Effect of 3 vs. 3 Soccer Small-Sided Game on Various Performance, Inflammatory, Muscle Damage and Hormonal Indicators in Semi-Professional Players
Abstract
:1. Introduction
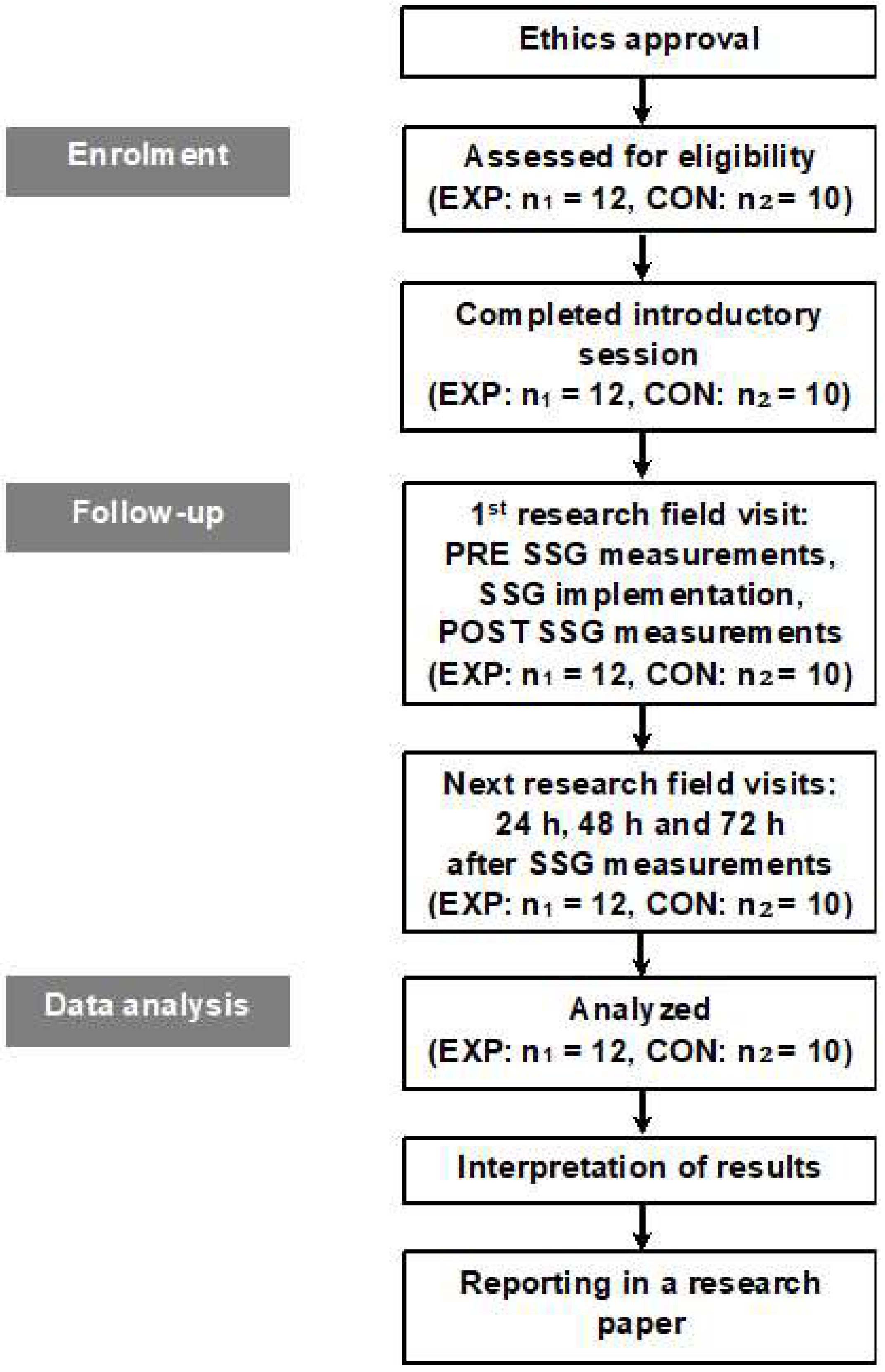
2. Materials and Methods
2.1. Subjects
2.2. Introductory Session
2.2.1. Anthropometric Characteristics
2.2.2. Maximum Heart Rate and Yo-Yo Intermittent Recovery Test Level 1
2.3. Experimental Procedure
2.3.1. SSG Training Format
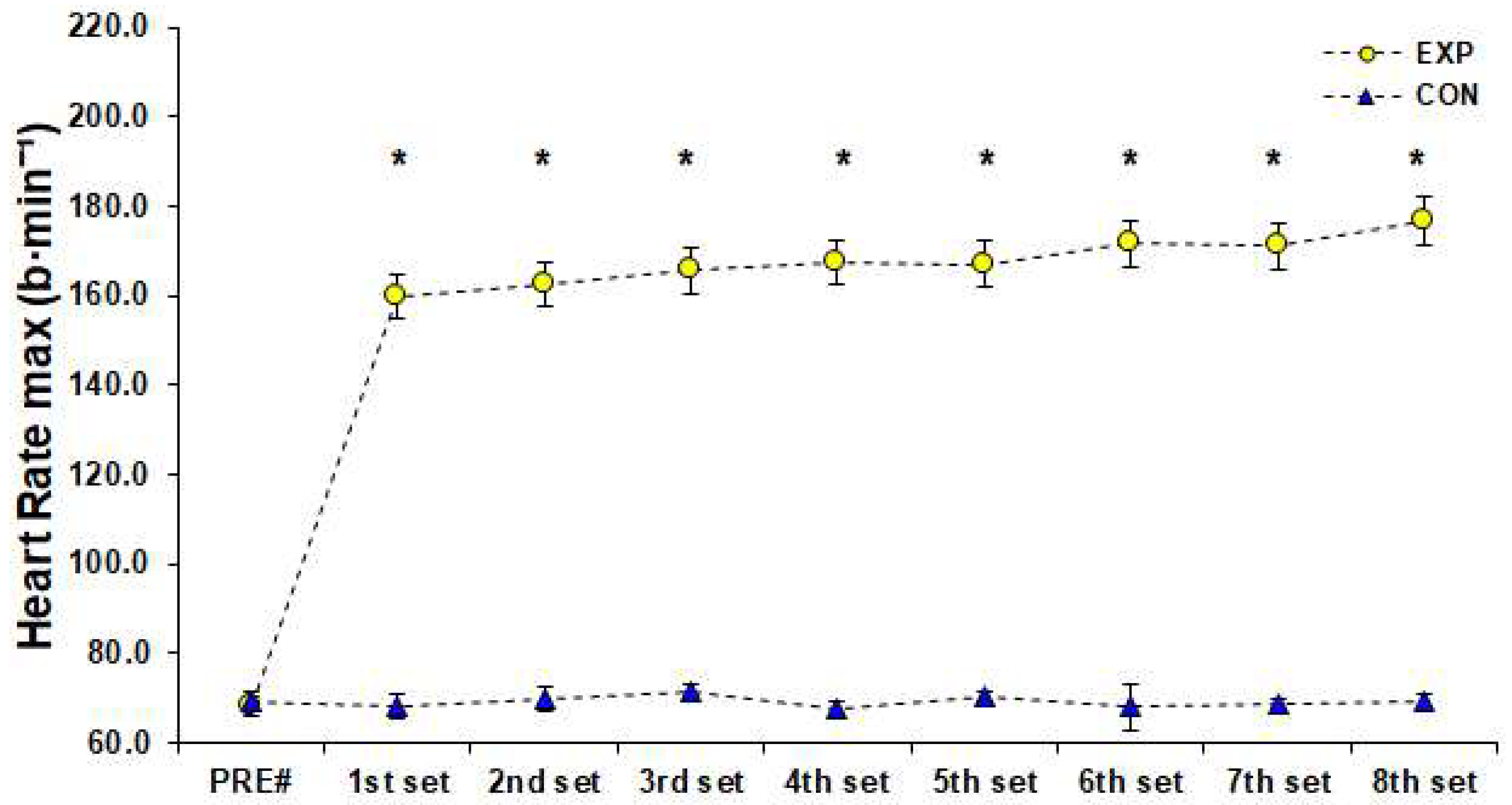
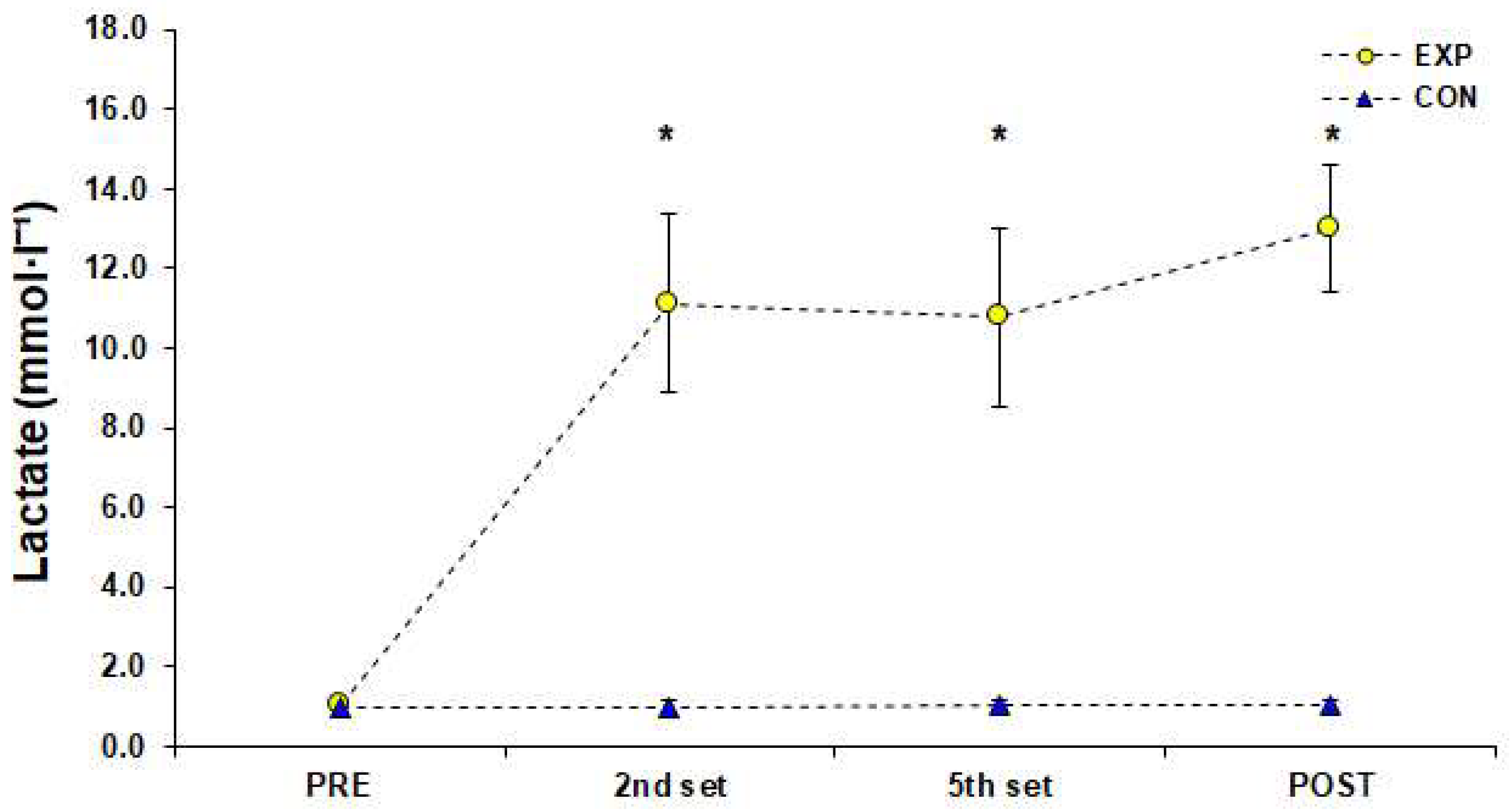
2.3.2. Heart Rate, Subjective Fatigue and Lactate Measurements
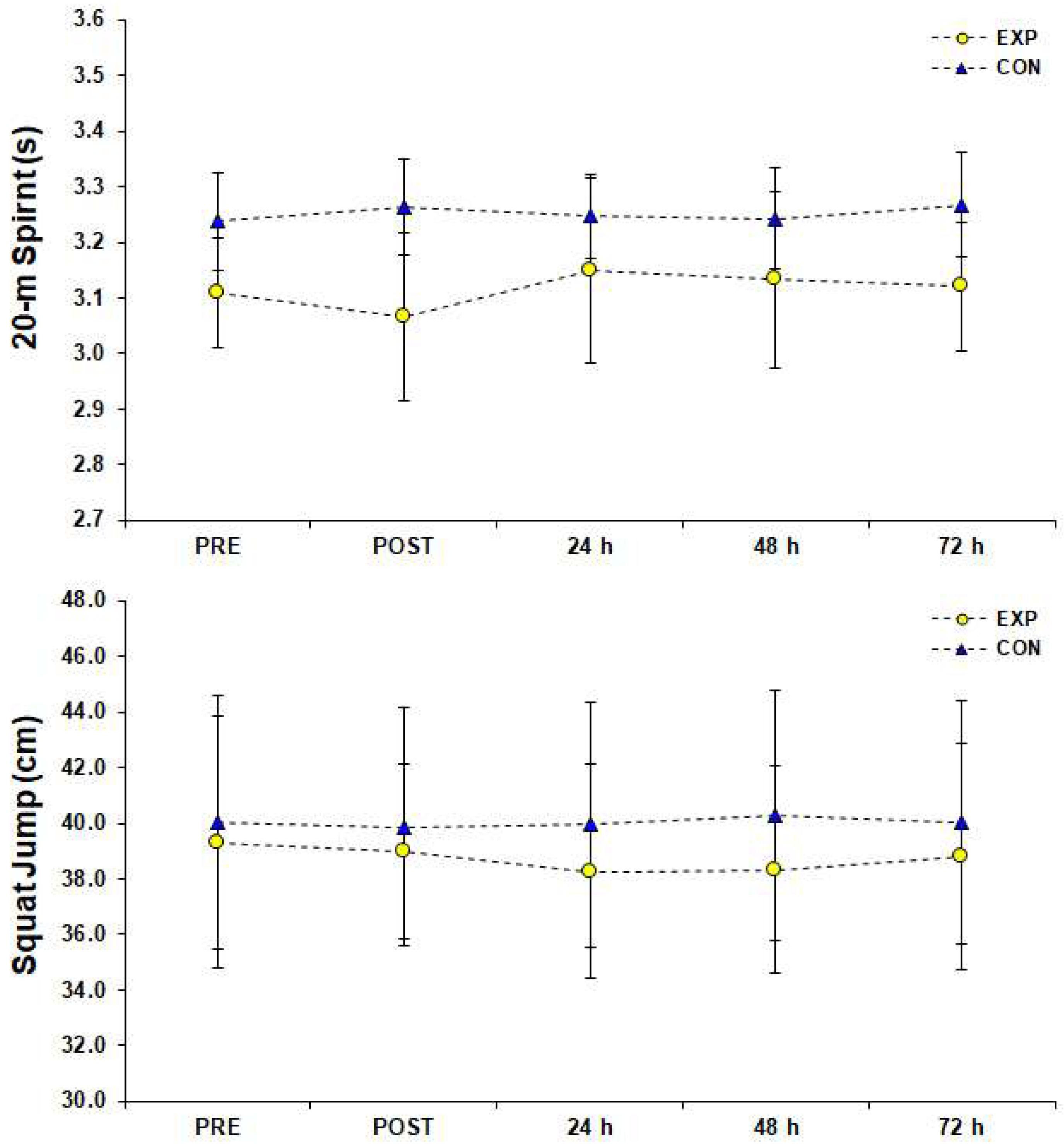
2.3.3. Jumping Test
2.3.4. Sprint Test
2.3.5. Hematological Measurements
2.4. Statistical Analysis
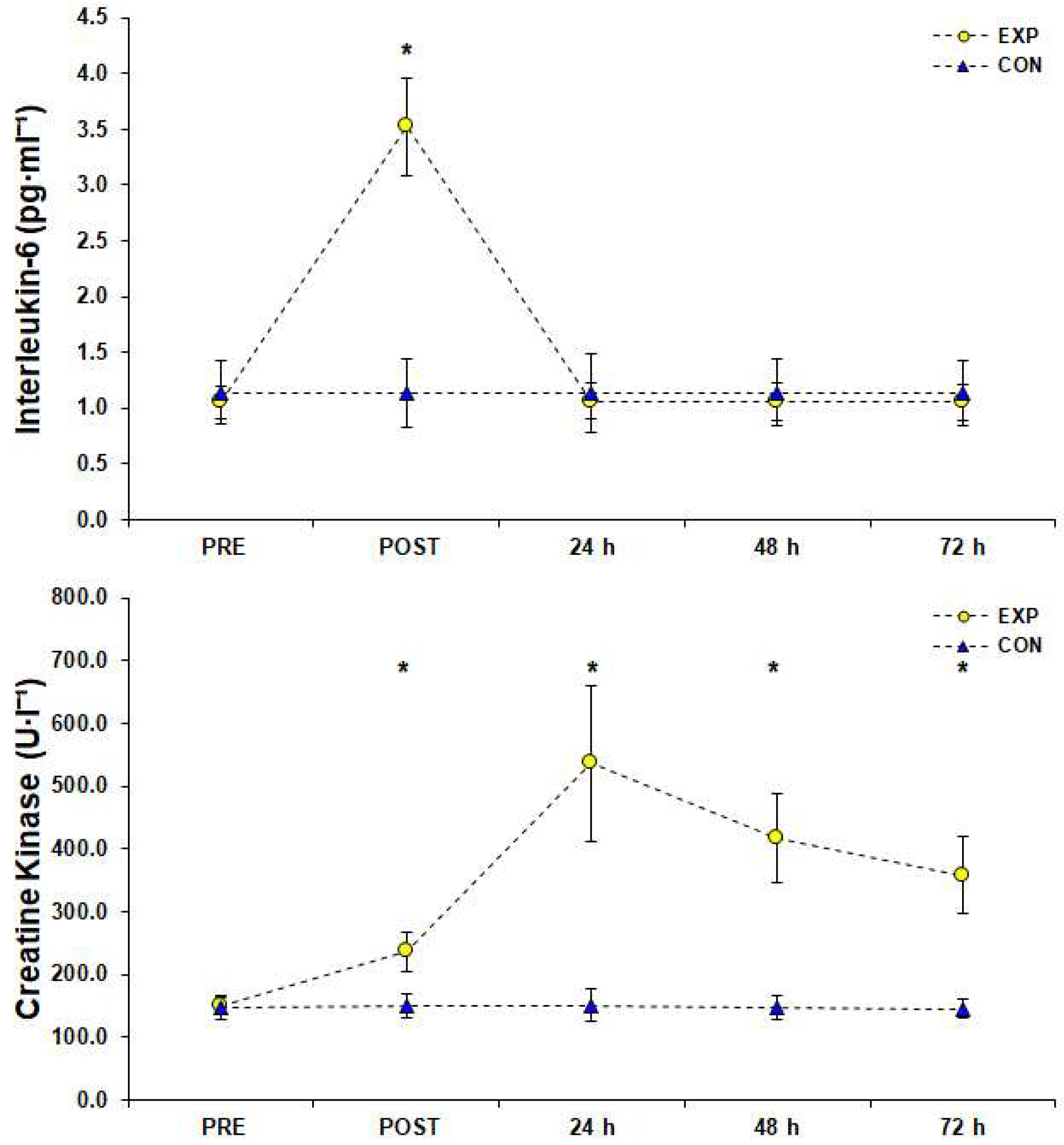
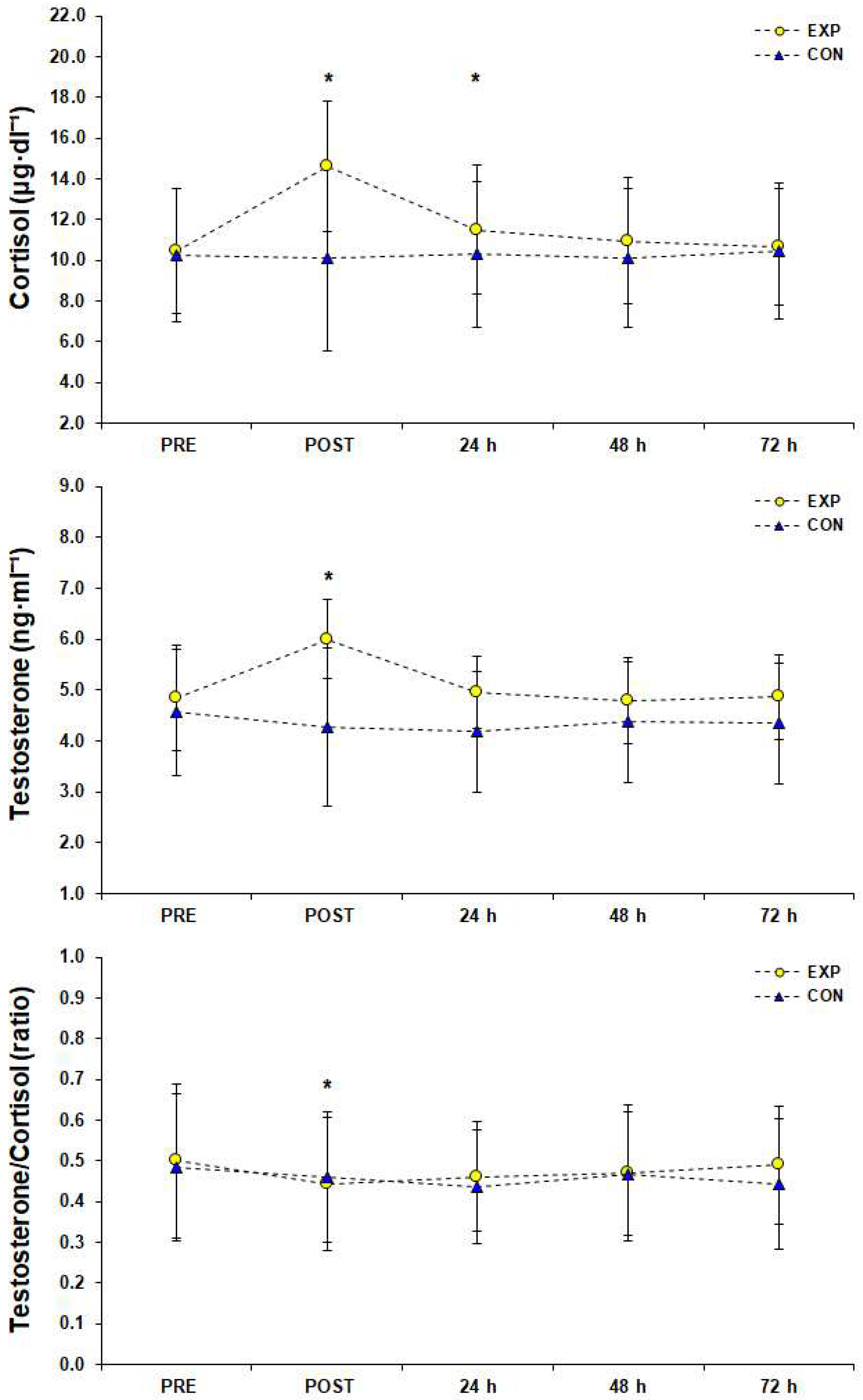
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Halouani, J.; Chtourou, H.; Gabbett, T.; Chaouachi, A.; Chamari, K. Small-sided games in team sports training: A brief review. J. Strength Cond. Res. 2014, 28, 3594–3618. [Google Scholar] [CrossRef] [PubMed]
- Hill-Haas, S.; Dawson, B.; Impellizzeri, F.; Coutts, A. Physiology of Small-Sided Games Training. Sport. Med. 2011, 41, 199–220. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, A.N.C.; Silva, P.; Rago, V.; Barreira, D.; Krustrup, P. Differences in strength and speed demands between 4v4 and 8v8 small-sided football games. J. Sport. Sci. 2016, 34, 2246–2254. [Google Scholar] [CrossRef] [PubMed]
- Casamichana, D.; Castellano, J. Time-motion, heart rate, perceptual and motor behaviour demands in small-sides soccer games: Effects of pitch size. J. Sport. Sci. 2010, 28, 1615–1623. [Google Scholar] [CrossRef] [PubMed]
- Hissey, S. Comparison of the Physical, Physiological and Perceptual Demands of Small-Sided Games and Match Play in Professional Football Players. Master’s Thesis, Edith Cowan University, Perth, Australia, 2014. [Google Scholar]
- Gaudino, P.; Iaia, F.M.; Alberti, G.; Hawkins, R.D.; Strudwick, A.J.; Gregson, W. Systematic bias between running speed and metabolic power data in elite soccer players: Influence of drill type. Int. J. Sport. Med. 2014, 35, 489–493. [Google Scholar] [CrossRef]
- Dellal, A.; Hill-Haas, S.; Lago-Penas, C.; Chamari, K. Small-sided games in soccer: Amateur vs. professional players’ physiological responses, physical, and technical activities. J. Strength Cond. Res. 2011, 25, 2371–2381. [Google Scholar] [CrossRef]
- Köklü, Y. A comparison of physiological responses to various intermittent and continuous small-sided games in young soccer players. J. Hum. Kinet. 2012, 31, 89–96. [Google Scholar] [CrossRef]
- Little, T. Optimizing the use of soccer drills for physiological development. Strength Cond. J. 2009, 31, 67–74. [Google Scholar] [CrossRef]
- Rampinini, E.; Impellizzeri, F.M.; Castagna, C.; Abt, G.; Chamari, K.; Sassi, A.; Marcora, S.M. Factors influencing physiological responses to small-sided soccer games. J. Sport. Sci. 2007, 25, 659–666. [Google Scholar] [CrossRef]
- Bekris, E.; Mylonis, E.; Sarakinos, A.; Gissis, I.; Anagnostakos, K.; Kombodieta, N. Supernumerary in small sided games 3Vs3 & 4Vs4. J. Phys. Educ. Sport 2012, 12, 398–406. [Google Scholar] [CrossRef]
- Brancaccio, P.; Maffulli, N.; Buonauro, R.; Limongelli, F.M. Serum Enzyme Monitoring in Sports Medicine. Clin. Sport. Med. 2008, 27, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Dolci, A.; Nanni, G.; Sisca, G.; Costantino, B.; Baldari, A.; Palaia, G.; Banfi, G. Leukocyte counts in professional football players. Haematologica 2003, 88, e156. [Google Scholar]
- Silva, J.R.; Ascensão, A.; Marques, F.; Seabra, A.; Rebelo, A.; Magalhães, J. Neuromuscular function, hormonal and redox status and muscle damage of professional soccer players after a high-level competitive match. Eur. J. Appl. Physiol. 2013, 113, 2193–2201. [Google Scholar] [CrossRef]
- Mougios, V. Reference intervals for serum creatine kinase in athletes. Br. J. Sport. Med. 2007, 41, 674–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruunsgaard, H.; Galbo, H.; Halkjaer-Kristensen, J.; Johansen, T.L.; MacLean, D.A.; Pedersen, B.K. Exercise-induced increase in serum inferleukin-6 in humans is related to muscle damage. J. Physiol. 1997, 499, 833–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croisier, J.L.; Camus, G.; Venneman, I.; Deby-Dupont, G.; Juchmès-Ferir, A.; Lamy, M.; Crielaard, J.M.; Deby, C.; Duchateau, J. Effects of Training on Exercise-Induced Muscle Damage and Interleukin 6 Production. Muscle Nerve 1999, 6, 208–212. [Google Scholar] [CrossRef]
- Reihmane, D.; Dela, F. Interleukin-6: Possible biological roles during exercise. Eur. J. Sport Sci. 2014, 14, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Banfi, G.; Marinelli, M.; Roi, G.S.; Agape, V. Usefulness of free testosterone/cortisol ratio during a season of elite speed skating athletes. Int. J. Sport. Med. 1993, 14, 373–379. [Google Scholar] [CrossRef]
- Vervoorn, C.; Quist, A.M.; Vermulst, L.J.M.; Erich, W.B.M.; De Vries, W.R.; Thijssen, J.H.H. The behaviour of the plasma free testosterone/cortisol ratio during a season of elite rowing training. Int. J. Sport. Med. 1991, 12, 257–263. [Google Scholar] [CrossRef]
- Ascensão, A.; Rebelo, A.; Oliveira, E.; Marques, F.; Pereira, L.; Magalhães, J. Biochemical impact of a soccer match—Analysis of oxidative stress and muscle damage markers throughout recovery. Clin. Biochem. 2008, 41, 841–851. [Google Scholar] [CrossRef]
- Bourdas, D.I.; Zacharakis, E.D. Impact of COVID-19 Lockdown on Physical Activity in a Sample of Greek Adults. Sports 2020, 8, 139. [Google Scholar] [CrossRef] [PubMed]
- Bourdas, D.I.; Zacharakis, E.D. Evolution of changes in physical activity over lockdown time: Physical activity datasets of four independent adult sample groups corresponding to each of the last four of the six COVID-19 lockdown weeks in Greece. Data Br. 2020, 32, 106301. [Google Scholar] [CrossRef] [PubMed]
- Warburton, D.; Jamnik, V.; Bredin, S.; Gledhill, N. The 2018 Physical Activity Readiness Questionnaire for Everyone (PAR-Q+) and electronic Physical Activity Readiness Medical Examination (ePARmed-X+). Health Fit. J. Can. 2018, 11, 31–34. [Google Scholar] [CrossRef]
- Bourdas, D.I.; Zacharakis, E.D.; Travlos, A.K.; Souglis, A.; Georgali, T.I.; Gofas, D.C.; Ktistakis, I.E.; Deltsidou, A. Impact of lockdown on smoking and sleeping in the early COVID-19 presence: Datasets of Greek Adults sample. Data Br. 2021, 39, 107480. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association Declaration of Helsinki, Ethical Principles for Scientific Requirements and Research Protocols. Bull. World Health Organ. 2013, 79, 373.
- Jackson, A.S.; Pollock, M.L. Generalized equations for predicting body density of men. Br. J. Nutr. 1978, 40, 497–504. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, B.; Pfeifer, C.; Kreitz, K.; Borowski, M.; Faldum, A.; Brand, S.M. The Yo-Yo intermittent tests: A systematic review and structured compendium of test results. Front. Physiol. 2018, 9, 870. [Google Scholar] [CrossRef] [Green Version]
- Castagna, C.; Impellizzeri, F.M.; Chamari, K.; Carlomagno, D.; Rampinini, E. Aerobic fitness and yo-yo continuous and intermittent tests performances in soccer players: A correlation study. J. Strength Cond. Res. 2006, 20, 320–325. [Google Scholar] [CrossRef] [Green Version]
- Bangsbo, J.; Iaia, F.M.; Krustrup, P. The Yo-Yo Intermittent Recovery Test: A Useful Tool for Evaluation of Physical Performance in Intermittent Sports. Sport. Med. 2008, 38, 37–51. [Google Scholar] [CrossRef]
- Bourdas, D.I.; Souglis, A.; Zacharakis, E.D.; Geladas, N.D.; Travlos, A.K. Meta-Analysis of Carbohydrate Solution Intake during Prolonged Exercise in Adults: From the Last 45+ Years’ Perspective. Nutrients 2021, 13, 4223. [Google Scholar] [CrossRef]
- Deltsidou, A.; Zarikas, V.; Mastrogiannis, D.; Kapreli, E.; Bourdas, D.; Raftopoulos, V.; Noula, M.; Lykeridou, K. Data on advanced glycation end-products concentrations and haemodynamic parameters following caffeine and nicotine consumption in nursing students. Data Br. 2020, 32, 106063. [Google Scholar] [CrossRef] [PubMed]
- Havenetidis, K.; Bourdas, D. Creatine supplementation: Effects on urinary excretion and anaerobic performance. J. Sport. Med. Phys. Fit. 2003, 43, 347–355. [Google Scholar]
- Borg, G.A.V. Psychophysical bases of perceived exertion. Med. Sci. Sport. Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Clemente, F.M.; Nikolaidis, P.T.; Rosemann, T.; Knechtle, B. Dose-response relationship between external load variables, body composition, and fitness variables in professional soccer players. Front. Physiol. 2019, 10, 443. [Google Scholar] [CrossRef] [PubMed]
- Roche Diagnostics GmbH Elecsys Testosteron, Method Sheet 05200067190V9.0. Available online: https://labogids.sintmaria.be/sites/default/files/files/testosteron_ii_2017-11_v9.pdf (accessed on 4 March 2022).
- Roche Diagnostics GmbH Elecsys Cortisol, Method Sheet 06687733190V4.0. Available online: http://labogids.sintmaria.be/sites/default/files/files/cortisol_ii_2017-05_v4.pdf (accessed on 4 March 2022).
- Keppel, G.; Wickens, T.D. Design and Analysis: A Researcher’s Handbook, 4th ed.; Pearson Education, Inc.: Upper Saddle River, NJ, USA, 2004; ISBN 978-0135159415. [Google Scholar]
- Meyers, L.S.; Gamst, G.; Guarino, A.J. Applied Multivariate Research Design and Interpretation, 3rd ed.; SAGE Publications Inc.: Los Angeles, CA, USA, 2016; ISBN 978-1506329765. [Google Scholar]
- Andersson, H.; Randers, M.; Heiner-Møller, A.; Krustrup, P.; Mohr, M. Elite female soccer players perform more high-intensity running when playing in international games compared with domestic league games. J. Strength Cond. Res. 2010, 24, 912–919. [Google Scholar] [CrossRef]
- Febbraio, M.A.; Pedersen, B.K. Muscle-derived interleukin-6: Mechanisms for activation and possible biological roles. FASEB J. 2002, 16, 1335–1347. [Google Scholar] [CrossRef]
- Ispirlidis, I.; Fatouros, I.G.; Jamurtas, A.Z.; Nikolaidis, M.G.; Michailidis, I.; Douroudos, I.; Margonis, K.; Chatzinikolaou, A.; Kalistratos, E.; Katrabasas, I.; et al. Time-course of changes in inflammatory and performance responses following a soccer game. Clin. J. Sport Med. 2008, 18, 423–431. [Google Scholar] [CrossRef]
- Naclerio, F.; Larumbe-Zabala, E.; Cooper, R.; Allgrove, J.; Earnest, C.P. A multi-ingredient containing carbohydrate, proteins L-glutamine and L-carnitine attenuates fatigue perception with no effect on performance, muscle damage or immunity in soccer players. PLoS ONE 2015, 10, e0125188. [Google Scholar] [CrossRef]
- Brancaccio, P.; Maffulli, N.; Limongelli, F.M. Creatine kinase monitoring in sport medicine. Br. Med. Bull. 2007, 81–82, 209–230. [Google Scholar] [CrossRef]
- Chen, T.C.; Hsieh, S.S. Effects of a 7-day eccentric training period on muscle damage and inflammation. Med. Sci. Sport. Exerc. 2001, 33, 1732–1738. [Google Scholar] [CrossRef]
- Byrne, C.; Twist, C.; Eston, R. Neuromuscular Function after Exercise-Induced Muscle Damage: Theoretical and Applied Implications. Sport. Med. 2004, 34, 49–69. [Google Scholar] [CrossRef] [PubMed]
- Howatson, G.; Milak, A. Exercise-induced muscle damage following a bout of sport specific repeated sprints. J. Strength Cond. Res. 2009, 23, 2419–2424. [Google Scholar] [CrossRef] [PubMed]
- Nedelec, M.; McCall, A.; Carling, C.; Legall, F.; Berthoin, S.; Dupont, G. Recovery in Soccer: Part I-post-match fatigue and time course of recovery. Sport. Med. 2012, 42, 997–1015. [Google Scholar] [CrossRef]
- Haneishi, K.; Fry, A.C.; Moore, C.A.; Schilling, B.K.; Yuhua, L.; Fry, M.D. Cortisol and stress responses during a game and practice in female collegiate soccer players. J. Strength Cond. Res. 2007, 21, 583–588. [Google Scholar] [CrossRef]
- Thorpe, R.; Sunderland, C. Muscle damage, endocrine, and immune marker response to a soccer match. J. Strength Cond. Res. 2012, 26, 2783–2790. [Google Scholar] [CrossRef]
- Derbré, F.; Vincent, S.; Maitel, B.; Jacob, C.; Delamarche, P.; Delamarche, A.; Zouhal, H. Androgen responses to sprint exercise in young men. Int. J. Sport. Med. 2010, 31, 291–297. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Marchitelli, L.; Gordon, S.E.; Harman, E.; Dziados, J.E.; Mello, R.; Frykman, P.; McCurry, D.; Fleck, S.J. Hormonal and growth factor responses to heavy resistance exercise protocols. J. Appl. Physiol. 1990, 69, 1442–1450. [Google Scholar] [CrossRef]
- Balachandar, R.; Bagepally, B.S.; Kalahasthi, R.; Haridoss, M. Blood lead levels and male reproductive hormones: A systematic review and meta-analysis. Toxicology 2020, 443, 152574. [Google Scholar] [CrossRef]
- Portaluppi, F.; Bagni, B.; degli Uberti, E.; Montanari, L.; Cavallini, R.; Trasforini, G.; Margutti, A.; Ferlini, M.; Zanella, M.; Parti, M. Circadian rhythms of atrial natriuretic peptide, renin, aldosterone, cortisol, blood pressure and heart rate in normal and hypertensive subjects. J. Hypertens. 1990, 8, 85–95. [Google Scholar] [CrossRef]
- Bourdas, D.I.; Zacharakis, E.D.; Travlos, A.K.; Souglis, A. Return to Basketball Play Following COVID-19 Lockdown. Sports 2021, 9, 81. [Google Scholar] [CrossRef]

| EXP | CON | |
|---|---|---|
| Age (year) | 22.42 ± 3.96 [20.17–24.66] | 22.20 ± 4.02 [19.71–24.70] |
| TP (year) | 6.08 ± 3.42 [4.15–8.02] | 6.00 ± 3.60 [3.77–8.22] |
| H (cm) | 182.41 ± 5.52 [179.28–185.53] | 180.15 ± 6.54 [176.10–184.20] |
| BM (kg) | † 82.68 ± 7.54 [78.41–86.95] | 80.23 ± 7.18 [75.78–84.68] |
| BM (kg) | ‡ 82.53 ± 7.55 [78.26–86.80] | - |
| BF (%) | † 15.31 ± 5.01 [12.48–18.15] | 13.04 ± 3.53 [10.85–15.23] |
| ADC (m) # | 2400.00 ± 574.87 [2074.74–2725.26] | 2464.00 ± 612.52 [2084.36–2843.64] |
| S (km·h−1) # | 17.29 ± 0.96 [16.75–17.84] | 17.40 ± 1.02 [16.77–18.03] |
| HRmax (b·min−1) # | 196 ± 6 [193–199] | 195 ± 5 [192–199] |
| HR (b·min−1) | † 68 ± 2 [67–70] | 69 ± 2 [68–70] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bekris, E.; Bourdas, D.I.; Mylonis, E.; Ispirlidis, I.; Zacharakis, E.D.; Katis, A. Effect of 3 vs. 3 Soccer Small-Sided Game on Various Performance, Inflammatory, Muscle Damage and Hormonal Indicators in Semi-Professional Players. Sports 2022, 10, 102. https://doi.org/10.3390/sports10070102
Bekris E, Bourdas DI, Mylonis E, Ispirlidis I, Zacharakis ED, Katis A. Effect of 3 vs. 3 Soccer Small-Sided Game on Various Performance, Inflammatory, Muscle Damage and Hormonal Indicators in Semi-Professional Players. Sports. 2022; 10(7):102. https://doi.org/10.3390/sports10070102
Chicago/Turabian StyleBekris, Evangelos, Dimitrios I. Bourdas, Eleftherios Mylonis, Ioannis Ispirlidis, Emmanouil D. Zacharakis, and Athanasios Katis. 2022. "Effect of 3 vs. 3 Soccer Small-Sided Game on Various Performance, Inflammatory, Muscle Damage and Hormonal Indicators in Semi-Professional Players" Sports 10, no. 7: 102. https://doi.org/10.3390/sports10070102
APA StyleBekris, E., Bourdas, D. I., Mylonis, E., Ispirlidis, I., Zacharakis, E. D., & Katis, A. (2022). Effect of 3 vs. 3 Soccer Small-Sided Game on Various Performance, Inflammatory, Muscle Damage and Hormonal Indicators in Semi-Professional Players. Sports, 10(7), 102. https://doi.org/10.3390/sports10070102






