 6 months |
| Roberts et al., 2024 | Platform Group: Participants performed squat exercises on a vibratory platform 3 times per week, on alternate days, for 12 weeks. The exercise involved squatting from approximately 10° to 60° of knee flexion, with standardized time and verbal encouragement for consistency. The vibration parameters were 35–40 Hz frequency, 4 mm amplitude, and 2.78–3.26 g acceleration. | Control Group: Daily oral diclofenac sodium (75 mg twice daily) without kinesitherapy. | IL-6, CRP, TNF-α, IL-17 | NA | Spinal pain, body fat percentage, waist circumference, blood pressure (systolic and diastolic), physical function (sit-to-stand tests), BASDAI, BASFI, WPAI, ASAS-HI | 12 weeks |
| Certainty Assessment | № of Patients (Musculoskeletal Pain) | Effect | Certainty | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| № of Studies | Study Design | Risk of Bias | In-consistency | In-directness | Imprecision | Other Considerations | Isokinetic Exercises | General Exercises | Absolute (95% CI) | |
| CRP (follow-up: range 4 weeks to 6 weeks) | ||||||||||
| 3 | randomized trials | not serious | not serious | not serious | not serious | none | 52 | 53 | MD 0.4 mg/L lower (0.44 lower to 0.36 lower) | ⨁⨁⨁⨁ High |
| TNF (follow-up: range 4 weeks to 6 weeks) | ||||||||||
| 3 | randomized trials | not serious | serious a | not serious | serious b | none | 52 | 53 | MD 4.24 pg/mL lower (5.13 lower to 3.36 lower) | ⨁⨁ Low a,b |
| IL-6 (follow-up: range 4 weeks to 6 weeks) | ||||||||||
| 4 | randomized trials | not serious | serious a | not serious | serious c | none | 73 | 73 | MD 1.59 pg/mL lower (2.61 lower to 0.56 lower) | ⨁⨁ Low a,c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo, C.N.; Wong, N.E.J.W.; Ho, S.; Ang, E.J.H.; Leung, B.P.L. Evaluating the Effects of Exercise on Inflammation Markers in Musculoskeletal Pain: A Systematic Review and Meta-Analysis. Sports 2025, 13, 168. https://doi.org/10.3390/sports13060168
Lo CN, Wong NEJW, Ho S, Ang EJH, Leung BPL. Evaluating the Effects of Exercise on Inflammation Markers in Musculoskeletal Pain: A Systematic Review and Meta-Analysis. Sports. 2025; 13(6):168. https://doi.org/10.3390/sports13060168
Chicago/Turabian StyleLo, Chi Ngai, Nicole Elizabeth Jing Wen Wong, Shina Ho, Elicia Jia Hui Ang, and Bernard Pui Lam Leung. 2025. "Evaluating the Effects of Exercise on Inflammation Markers in Musculoskeletal Pain: A Systematic Review and Meta-Analysis" Sports 13, no. 6: 168. https://doi.org/10.3390/sports13060168
APA StyleLo, C. N., Wong, N. E. J. W., Ho, S., Ang, E. J. H., & Leung, B. P. L. (2025). Evaluating the Effects of Exercise on Inflammation Markers in Musculoskeletal Pain: A Systematic Review and Meta-Analysis. Sports, 13(6), 168. https://doi.org/10.3390/sports13060168






