Can Bodybuilding Peak Week Manipulations Favorably Affect Muscle Size, Subcutaneous Thickness, and Related Body Composition Variables? A Case Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Subject
2.3. Procedures
2.3.1. Dietary Intake and Fluid Consumption
2.3.2. Body Composition, Bioimpedance, and Anthropometry
2.3.3. Subcutaneous Thickness and Muscle Thickness
2.3.4. Subjective Fullness
2.3.5. Urine Specific Gravity
2.4. Statistical Analyses
3. Results
3.1. Dietary Intake
3.2. Body Composition
3.3. Muscle Thickness
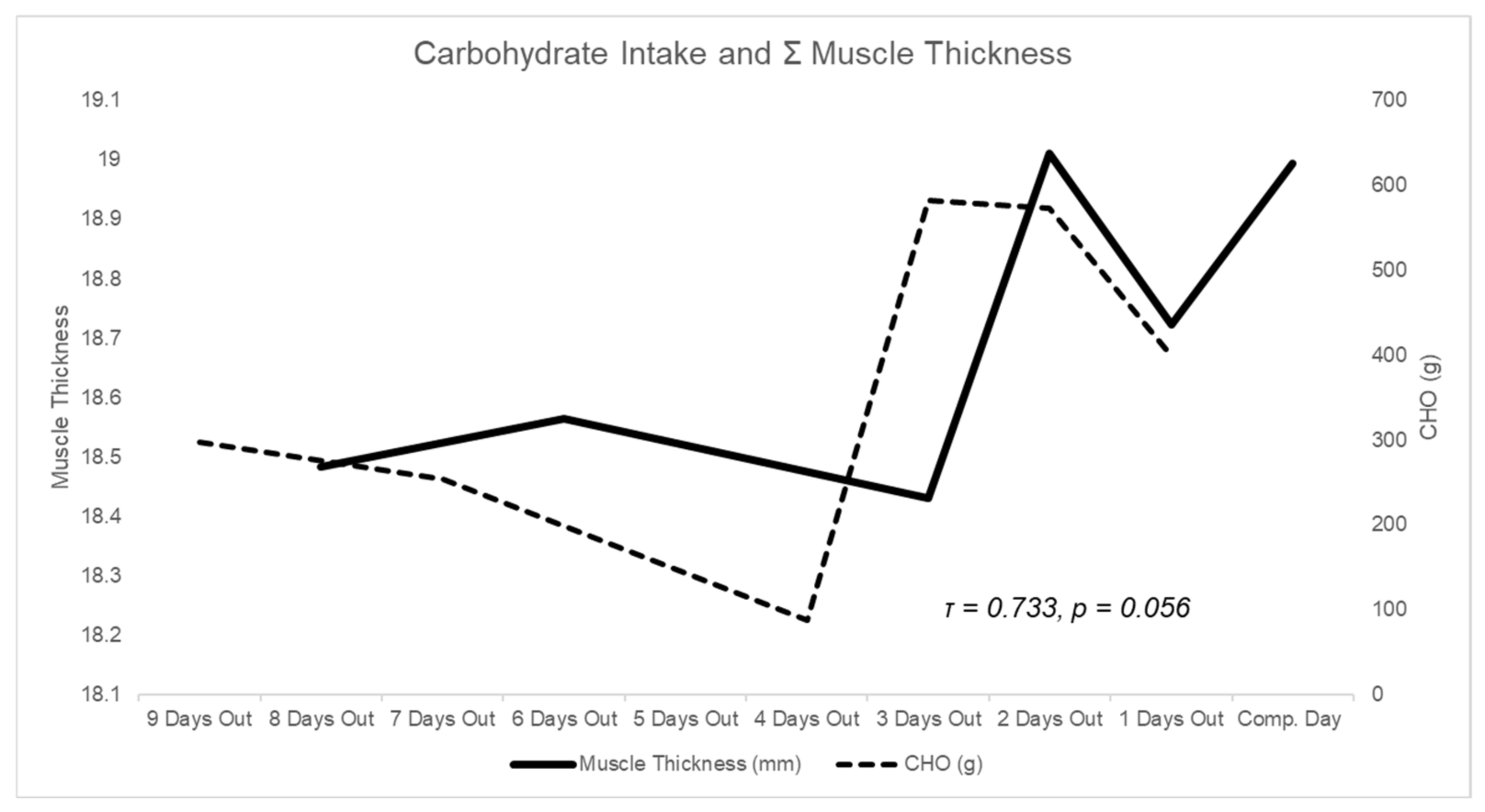
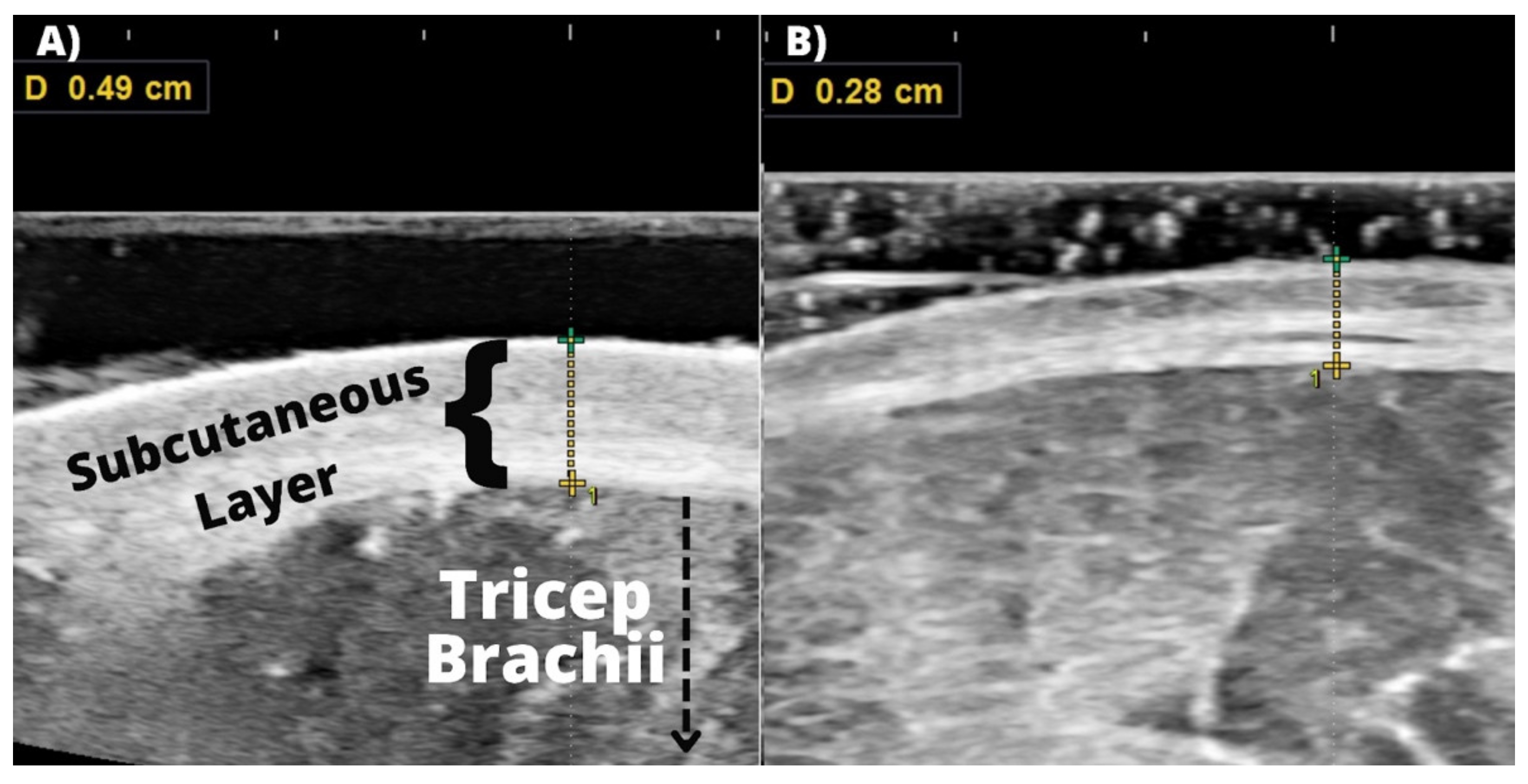
3.4. Subcutaneous Thickness
3.5. Body Fluids
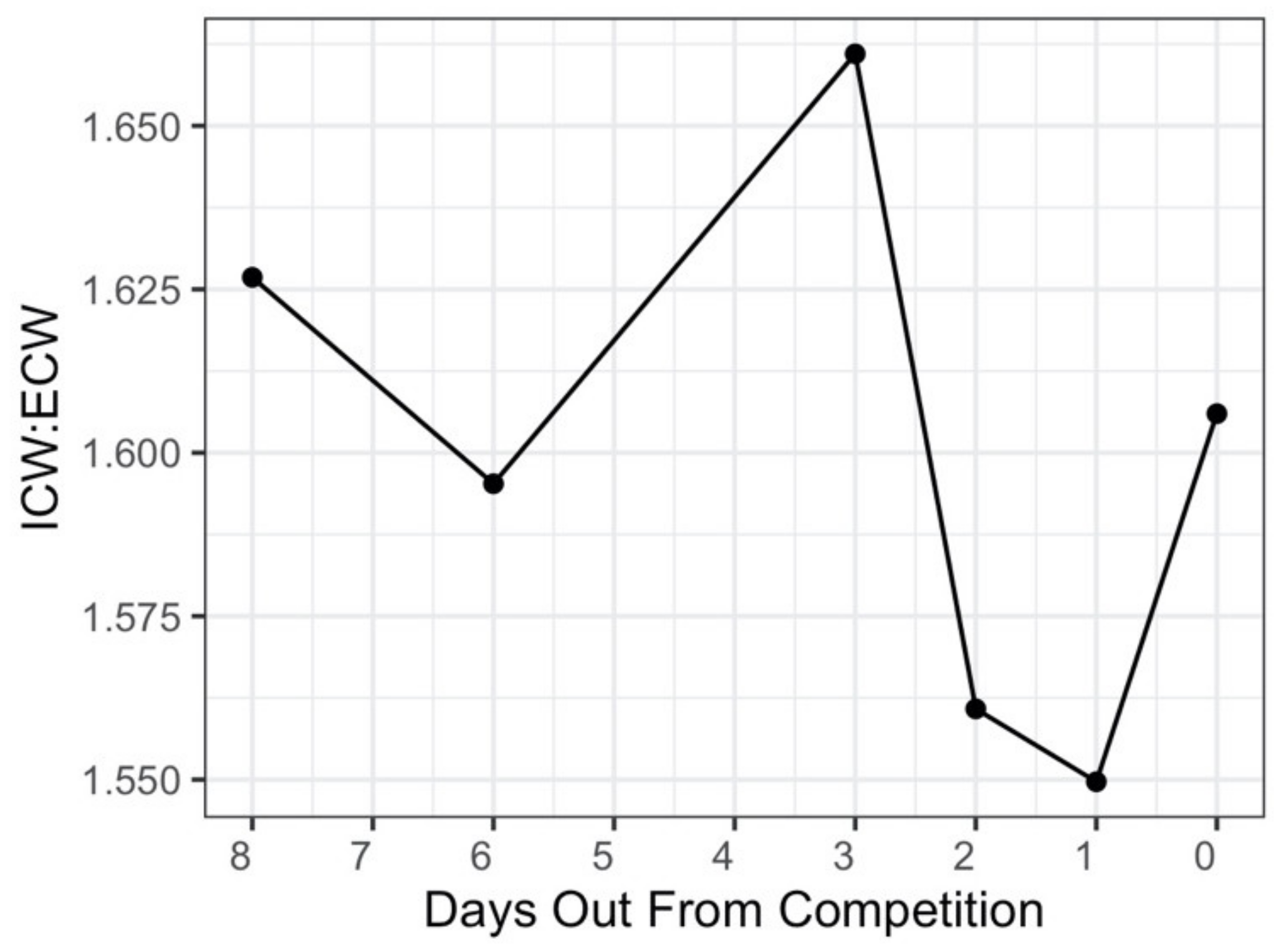
3.6. Raw Bioimpedance
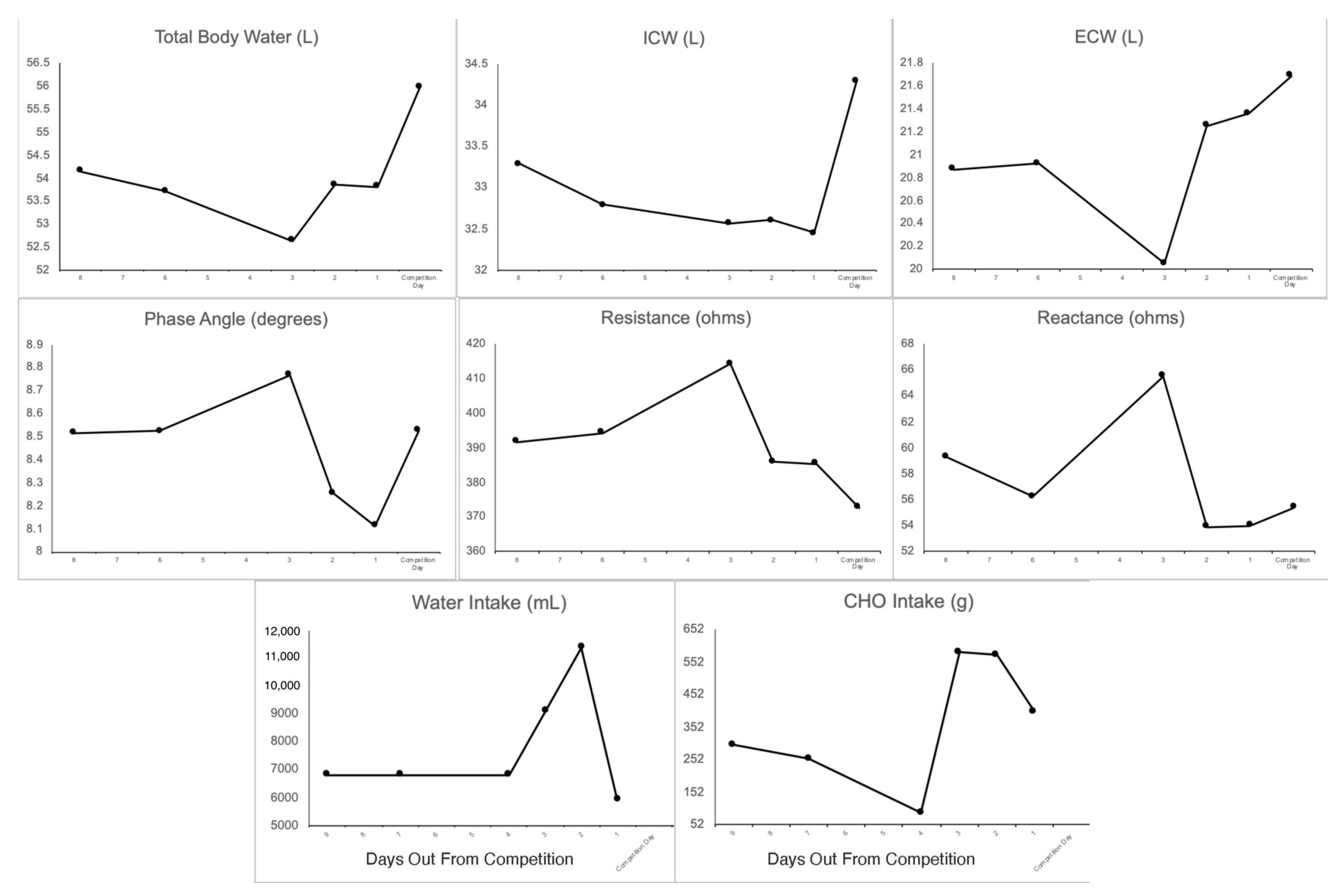
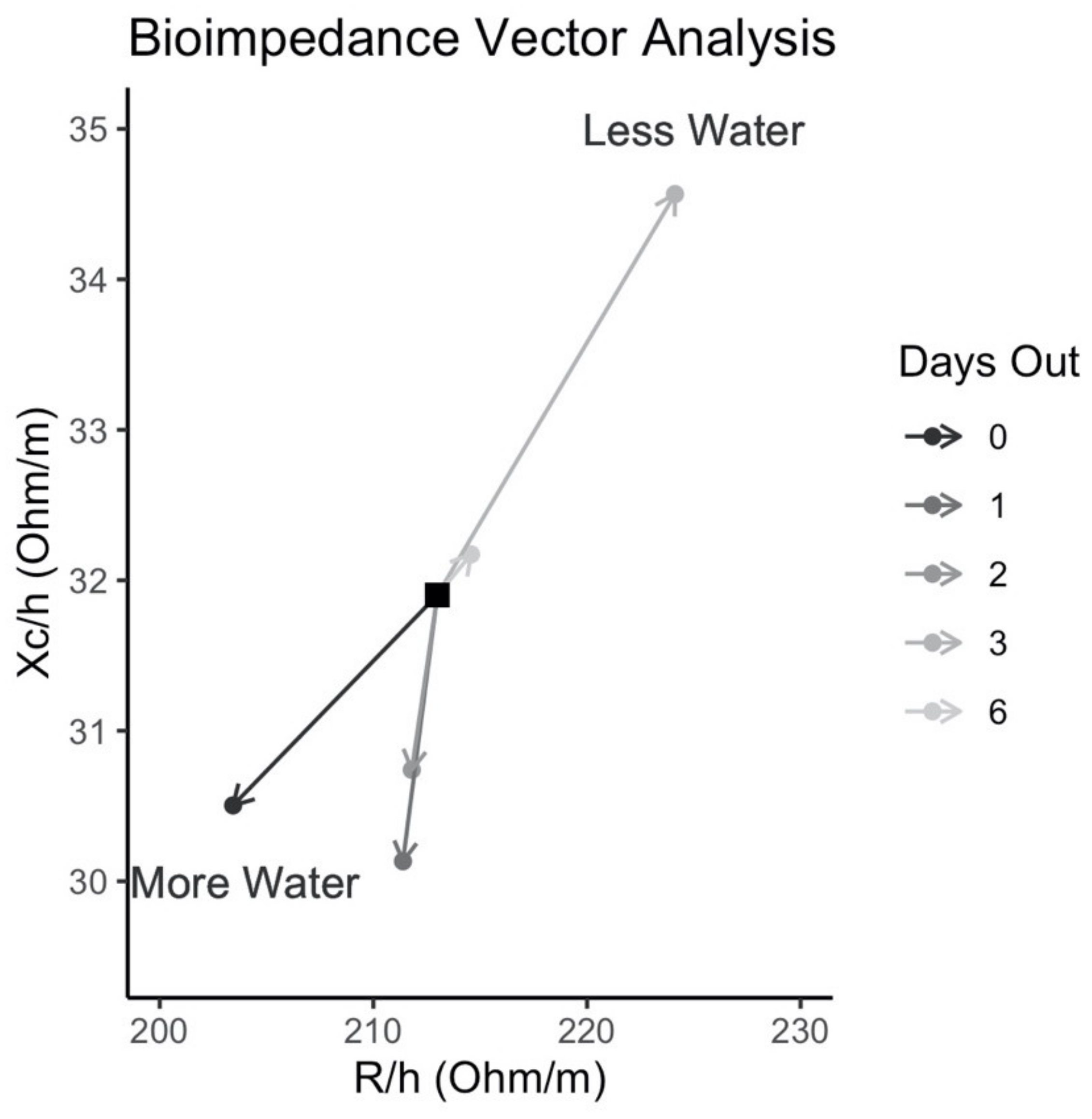
3.7. Hydration Status
3.8. Subjective Muscless Fullness and Arm Circumference
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iraki, J.; Fitschen, P.; Espinar, S.; Helms, E. Nutrition Recommendations for Bodybuilders in the Off-Season: A Narrative Review. Sports 2019, 7, 154. [Google Scholar] [CrossRef] [Green Version]
- Helms, E.R.; Aragon, A.A.; Fitschen, P.J. Evidence-based recommendations for natural bodybuilding contest preparation: Nutrition and supplementation. J. Int. Soc. Sports Nutr. 2014, 11, 20. [Google Scholar] [CrossRef] [Green Version]
- Helms, E.R.; Fitschen, P.J.; Aragon, A.A.; Cronin, J.; Schoenfeld, B.J. Recommendations for natural bodybuilding contest preparation: Resistance and cardiovascular training. J. Sports Med. Phys. Fitness 2014, 55, 164–178. [Google Scholar] [PubMed]
- Escalante, G.; Stevenson, S.W.; Barakat, C.; Aragon, A.A.; Schoenfeld, B.J. Peak week recommendations for bodybuilders: An evidence based approach. BMC Sports Sci. Med. Rehabil. 2021, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J.; Alto, A.; Grgic, J.; Tinsley, G.; Haun, C.T.; Campbell, B.I.; Escalante, G.; Sonmez, G.T.; Cote, G.; Francis, A.; et al. Alterations in Body Composition, Resting Metabolic Rate, Muscular Strength, and Eating Behavior in Response to Natural Bodybuilding Competition Preparation: A Case Study. J. Strength Cond. Res. 2020, 34, 3124–3138. [Google Scholar] [CrossRef] [PubMed]
- Nunes, J.P.; Araújo, J.P.M.; Ribeiro, A.S.; Campa, F.; Schoenfeld, B.J.; Cyrino, E.S.; Trindade, M.C.C. Changes in Intra-to-Extra-Cellular Water Ratio and Bioelectrical Parameters from Day-Before to Day-Of Competition in Bodybuilders: A Pilot Study. Sports 2022, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Chappell, A.J.; Simper, T.N. Nutritional Peak Week and Competition Day Strategies of Competitive Natural Bodybuilders. Sports 2018, 6, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spendlove, J.; Mitchell, L.; Gifford, J.; Hackett, D.; Slater, G.; Cobley, S.; O’Connor, H. Dietary Intake of Competitive Bodybuilders. Sports Med. 2015, 45, 1041–1063. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, L.; Hackett, D.; Gifford, J.; Estermann, F.; O’Connor, H. Do Bodybuilders Use Evidence-Based Nutrition Strategies to Manipulate Physique? Sports 2017, 5, 76. [Google Scholar] [CrossRef] [PubMed]
- Escalante, G.; Barakat, C.; Tinsley, G.; Schoenfeld, B. Nutrition, Training, Supplementation, and Performance-Enhancing Drug Practices of Male and Female Physique Athletes Peaking for Competition. J. Strength Cond. Res. 2022; ahead of print. [Google Scholar]
- De Moraes, W.M.A.M.; de Almeida, F.N.; Dos Santos, L.E.A.; Cavalcante, K.D.G.; Santos, H.O.; Navalta, J.W. Carbohydrate Loading Practice in Bodybuilders: Effects on Muscle Thickness, Photo Silhouette Scores, Mood States and Gastrointestinal Symptoms. J. Sports Sci. Med. 2019, 18, 772–779. [Google Scholar] [PubMed]
- Norton, L.; Baker, P. The Complete Contest Prep Guide (Male Cover); CreateSpace Independent Publishing Platform: Scotts Valley, CA, USA, 2018. [Google Scholar]
- Aceto, C. Championship Bodybuilding, 5th ed.; Nutramedia, 2001. [Google Scholar]
- Shiose, K.; Yamada, Y.; Motonaga, K.; Sagayama, H.; Higaki, Y.; Tanaka, H.; Takahashi, H. Segmental extracellular and intracellular water distribution and muscle glycogen after 72-h carbohydrate loading using spectroscopic techniques. J. Appl. Physiol. 2016, 121, 205–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gentil, P.; de Lira, C.A.B.; Paoli, A.; Dos Santos, J.A.B.; da Silva, R.D.T.; Junior, J.R.P. Nutrition, Pharmacological and Training Strategies Adopted by Six Bodybuilders: Case Report and Critical Review. Eur. J. Transl. Myol. 2017, 27, 6247. [Google Scholar] [CrossRef]
- Evenepoel, C.; Clevers, E.; Deroover, L.; Van Loo, W.; Matthys, C.; Verbeke, K. Accuracy of Nutrient Calculations Using the Consumer-Focused Online App MyFitnessPal: Validation Study. J. Med. Internet Res. 2020, 22, e18237. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, G.M.; Graybeal, A.J.; Moore, M.L.; Nickerson, B.S. Fat-free Mass Characteristics of Muscular Physique Athletes. Med. Sci. Sports Exerc. 2019, 51, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Baranauskas, M.N.; Johnson, K.E.; Juvancic-Heltzel, J.A.; Kappler, R.M.; Richardson, L.; Jamieson, S.; Otterstetter, R. Seven-site versus three-site method of body composition using BodyMetrix ultrasound compared to dual-energy X-ray absorptiometry. Clin. Physiol. Funct. Imaging 2015, 37, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Measurement Guide—Body Composition Analysis; ImpediMed Ltd.: Carlsbad, CA, USA, 2009. Available online: http://impedimed.com/wp-content/uploads/2019/07/SFB7MeasurementGuide.pdf (accessed on 7 June 2022).
- Müller, W.; Horn, M.; Fürhapter-Rieger, A.; Kainz, P.; Kröpfl, J.; Maughan, R.J.; Ahammer, H. Body composition in sport: A comparison of a novel ultrasound imaging technique to measure subcutaneous fat tissue compared with skinfold measurement. Br. J. Sports Med. 2013, 47, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Brossart, D.F.; Laird, V.C.; Armstrong, T.W. Interpreting Kendall’s Tau and Tau-U for single-case experimental designs. Cogent Psychol. 2018, 5, 1518687. [Google Scholar] [CrossRef]
- Casa, D.J.; Armstrong, L.E.; Hillman, S.K.; Montain, S.J.; Reiff, R.V.; Rich, B.S.E.; Roberts, W.O.; Stone, J.A. National athletic trainers’ association position statement: Fluid replacement for athletes. J. Athl. Train. 2000, 35, 212–224. [Google Scholar] [PubMed]
- Murray, B.; Rosenbloom, C. Fundamentals of glycogen metabolism for coaches and athletes. Nutr. Rev. 2018, 76, 243–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burke, L.M.; van Loon, L.J.C.; Hawley, J.A. Postexercise muscle glycogen resynthesis in humans. J. Appl. Physiol. 2017, 122, 1055–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef] [PubMed]
- Hultman, E.; Bergström, J. Muscle glycogen synthesis in relation to diet studied in normal subjects. Acta Med. Scand. 1967, 182, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Sherman, W.M.; Costill, D.L.; Fink, W.J.; Miller, J.M. Effect of Exercise-Diet Manipulation on Muscle Glycogen and Its Subsequent Utilization During Performance. Int. J. Sports Med. 1981, 2, 114–118. [Google Scholar] [CrossRef]
- Zderic, T.W.; Davidson, C.J.; Schenk, S.; Byerley, L.O.; Coyle, E.F. High-fat diet elevates resting intramuscular triglyceride concentration and whole body lipolysis during exercise. Am. J. Physiol. Metab. 2004, 286, E217–E225. [Google Scholar] [CrossRef] [Green Version]
- Van Loon, L.J.C. Use of intramuscular triacylglycerol as a substrate source during exercise in humans. J. Appl. Physiol. 2004, 97, 1170–1187. [Google Scholar] [CrossRef] [Green Version]
- Décombaz, J. Nutrition and recovery of muscle energy stores after exercise. Schweiz. Z. Sportmed. Sporttraumatol. 2003, 51, 31–38. [Google Scholar]
- Hector, A.J.; McGlory, C.; Damas, F.; Mazara, N.; Baker, S.K.; Phillips, S.M. Pronounced energy restriction with elevated protein intake results in no change in proteolysis and reductions in skeletal muscle protein synthesis that are mitigated by resistance exercise. FASEB J. 2017, 32, 265–275. [Google Scholar] [CrossRef] [Green Version]
- McGlory, C.; Devries, M.C.; Phillips, S.M. Skeletal muscle and resistance exercise training; the role of protein synthesis in recovery and remodeling. J. Appl. Physiol. 2017, 122, 541–548. [Google Scholar] [CrossRef]
- Longland, T.M.; Oikawa, S.Y.; Mitchell, C.J.; Devries, M.C.; Phillips, S.M. Higher compared with lower dietary protein during an energy deficit combined with intense exercise promotes greater lean mass gain and fat mass loss: A randomized trial. Am. J. Clin. Nutr. 2016, 103, 738–746. [Google Scholar] [CrossRef]
- Lukaski, H.C.; Diaz, N.V.; Talluri, A.; Nescolarde, L. Classification of Hydration in Clinical Conditions: Indirect and Direct Approaches Using Bioimpedance. Nutrients 2019, 11, 809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, J.E.; Hall, M.E. Guyton and Hall Textbook of Medical Physiology, 13th ed.; Elsevier: Philadelphia, PA, USA, 2016. [Google Scholar]
- Goforth, H.W., Jr.; Arnall, D.A.; Bennett, B.L.; Law, P.G. Persistence of supercompensated muscle glycogen in trained subjects after carbohydrate loading. J. Appl. Physiol. 1997, 82, 342–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bone, J.L.; Ross, M.L.; Tomcik, K.A.; Jeacocke, N.A.; Hopkins, W.G.; Burke, L.M. Manipulation of Muscle Creatine and Glycogen Changes Dual X-ray Absorptiometry Estimates of Body Composition. Med. Sci. Sports Exerc. 2017, 49, 1029–1035. [Google Scholar] [CrossRef] [Green Version]
- Bussau, V.A.; Fairchild, T.J.; Rao, A.; Steele, P.; Fournier, P.A. Carbohydrate loading in human muscle: An improved 1 day protocol. Eur. J. Appl. Physiol. 2002, 87, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Tarnopolsky, M.A.; Atkinson, S.A.; Phillips, S.; MacDougall, J.D. Carbohydrate loading and metabolism during exercise in men and women. J. Appl. Physiol. 1995, 78, 1360–1368. [Google Scholar] [CrossRef] [PubMed]
- Jeukendrup, A. A Step Towards Personalized Sports Nutrition: Carbohydrate Intake During Exercise. Sports Med. 2014, 44, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Jeukendrup, A.E.; McLaughlin, J. Carbohydrate Ingestion during Exercise: Effects on Performance, Training Adaptations and Trainability of the Gut. In Nestlé Nutrition Institute Workshop Series; 2011; pp. 1–17. Available online: https://www.karger.com/Article/Abstract/329268 (accessed on 7 June 2022).
- Wright, E.M.; Loo, D.D.F.; Hirayama, B.A. Biology of Human Sodium Glucose Transporters. Physiol. Rev. 2011, 91, 733–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeukendrup, A.E. Training the Gut for Athletes. Sports Med. 2017, 47, 101–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mullins, V.; Houtkooper, L.B.; Howell, W.H.; Going, S.B.; Brown, C.H. Nutritional status of U.S. elite female heptathletes during training. Int. J. Sport Nutr. Exerc. Metab. 2001, 11, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.; Orme, P.; Naughton, R.J.; Close, G.L.; Milsom, J.; Rydings, D.; O’Boyle, A.; Di Michele, R.; Louis, J.; Hambley, C.; et al. Energy Intake and Expenditure of Professional Soccer Players of the English Premier League: Evidence of Carbohydrate Periodization. Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Décombaz, J.; Fleith, M.; Hoppeler, H.; Kreis, R.; Boesch, C. Effect of diet on the replenishment of intramyocellular lipids after exercise. Eur. J. Nutr. 2000, 39, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Reale, R.; Slater, G.; Cox, G.R.; Dunican, I.C.; Burke, L.M. The Effect of Water Loading on Acute Weight Loss Following Fluid Restriction in Combat Sports Athletes. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 565–573. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.R.; Hu, F.B. Short sleep duration and weight gain: A systematic review. Obesity 2008, 16, 643–653. [Google Scholar] [CrossRef] [Green Version]
- Mayr, F.B.; Domanovits, H.; Laggner, A.N. Hypokalemic paralysis in a professional bodybuilder. Am. J. Emerg. Med. 2012, 30, 1324.e5–1324.e8. [Google Scholar] [CrossRef]
- Kingston, M. Dangers of ripping in body building. Intern. Med. J. 2011, 41, 708–709. [Google Scholar] [CrossRef] [PubMed]
- Matias, C.N.; Santos, D.; Júdice, P.B.; Magalhães, J.P.; Minderico, C.S.; Fields, D.A.; Lukaski, H.C.; Sardinha, L.B.; Silva, A.M. Estimation of total body water and extracellular water with bioimpedance in athletes: A need for athlete-specific prediction models. Clin. Nutr. 2016, 35, 468–474. [Google Scholar] [CrossRef]
- Moon, J.R. Body composition in athletes and sports nutrition: An examination of the bioimpedance analysis technique. Eur. J. Clin. Nutr. 2013, 67 (Suppl. S1), S54–S59. [Google Scholar] [CrossRef] [Green Version]
- Stratton, M.T.; Smith, R.W.; Harty, P.S.; Rodriguez, C.; Johnson, B.A.; Dellinger, J.R.; Williams, A.D.; White, S.J.; Benavides, M.L.; Tinsley, G.M. Longitudinal agreement of four bioimpedance analyzers for detecting changes in raw bioimpedance during purposeful weight gain with resistance training. Eur. J. Clin. Nutr. 2021, 75, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.W. The chemical composition of adipose tissue of man and mice. Q. J. Exp. Physiol. Cogn. Med. Sci. 1962, 47, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Probert, A.; Palmer, F.; Leberman, S. The Fine Line: An insight into ‘risky’ practices of male and female competitive bodybuilders. Ann. Leis. Res. 2007, 10, 272–290. [Google Scholar] [CrossRef]
- Haun, C.T.; Vann, C.G.; Roberts, B.M.; Vigotsky, A.D.; Schoenfeld, B.J.; Roberts, M.D. A Critical Evaluation of the Biological Construct Skeletal Muscle Hypertrophy: Size Matters but So Does the Measurement. Front. Physiol. 2019, 10, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, J.R.; Tobkin, S.E.; Roberts, M.D.; Dalbo, V.J.; Kerksick, C.M.; Bemben, M.G. Total body water estimations in healthy men and women using bioimpedance spectroscopy: A deuterium oxide comparison. Nutr. Metab. 2008, 5, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marles, R.J. Mineral nutrient composition of vegetables, fruits and grains: The context of reports of apparent historical declines. J. Food Compos. Anal. 2017, 56, 93–103. [Google Scholar] [CrossRef]
- Spritzler, F. How Cooking Affects the Nutrient Content of Foods; Healthline Media, Inc.: San Francisco, CA, USA, 2017. [Google Scholar]
| Nutrition (The Day before Assessments) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Days Out | Fat (g) | Carb (g) | Pro (g) | Kcal | Water (mL) | Na+ via Added Salt (mg) | Na+ from Food (mg) | ∑ Na+ (mg) | K+ from Food (mg) |
| Nine | 39 | 297 | 249 | 2535 | 6819 | 2300 | 1644 | 3944 | 1775 |
| Seven | 53 | 254 | 207 | 2321 | 6819 | 2300 | 1011 | 3311 | 1567 |
| Six | 132 | 88 | 217 | 2408 | 6819 | 2300 | 2647 | 4947 | 2103 |
| Five | 86 | 73 | 285 | 2206 | 6819 | 2300 | 5227 | 7527 | 2153 |
| Four | 125 | 88 | 253 | 2489 | 6819 | 2300 | 2926 | 5226 | 3375 |
| Three | 46 | 582 | 195 | 3522 | 9092 | 3059 | 1384 | 4443 | 6522 |
| Two | 55 | 573 | 219 | 3663 | 11,365 | 3059 | 2242 | 5301 | 5971 |
| One | 81 | 399 | 228 | 3237 | 5909 | 2300 | 1927 | 4227 | 5862 |
| DEXA | JP-7 via US | ||||||
|---|---|---|---|---|---|---|---|
| Body Mass (kg) | Body Fat % | FM (kg) | FFM (kg) | BMC (kg) | Soft LBM (kg) | Body Fat % | |
| Eight Days Out | 72.45 | 5.5 | 3.99 | 68.47 | 2.591 | 65.88 | n/a |
| Six Days Out | 73.45 | 6.0 | 4.41 | 69.05 | 2.606 | 66.44 | 3.6 |
| Three Days Out | 72.64 | 6.2 | 4.50 | 68.13 | 2.573 | 65.56 | 4.1 |
| Two Days Out | 73.18 | 5.6 | 4.10 | 69.08 | 2.610 | 66.47 | 4.2 |
| One Day Out | 73.45 | 5.4 | 3.97 | 69.49 | 2.608 | 66.88 | 3.8 |
| Competition Day | 73.55 | 6.3 | 4.63 | 68.91 | 2.591 | 66.32 | 3.6 |
| Distal Quad | Proximal Quad | Chest | Elbow Flexors | SUM | |
|---|---|---|---|---|---|
| Eight Days Out | 5.42 | 6.55 | 2.58 | 3.93 | 18.48 |
| Six Days Out | 5.39 | 6.52 | 2.56 | 4.10 | 18.56 |
| Three Days Out | 5.39 | 6.54 | 2.53 | 3.96 | 18.43 |
| Two Days Out | 5.70 | 6.78 | 2.53 | 4.00 | 19.01 |
| One Day Out | 5.52 | 6.66 | 2.60 | 3.94 | 18.72 |
| Competition Day | 5.60 | 6.63 | 2.81 | 3.95 | 18.99 |
| Chest (mm) | SubScap (mm) | Triceps (mm) | MidAx (mm) | Hip (SA) (mm) | Ab (mm) | Thigh (mm) | Total Sum | |
|---|---|---|---|---|---|---|---|---|
| Six Days Out | 3.7 | 5.4 | 3.0 | 4.3 | 3.8 | 5.0 | 4.6 | 29.8 |
| Three Days Out | 3.5 | 5.8 | 4.9 | 5.2 | 3.1 | 4.2 | 5.8 | 32.5 |
| Two Days Out | 3.5 | 6.0 | 3.5 | 5.0 | 3.8 | 6.0 | 5.1 | 32.9 |
| One Day Out | 3.4 | 5.8 | 3.1 | 4.1 | 3.8 | 5.6 | 4.8 | 30.6 |
| Competition Day | 3.3 | 5.9 | 2.8 | 4.0 | 4.2 | 4.9 | 4.5 | 29.6 |
| Refractometer | ||
|---|---|---|
| Eight Days Out | Upon Waking | 1.012 |
| Pre-Assessment | 1.018 | |
| Six Days Out | Upon Waking | 1.010 |
| Pre-Assessment | 1.012 | |
| Three Days Out | Upon Waking | 1.012 |
| Pre-Assessment | 1.011 | |
| Two Days Out | Upon Waking | n/a |
| Pre-Assessment | 1.012 | |
| One Day Out | Upon Waking | 1.010 |
| Pre-Assessment | 1.010 | |
| Competition Day | Upon Waking | 1.018 |
| Pre-Assessment | 1.012 | |
| Subjective Muscle Fullness (1–10) | R Arm Circumference (cm) | |
|---|---|---|
| Three Days Out | 1 | 40.5 |
| Two Days Out | 4.5 | 40.5 |
| One Day Out | 7 | 40.5 |
| Competition Day | 8 | 40.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barakat, C.; Escalante, G.; Stevenson, S.W.; Bradshaw, J.T.; Barsuhn, A.; Tinsley, G.M.; Walters, J. Can Bodybuilding Peak Week Manipulations Favorably Affect Muscle Size, Subcutaneous Thickness, and Related Body Composition Variables? A Case Study. Sports 2022, 10, 106. https://doi.org/10.3390/sports10070106
Barakat C, Escalante G, Stevenson SW, Bradshaw JT, Barsuhn A, Tinsley GM, Walters J. Can Bodybuilding Peak Week Manipulations Favorably Affect Muscle Size, Subcutaneous Thickness, and Related Body Composition Variables? A Case Study. Sports. 2022; 10(7):106. https://doi.org/10.3390/sports10070106
Chicago/Turabian StyleBarakat, Christopher, Guillermo Escalante, Scott W. Stevenson, Joshua T. Bradshaw, Andrew Barsuhn, Grant M. Tinsley, and Joseph Walters. 2022. "Can Bodybuilding Peak Week Manipulations Favorably Affect Muscle Size, Subcutaneous Thickness, and Related Body Composition Variables? A Case Study" Sports 10, no. 7: 106. https://doi.org/10.3390/sports10070106
APA StyleBarakat, C., Escalante, G., Stevenson, S. W., Bradshaw, J. T., Barsuhn, A., Tinsley, G. M., & Walters, J. (2022). Can Bodybuilding Peak Week Manipulations Favorably Affect Muscle Size, Subcutaneous Thickness, and Related Body Composition Variables? A Case Study. Sports, 10(7), 106. https://doi.org/10.3390/sports10070106






