Vascular Function in Norwegian Female Elite Runners: A Cross-Sectional, Controlled Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Participants
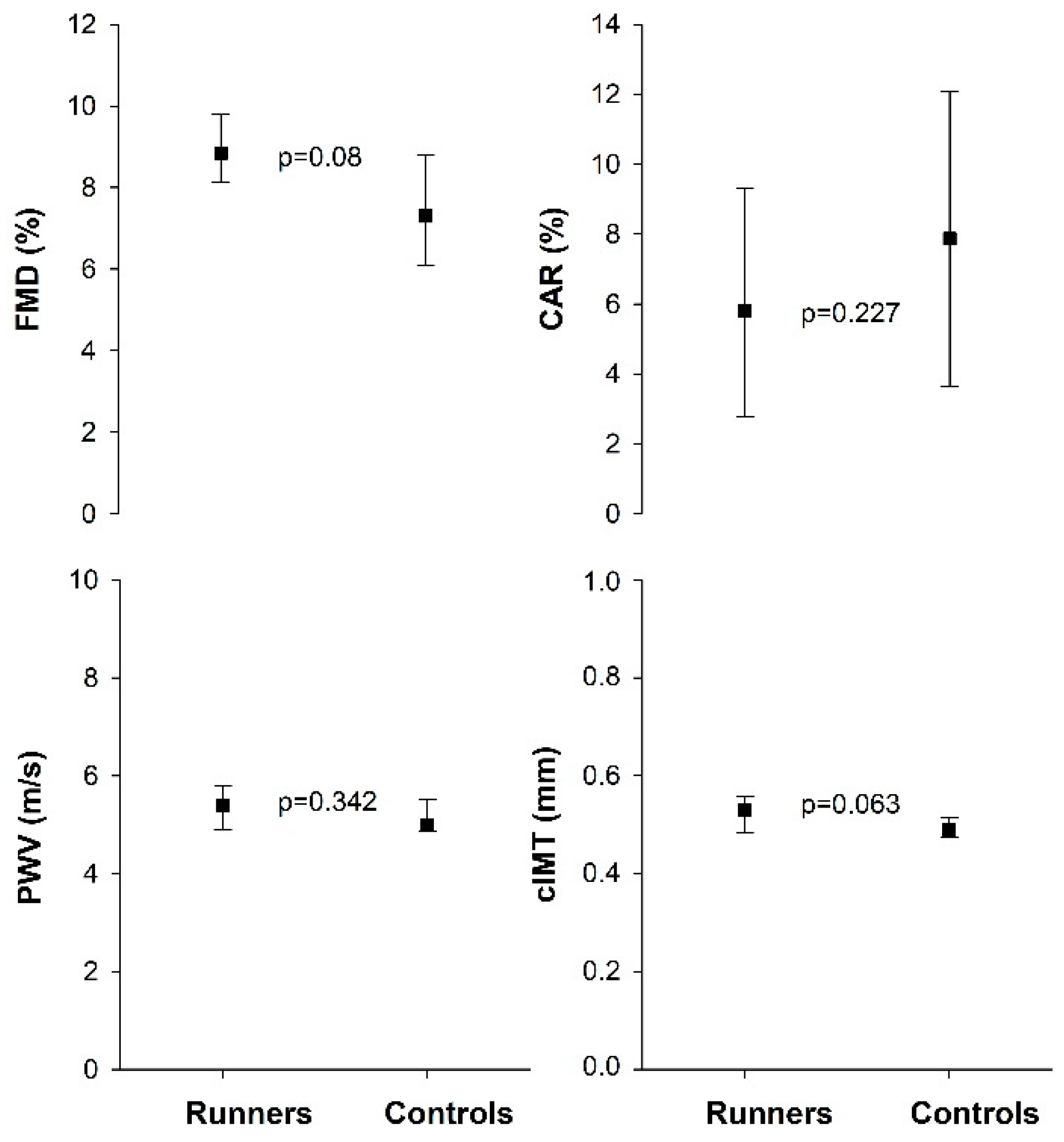
3.2. Vascular Function and Vascular Morphology
3.3. Endocrine and Metabolic Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Powell, K.E.; Thompson, P.D.; Caspersen, C.J.; Kendrick, J.S. Physical activity and the incidence of coronary heart disease. Annu. Rev. Public Health 1987, 8, 253–287. [Google Scholar] [CrossRef] [PubMed]
- Kasikcioglu, E.; Oflaz, H.; Kasikcioglu, H.A.; Kayserilioglu, A.; Umman, S.; Meric, M. Endothelial Flow-Mediated Dilatation and Exercise Capacity in Highly Trained Endurance Athletes. Tohoku J. Exp. Med. 2005, 205, 45–51. [Google Scholar] [CrossRef]
- Moe, I.T.; Hoven, H.; Hetland, E.V.; Rognmo, Ø.; Slørdahl, S.A. Endothelial function in highly endurance-trained and sedentary, healthy young women. Vasc. Med. 2005, 10, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Green, D.J.; Rowley, N.; Spence, A.; Carter, H.; Whyte, G.; George, K.; Naylor, L.H.; Cable, N.T.; Dawson, E.A.; Thijssen, D.H. Why isn’t flow-mediated dilation enhanced in athletes? Med. Sci. Sports Exerc. 2013, 45, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Lanser, E.M.B.S.; Zach, K.N.M.D.; Hoch, A.Z.D.O. The Female Athlete Triad and Endothelial Dysfunction. PMR 2011, 3, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.L.M.D.; Hoch, A.Z.D.O. The Female Runner: Gender Specifics. Clin. Sports Med. 2010, 29, 477–498. [Google Scholar] [CrossRef] [PubMed]
- Mountjoy, M.; Sundgot-Borgen, J.; Burke, L.; Carter, S.; Constantini, N.; Lebrun, C.; Meyer, N.; Sherman, R.; Steffen, K.; Budgett, R.; et al. The IOC consensus statement: Beyond the Female Athlete Triad--Relative Energy Deficiency in Sport (RED-S). Br. J. Sports Med. 2014, 48, 491–497. [Google Scholar] [CrossRef]
- Mountjoy, M.; Sundgot-Borgen, J.; Burke, L.; Ackerman, K.E.; Blauwet, C.; Constantini, N.; Lebrun, C.; Lundy, B.; Melin, A.; Meyer, N.; et al. International Olympic Committee (IOC) Consensus Statement on Relative Energy Deficiency in Sport (RED-S): 2018 Update. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 316–331. [Google Scholar] [CrossRef] [PubMed]
- Rickenlund, A.; Eriksson, M.J.; Schenck-Gustafsson, K.; Hirschberg, A.L. Amenorrhea in Female Athletes Is Associated with Endothelial Dysfunction and Unfavorable Lipid Profile. J. Clin. Endocrinol. Metab. 2005, 90, 1354–1359. [Google Scholar] [CrossRef]
- Hoch, A.Z.; Papanek, P.; Szabo, A.; Widlansky, M.E.; Schimke, J.E.; Gutterman, D.D. Association Between the Female Athlete Triad and Endothelial Dysfunction in Dancers. Clin. J. Sport Med. 2011, 21, 119–125. [Google Scholar] [CrossRef]
- Mendelsohn, M.E. Protective effects of estrogen on the cardiovascular system. Am. J. Cardiol. 2002, 89, 12–17. [Google Scholar] [CrossRef]
- Thijssen, D.H.; Bruno, R.M.; van Mil, A.C.; Holder, S.M.; Faita, F.; Greyling, A.; Zock, P.L.; Taddei, S.; Deanfield, J.E.; Luscher, T.; et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur. Heart J. 2019, 40, 2534–2547. [Google Scholar] [CrossRef] [PubMed]
- Van Mil, A.C.C.M.; Pouwels, S.; Wilbrink, J.; Warle, M.C.; Thijssen, D.H.J. Carotid Artery Reactivity Predicts Events in Peripheral Arterial Disease Patients. Ann. Surg. 2019, 269, 767–773. [Google Scholar] [CrossRef] [PubMed]
- AtCorMedical. Technical Notes. 2014. Available online: http://atcormedical.com/technicalnotes.html (accessed on 24 January 2022).
- Melin, A.; Tornberg, Å.B.; Skouby, S.; Faber, J.; Ritz, C.; Sjödin, A.; Sundgot-Borgen, J. The LEAF questionnaire: A screening tool for the identification of female athletes at risk for the female athlete triad. Br. J. Sports Med. 2014, 48, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Miele, E.M.; Headley, S.A.E. The Effects of Chronic Aerobic Exercise on Cardiovascular Risk Factors in Persons with Diabetes Mellitus. Curr. Diab. Rep. 2017, 17, 97. [Google Scholar] [CrossRef]
- Beck, D.T.; Martin, J.S.; Casey, D.P.; Braith, R.W. Exercise training improves endothelial function in resistance arteries of young prehypertensives. J. Hum. Hypertens. 2014, 28, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Franzoni, F.; Ghiadoni, L.; Galetta, F.; Plantinga, Y.; Lubrano, V.; Huang, Y.; Salvetti, G.; Regoli, F.; Taddei, S.; Santoro, G.; et al. Physical activity, plasma antioxidant capacity, and endothelium-dependent vasodilation in young and older men. Am. J. Hypertens. 2005, 18, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt, C.R.; Izar, M.C.; Schwerz, V.L.; Póvoa, R.M.; Fonseca, H.A.; Fonseca, M.I.; Bianco, H.T.; Franca, C.N.; Ferreira, C.E.; Fonseca, F.A. Effects of high-intensity training of professional runners on myocardial hypertrophy and subclinical atherosclerosis. PLoS ONE 2016, 11, e0166009. [Google Scholar] [CrossRef]
- Tanriverdi, H.; Evrengul, H.; Tanriverdi, S.; Turgut, S.; Akdag, B.; Kaftan, H.A.; Semiz, E. Improved Endothelium Dependent Vasodilation in Endurance Athletes and Its Relation With ACE I/D Polymorphism. Circ. J. 2005, 69, 1105–1110. [Google Scholar] [CrossRef][Green Version]
- Pyke, K.E.; Dwyer, E.M.; Tschakovsky, M.E. Impact of controlling shear rate on flow-mediated dilation responses in the brachial artery of humans. J. Appl. Physiol. 2004, 97, 499–508. [Google Scholar] [CrossRef]
- Van Mil, A.C.; Hartman, Y.; Van Oorschot, F.; Heemels, A.; Bax, N.; Dawson, E.A.; Hopkins, N.; Hopman, M.T.; Green, D.J.; Oxborough, D.L.; et al. Correlation of carotid artery reactivity with cardiovascular risk factors and coronary artery vasodilator responses in asymptomatic, healthy volunteers. J. Hypertens. 2017, 35, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Kardara, D.; Anastasakis, A.; Baou, K.; Terentes-Printzios, D.; Tousoulis, D.; Stefanadis, C. Arterial Stiffness and Wave Reflections in Marathon Runners. Am. J. Hypertens. 2010, 23, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Namgoong, H.; Lee, D.; Hwang, M.-H.; Lee, S. The relationship between arterial stiffness and maximal oxygen consumption in healthy young adults. J. Exerc. Sci. Fit. 2018, 16, 73–77. [Google Scholar] [CrossRef]
- Bjarnegård, N.; Länne, T.; Cinthio, M.; Ekstrand, J.; Hedman, K.; Nylander, E.; Henriksson, J. Vascular characteristics in young women—Effect of extensive endurance training or a sedentary lifestyle. Acta Physiol. 2018, 223, e13041. [Google Scholar] [CrossRef] [PubMed]
- Denham, J.; Brown, N.J.; Tomaszewski, M.; Williams, B.; O’Brien, B.J.; Charchar, F.J. Aortic augmentation index in endurance athletes: A role for cardiorespiratory fitness. Eur. J. Appl. Physiol. 2016, 116, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, Z.M.; Phillipou, A.; Castle, D.J.; Eikelis, N.; Lambert, E.A. Arterial stiffness in underweight and weight-restored anorexia nervosa. Psychophysiology 2021, 58, e13913. [Google Scholar] [CrossRef] [PubMed]
- Birmingham, C.L.; Lear, S.A.; Kenyon, J.; Chan, S.Y.M.; Mancini, G.B.J.; Frohlich, J. Coronary atherosclerosis in anorexia nervosa. Int. J. Eat. Disord. 2003, 34, 375–377. [Google Scholar] [CrossRef]
- Logue, D.M.; Madigan, S.M.; Melin, A.; Delahunt, E.; Heinen, M.; Donnell, S.J.; Corish, C.A. Low energy availability in athletes 2020: An updated narrative review of prevalence, risk, within-day energy balance, knowledge, and impact on sports performance. Nutrients 2020, 12, 835. [Google Scholar] [CrossRef]
- Melin, A.K.; Heikura, I.A.; Tenforde, A.; Mountjoy, M. Energy Availability in Athletics: Health, Performance, and Physique. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 152–164. [Google Scholar] [CrossRef]
- Pollock, N.; Grogan, C.; Perry, M.; Pedlar, C.; Cooke, K.; Morrissey, D.; Dimitriou, L. Bone Mineral Density And Aspects Of The Female Athlete Triad In Elite Endurance Runners: 1562 Board #218 June 2 11:00 AM–12:30 PM. Med. Sci. Sports Exerc. 2010, 42, 318–319. [Google Scholar] [CrossRef]
- Melin, A.; Tornberg, Å.B.; Skouby, S.; Møller, S.S.; Sundgot-Borgen, J.; Faber, J.; Sidelmann, J.J.; Aziz, M.; Sjödin, A. Energy availability and the female athlete triad in elite endurance athletes. Scand. J. Med. Sci. Sports 2015, 25, 610–622. [Google Scholar] [CrossRef] [PubMed]
- Soleimany, G.; Dadgostar, H.; Lotfian, S.; Moradi-Lakeh, M.; Dadgostar, E.; Movaseghi, S. Bone mineral changes and cardiovascular effects among female athletes with chronic menstrual dysfunction. Asian J. Sports Med. 2012, 3, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Santiago, K.A.; Abutalib, Z.; Temme, K.E.; Hulme, A.; Goolsby, M.A.; Esopenko, C.L.; Casey, E.K. Menstrual Irregularity, Hormonal Contraceptive Use, and Bone Stress Injuries in Collegiate Female Athletes in the United States. PMR 2021, 13, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
| Groups | Runners (n = 16) | Controls (n = 17) | p-Value |
|---|---|---|---|
| Age (yr) | 27.0 (24.3–30.0) | 26.0 (24.0–27.5) | 0.196 |
| Height (cm) | 169 (164–176) | 172 (166–178) | 0.328 |
| Weight (kg) | 55.9 (54.3–60.7) | 63.0 (60.8–70.2) | <0.001 |
| BMI (km/m2) | 19.9 (18.9–21.0) | 22.0 (20.6–24.0) | <0.001 |
| SP (mmHg) | 110 (110–118) | 110 (110–115) | 0.789 |
| DP (mmHg) | 70 (70–78) | 70 (70–70) | 0.542 |
| VO2max (mL·kg−1·min−1) | 64.3 (62.5–66.7) | 44.8 (41.8–45.4) | <0.001 |
| Endurance training (hrs·wk−1) | 11.0 (9.0–14.5) | 1.0 (0.0–1.0) | <0.001 |
| Fat mass (%) | 16.9 (15.3–19.0) | 29.7 (25.6–33.7) | <0.001 |
| Fat mass (kg) | 9.1 (8.2–10.7) | 19.3 (16.1–21.9) | <0.001 |
| LEAF-Q (total score) | 7.0 (4.3–9.0) | 3.0 (1.0–6.0) | 0.004 |
| Groups | Runners (n = 16) | Controls (n = 17) | p-Value |
|---|---|---|---|
| Total cholesterol (mmol/L) | 4.4 (4.0–5.125 | 4.0 (3.6–4.6) | 0.101 |
| HDL cholesterol (mmol/L) | 1.9 (1.7–2.4) | 1.5 (1.4–1.9) | 0.017 |
| LDL-cholesterol (mmol/L) Ratio total cholesterol/HDL | 2.4 (1.9–2.9) 2.2 (2.1–2.5) | 2.3 (1.9–3.0) 2.5 (2.2–2.8) | 0.900 0.101 |
| Triglycerides (mmol/L) | 0.7 (0.5–1.0) | 0.8 (0.6–1.050 | 0.446 |
| Apolipoprotein A | 1.82 (1.58–2.04) | 1.49 (1.37–1.75) | 0.027 |
| Apolipoprotein B | 0.73 (0.65–0.94) | 0.73 (0.61–0.88) | 0.914 |
| VCAM (ng/mL) | 695 (607–784) | 707 (634–817) | 0.577 |
| ICAM (ng/mL) | 200 (188–215) | 195 (178–215) | 0.552 |
| CRP (mg/L) | 0.31 (0.18–0.53) | 0.57 (0.22–1.79) | 0.097 |
| E-selectin (ng/mL) | 25.4 (18.8–39.2) | 30.9 (19.9–38.4) | 0.914 |
| vWF (%) | 93 (77–108) | 82 (70–90) | 0.084 |
| P-selectin (ng/mL) | 21.9 (20.7–25.8) | 21.5 (19.1–24.7) | 0.321 |
| L-arginin (µM) | 36.0 (30.5–41.1) | 30.6 (28.9–40.0) | 0.322 |
| ADMA (µM) | 0.36 (0.32–0.38) | 0.35 (0.32–0.38) | 0.677 |
| SDMA (µM) | 0.23 (0.18–0.24) | 0.20 (0.19–0.21) | 0.163 |
| Ratio L-arg/ADMA | 101 (89–114) | 94 (88–111) | 0.482 |
| Groups | Runners (n = 16) | Controls (n = 17) | p-Value |
|---|---|---|---|
| FSH (IU/L) | 5.1 (2.5–6.2) | 4.8 (3.6–5.6) | 0.692 |
| LH (IU/L) | 2.0 (1.0–3.9) | 5.1 (3.6–6.3) | 0.010 |
| Estradiol (nmol/L) | 0.08 (0.07–0.24) | 0.21 (0.14–0.33) | 0.090 |
| Testosterone (nmol/L) | 0.83 (0.62–1.10) | 1.00 (0.81–1.30) | 0.058 |
| SHBG (nmol/L) | 56.0 (46.3–67.0) | 65.0 (48.5–92.0) | 0.121 |
| TSH (IU/L) | 1.55 (1.23–2.20) | 2.30 (1.50–2.90) | 0.061 |
| fT4 (pmol/L) | 12.0 (11.0–14.0) | 13.0 (11.5–13.5) | 0.741 |
| Glucose (mmol/L) | 4.7 (4.6–5.0) | 4.8 (4.7–4.9) | 0.283 |
| HbA1c (mmol/L) | 29.0 (28.0–33.0) | 29.0 (28.0–31.0) | 0.714 |
| Insulin (pmol/L) | 29.0 (22.8–40.8) | 41.0 (34.5–58.5) | 0.022 |
| C-peptid (pmol/L) | 463 (402–511) | 529 (442–667) | 0.052 |
| HOMA-IR | 0.545 (0.417–0.765) | 0.760 (0.640–1.095) | 0.025 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyte, K.H.; Stensrud, T.; Berg, T.J.; Seljeflot, I.; Hisdal, J. Vascular Function in Norwegian Female Elite Runners: A Cross-Sectional, Controlled Study. Sports 2022, 10, 37. https://doi.org/10.3390/sports10030037
Kyte KH, Stensrud T, Berg TJ, Seljeflot I, Hisdal J. Vascular Function in Norwegian Female Elite Runners: A Cross-Sectional, Controlled Study. Sports. 2022; 10(3):37. https://doi.org/10.3390/sports10030037
Chicago/Turabian StyleKyte, Karoline Holsen, Trine Stensrud, Tore Julsrud Berg, Ingebjørg Seljeflot, and Jonny Hisdal. 2022. "Vascular Function in Norwegian Female Elite Runners: A Cross-Sectional, Controlled Study" Sports 10, no. 3: 37. https://doi.org/10.3390/sports10030037
APA StyleKyte, K. H., Stensrud, T., Berg, T. J., Seljeflot, I., & Hisdal, J. (2022). Vascular Function in Norwegian Female Elite Runners: A Cross-Sectional, Controlled Study. Sports, 10(3), 37. https://doi.org/10.3390/sports10030037






