Adverse Childhood Experiences, DNA Methylation, and Depressive Symptoms in Black Pregnant Women
Abstract
1. Introduction
1.1. Adverse Childhood Experiences
1.2. Toxic Stress
1.3. DNA Methylation
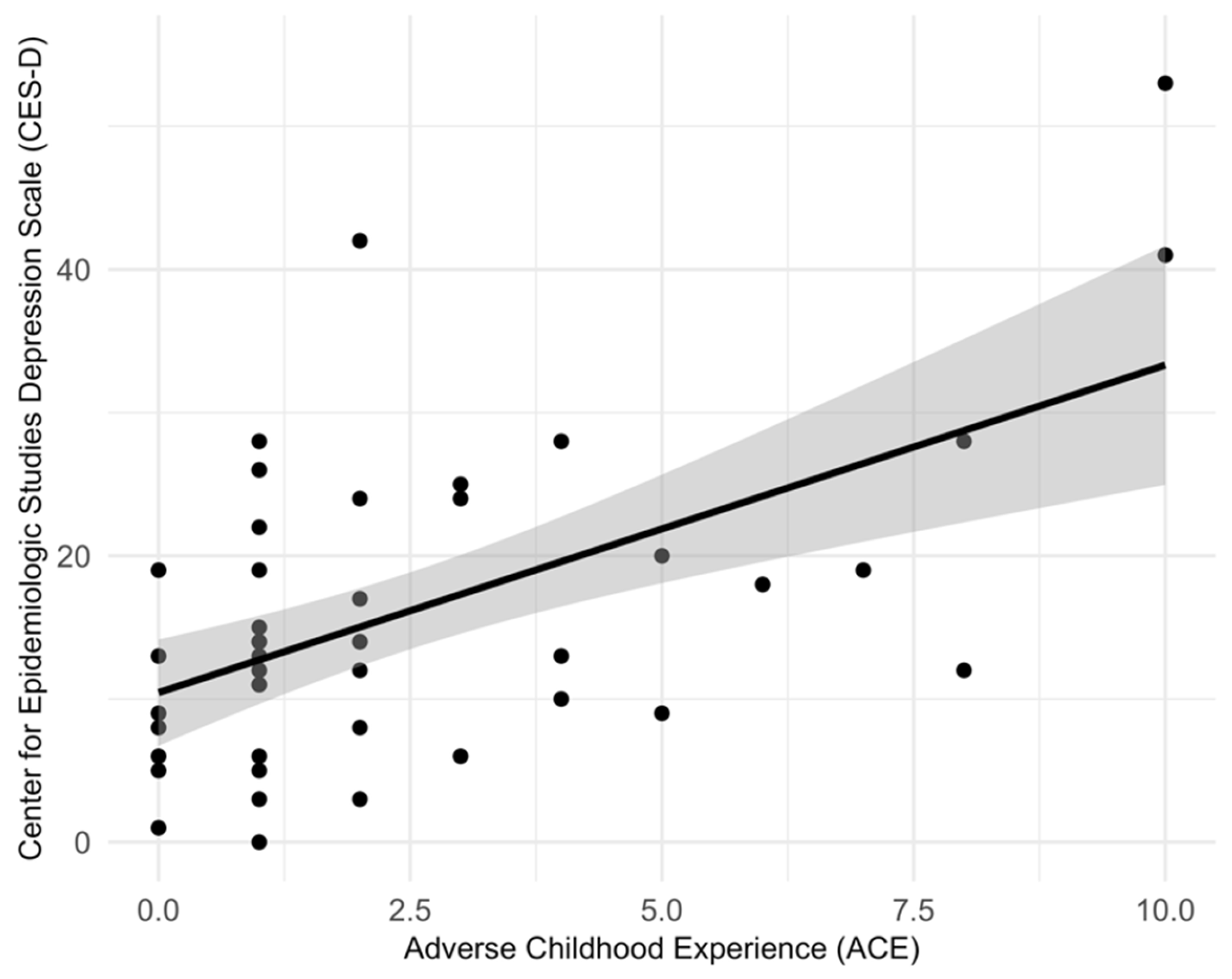
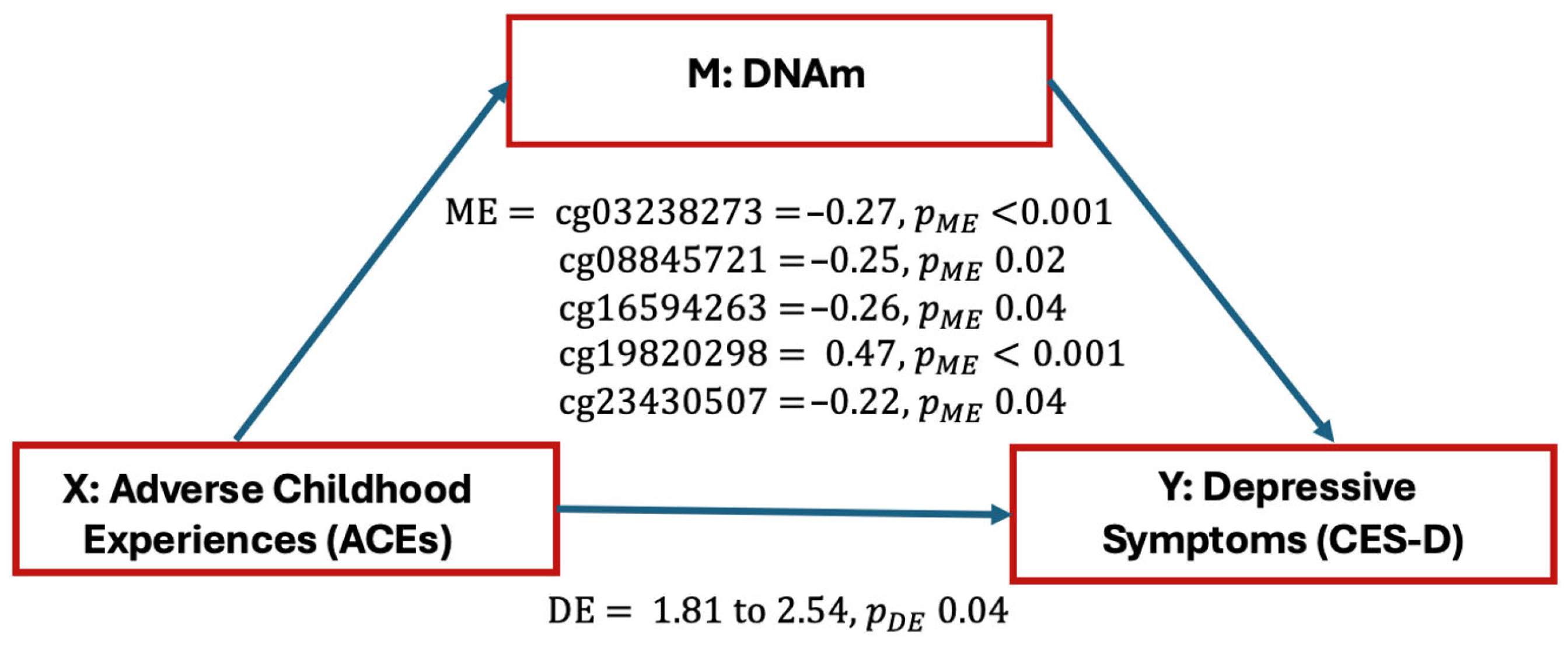
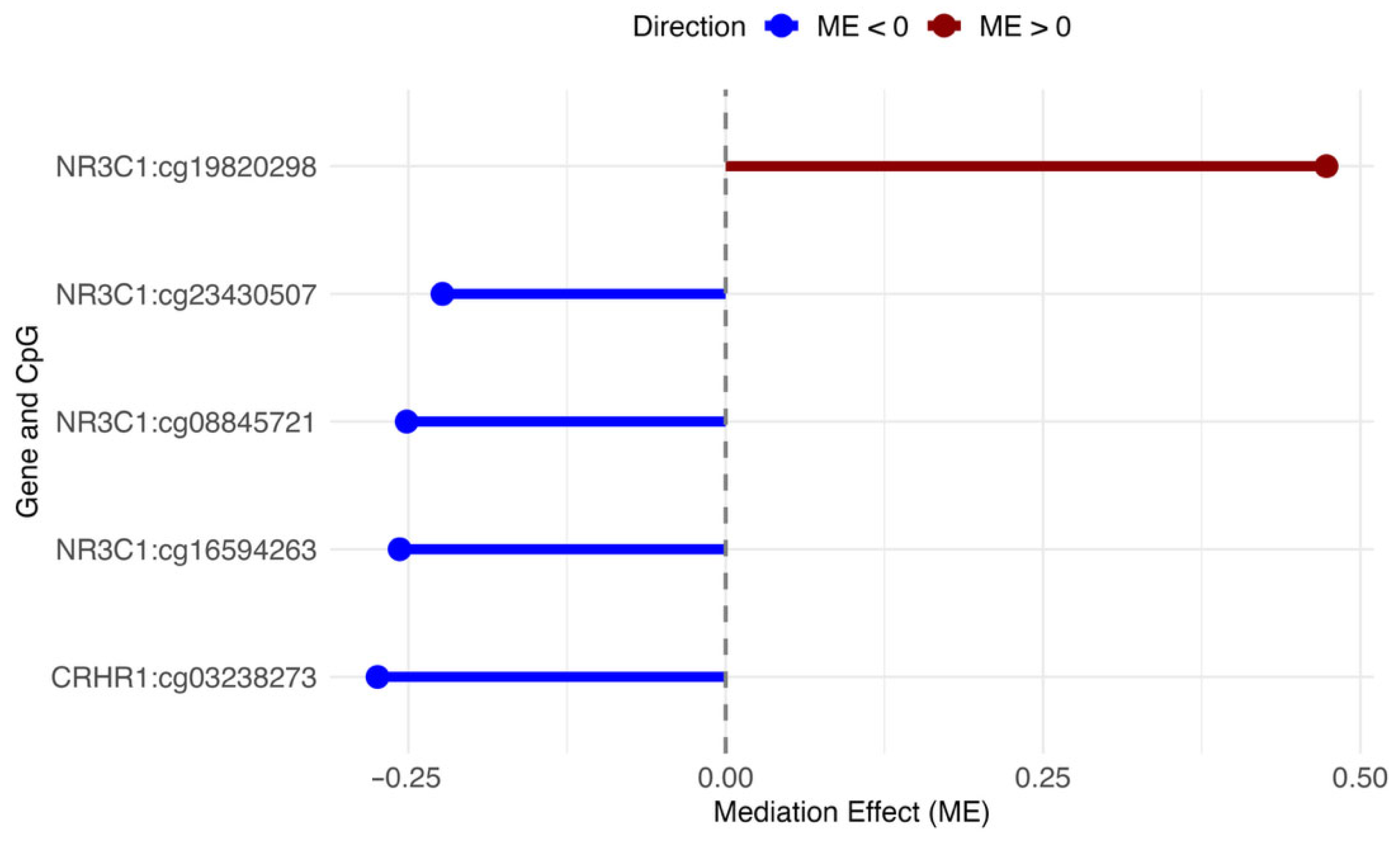
2. Results
3. Discussion
4. Materials and Methods
4.1. Design
4.2. Maternal Characteristics
4.3. Adverse Childhood Experiences
4.4. Depressive Symptoms
4.5. DNA Methylation
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alder, J.; Fink, N.; Bitzer, J.; Hösli, I.; Holzgreve, W. Depression and anxiety during pregnancy: A risk factor for obstetric, fetal and neonatal outcome? A critical review of the literature. J. Matern. Fetal Neonatal Med. 2007, 20, 189–209. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, U.; Papabathini, S.S.; Kawuki, J.; Obore, N.; Musa, T.H. Depression during pregnancy and the risk of low birth weight, preterm birth and intrauterine growth restriction—An updated meta-analysis. Early Hum. Dev. 2021, 152, 105243. [Google Scholar] [CrossRef] [PubMed]
- Gelaye, B.; Rondon, M.B.; Araya, R.; Williams, M.A. Epidemiology of maternal depression, risk factors, and child outcomes in low-income and middle-income countries. Lancet Psychiatry 2016, 3, 973–982. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Estriplet, T.; Morgan, I.; Davis, K.; Crear Perry, J.; Matthews, K. Black perinatal mental health: Prioritizing maternal mental health to optimize infant health and wellness. Front. Psychiatry 2022, 13, 807235. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mukherjee, S.; Trepka, M.; Pierre-Victor, D.; Behelah, R.; Avent, T. Racial/ethnic disparities in antenatal depression in the United States: A systematic review. Matern. Child Health J. 2016, 20, 1780–1797. [Google Scholar] [CrossRef]
- Sujan, A.C.; Nance, N.; Quesenberry, C.; Ridout, K.; Bhalala, M.; Avalos, L.A. Racial and ethnic differences in perinatal depression and anxiety. J. Affect. Disord. 2023, 334, 297–301. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Merrick, M.T.; Ford, D.C.; Ports, K.A.; Guinn, A.S. Prevalence of adverse childhood experiences from the 2011–2014 Behavioral Risk Factor Surveillance System in 23 states. JAMA Pediatr. 2018, 172, 1038–1044. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Felitti, V.J.; Anda, R.F.; Nordenberg, D.; Williamson, D.F.; Spitz, A.M.; Edwards, V.; Koss, M.P.; Marks, J.S. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am. J. Prev. Med. 1998, 14, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Chapman, D.P.; Whitfield, C.L.; Felitti, V.J.; Dube, S.R.; Edwards, V.J.; Anda, R.F. Adverse childhood experiences and the risk of depressive disorders in adulthood. J. Affect. Disord. 2004, 82, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Merrick, M.T.; Ports, K.A.; Ford, D.C.; Afifi, T.O.; Gershoff, E.T.; Grogan-Kaylor, A. Unpacking the impact of adverse childhood experiences on adult mental health. Child Abus. Negl. 2017, 69, 10–19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iob, E.; Lacey, R.; Giunchiglia, V.; Steptoe, A. Adverse childhood experiences and severity levels of inflammation and depression from childhood to young adulthood: A longitudinal cohort study. Mol. Psychiatry 2022, 27, 2255–2263. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Desch, J.; Mansuri, F.; Tran, D.; Schwartz, S.W.; Bakour, C. The association between adverse childhood experiences and depression trajectories in the Add Health study. Child Abus. Negl. 2023, 137, 106034. [Google Scholar] [CrossRef] [PubMed]
- Ångerud, K.; Annerbäck, E.M.; Tydén, T.; Boddeti, S.; Kristiansson, P. Adverse childhood experiences and depressive symptomatology among pregnant women. Acta Obstet. Gynecol. Scand. 2018, 97, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Racine, N.; Zumwalt, K.; McDonald, S.; Tough, S.; Madigan, S. Perinatal depression: The role of maternal adverse childhood experiences and social support. J. Affect. Disord. 2020, 263, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.C.; Goslawski, M.M.; Taple, B.J.; Sakowicz, A.; Alvarado-Goldberg, M.; Miller, E.S. The association between adverse childhood experiences and perinatal depression symptom trajectories. Am. J. Obstet. Gynecol. 2023, 5, 101039. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.G.; Theall, K.P.; Jackson, C.; Drury, S. Racial differences in the risk of prenatal depression among women experiencing childhood and adult stressors. Matern. Child Health J. 2022, 26, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Burford, N.G.; Webster, N.A.; Cruz-Topete, D. Hypothalamic-pituitary-adrenal axis aodulation of glucocorticoids in the cardiovascular aystem. Int. J. Mol. Sci. 2017, 18, 2150. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lightman, S.L. The neuroendocrinology of stress: A never ending story. J. Neuroendocrinol. 2008, 20, 880–884. [Google Scholar] [CrossRef] [PubMed]
- Hauger, R.L.; Risbrough, V.; Brauns, O.; Dautzenberg, F.M. Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: New molecular targets. CNS Neurol. Disord. Drug Targets 2006, 5, 453–479. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Smith, S.M.; Vale, W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006, 8, 383–395. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wochnik, G.M.; Rüegg, J.; Abel, G.A.; Schmidt, U.; Holsboer, F.; Rein, T. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J. Biol. Chem. 2005, 280, 4609–4616. [Google Scholar] [CrossRef] [PubMed]
- Ratman, D.; Vanden Berghe, W.; Dejager, L.; Libert, C.; Tavernier, J.; Becke, I.M.; De Bosscher, K. How glucocorticoid receptors modulate the activity of other transcription factors: A scope beyond tethering. Mol. Cell. Endocrinol. 2013, 380, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Rogac, M.; Peterlin, B. Epigenetic signature of chronic maternal stress load during pregnancy might be a potential biomarker for spontaneous preterm birth. Balk. J. Med. Genet. 2018, 21, 27–33. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Brain on stress: How the social environment gets under the skin. Proc. Natl. Acad. Sci. USA 2012, 109 (Suppl. 2), 17180–17185. [Google Scholar] [CrossRef]
- McEwen, B.S.; Wingfield, J.C. The concept of allostasis in biology and biomedicine. Horm. Behav. 2003, 43, 2–15. [Google Scholar] [CrossRef]
- Shonkoff, J.P.; Garner, A.S. The lifelong effects of early childhood adversity and toxic stress. Pediatrics 2012, 129, e232–e246. [Google Scholar] [CrossRef]
- Heim, C.; Newport, D.J.; Heit, S.; Graham, Y.P.; Wilcox, M.; Bonsall, R.; Miller, A.H.; Nemeroff, C.B. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA 2000, 284, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Heim, C.; Nemeroff, C.B. The role of childhood trauma in the neurobiology of mood and anxiety disorders: Preclinical and clinical studies. Biol. Psychiatry 2001, 49, 1023–1039. [Google Scholar] [CrossRef] [PubMed]
- Seth, S.; Lewis, A.J.; Galbally, M. Perinatal maternal depression and cortisol function in pregnancy and the postpartum period: A systematic literature review. BMC Pregnancy Childbirth 2016, 16, 124. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and its basic function. Neuropsychopharmacology 2012, 38, 23–38. [Google Scholar] [CrossRef]
- Jaenisch, R.; Bird, A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat. Genet. 2003, 33, 245–254. [Google Scholar] [CrossRef] [PubMed]
- McGowan, P.O.; Sasaki, A.; D’Alessio, A.; Dymov, S.; Labonté, B.; Szyf, M.; Turecki, G.; Meaney, M. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat. Neurosci. 2009, 12, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Palma-Gudiel, H.; Córdova-Palomera, A.; Eixarch, E.; Deuschle, M.; Fañanás, L. Maternal psychosocial stress during pregnancy alters the epigenetic signature of the glucocorticoid receptor gene promoter in their offspring: A meta-analysis. Epigenetics 2015, 10, 893–902. [Google Scholar] [CrossRef]
- Parade, S.H.; Huffhines, L.; Daniels, T.E.; Stroud, L.R.; Nugent, N.R.; Tyrka, A.R. A systematic review of childhood maltreatment and DNA methylation: Candidate gene and epigenome-wide approaches. Transl. Psychiatry 2021, 11, 134. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cecil, C.A.M.; Zhang, Y.; Nolte, T. Childhood maltreatment and DNA methylation: A systematic review. Neurosci. Biobehav. Rev. 2020, 112, 392–409. [Google Scholar] [CrossRef] [PubMed]
- Rubens, M.; Bruenig, D.; Adams, J.A.M.; Suresh, S.M.; Sathyanarayanan, A.; Haslam, D.; Shenk, C.E.; Mathews, B.; Mehta, D. Childhood maltreatment and DNA methylation: A systematic review. Neurosci. Biobehav. Rev. 2023, 147, 105079. [Google Scholar] [CrossRef] [PubMed]
- Tyrka, A.R.; Price, L.H.; Marsit, C.; Walters, O.C.; Carpenter, L.L. Childhood adversity and epigenetic modulation of the leukocyte glucocorticoid receptor: Preliminary findings in healthy adults. PLoS ONE 2012, 7, e30148. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parade, S.H.; Parent, J.; Rabemananjara, K.; Seifer, R.; Marsit, C.J.; Yang, B.Z.; Zhang, H.; Tyrka, A.R. Change in FK506 binding protein 5 (FKBP5) methylation over time among preschoolers with adversity. Dev. Psychopathol. 2017, 29, 1627–1634. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parent, J.; Parade, S.H.; Laumann, L.E.; Ridout, K.K.; Yang, B.Z.; Marsit, C.J.; Seifer, R.; Tyrka, A.R. Dynamic stress-related epigenetic regulation of the glucocorticoid receptor gene promoter during early development: The role of child maltreatment. Dev. Psychopathol. 2017, 29, 1635–1648. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Argentieri, M.A.; Nagarajan, S.; Seddighzadeh, B.; Baccarelli, A.A.; Shileds, A.E. Epigenetic pathways in human disease: The impact of DNA methylation on stress-related pathogenesis and current challenges in biomarker development. eBioMedicine 2017, 18, 327–350. [Google Scholar] [CrossRef]
- Liu, P.Z.; Nusslock, R. How stress gets under the skin: Early life adversity and glucocorticoid receptor epigenetic regulation. Curr. Genom. 2018, 19, 653–664. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Turecki, G.; Meaney, M.J. Effects of the Social Environment and Stress on Glucocorticoid Receptor Gene Methylation: A Systematic Review. Biol. Psychiatry 2016, 79, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Tyrka, A.R.; Parade, S.H.; Welch, E.S.; Ridout, K.K.; Price, L.H.; Marsit, C.; Philip, N.S.; Carpenter, L.L. Methylation of the leukocyte glucocorticoid receptor gene promoter in adults: Associations with early adversity and depressive, anxiety and substance-use disorders. Transl. Psychiatry 2016, 6, e848. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Watkeys, O.J.; Kremerskothen, K.; Quidé, Y.; Fullerton, J.M.; Green, M.J. Glucocorticoid receptor gene (NR3C1) DNA methylation in association with trauma, psychopathology, transcript expression, or genotypic variation: A systematic review. Neurosci. Biobehav. Rev. 2018, 95, 85–122. [Google Scholar] [CrossRef]
- Tyrka, A.R.; Ridout, K.K.; Parade, S.H.; Paquette, A.; Marsit, C.J.; Seifer, R. Childhood maltreatment and methylation of FK506 binding protein 5 gene (FKBP5). Dev. Psychopathol. 2015, 27, 1637–1645. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ramo-Fernández, L.; Boeck, C.; Koenig, A.M.; Schury, K.; Binder, E.B.; Gündel, H.; Fegert, J.M.; Karabatsiakis, A.; Kolassa, I.T. The effects of childhood maltreatment on epigenetic regulation of stress-response associated genes: An intergenerational approach. Sci. Rep. 2019, 9, 983. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Klengel, T.; Mehta, D.; Anacker, C.; Rex-Haffner, M.; Pruessner, J.C.; Pariante, C.M.; Pace, T.W.; Mercer, K.B.; Mayberg, H.S.; Bradley, B.; et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat. Neurosci. 2013, 16, 33–41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pariante, C.M.; Lightman, S.L. The HPA axis in major depression: Classical theories and new developments. Trends Neurosci. 2008, 31, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Benatar, S.; Cross-Barnet, C.; Johnston, E.; Hill, I. Prenatal depression: Assessment and outcomes among medicaid participants. J. Behav. Health Serv. Res. 2020, 47, 409–423. [Google Scholar] [CrossRef] [PubMed]
- Fekadu Dadi, A.; Miller, E.R.; Woodman, R.J.; Azale, T.; Mwanri, L. Effect of antenatal depression on adverse birth outcomes in Gondar town, Ethiopia: A community-based cohort study. PLoS ONE 2020, 15, e0234728. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bonari, L.; Pinto, N.; Ahn, E.; Einarson, A.; Steiner, M.; Koren, G. Perinatal risks of untreated depression during pregnancy. Can. J. Psychiatry 2004, 49, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.; Gomez, R.; Williams, G.; Lembke, A.; Lazzeroni, L.; Murphy, G.M., Jr.; Schatzberg, A.F. HPA axis in major depression: Cortisol, clinical symptomatology and genetic variation predict cognition. Mol. Psychiatry 2017, 22, 527–536. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ryan, J.; Mansell, T.; Fransquet, P.; Saffery, R. Does maternal mental well-being in pregnancy impact the early human epigenome? Epigenomics 2017, 9, 313–332. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.L.; Payne, J.L. Perinatal depression: A review and an update. Psychiatr. Clin. N. Am. 2023, 46, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Oberlander, T.F.; Weinberg, J.; Papsdorf, M.; Grunau, R.; Misri, S.; Devlin, A.M. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics 2008, 3, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Ying, X.; Zhai, M.; Li, J.; Liu, D.; Liu, X.; Yu, B.; Yan, H. The association between peritraumatic distress, perceived stress, depression in pregnancy, and NR3C1 DNA methylation among Chinese pregnant women who experienced COVID-19 lockdown. Front. Immunol. 2022, 13, 966522. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.T.A.; Gardini, E.; Iliadis, S.I.; Ehlert, U.; Kallak, T.K.; Skalkidou, A. Personality vulnerability to depression, resilience, and depressive symptoms: Epigenetic markers among perinatal women. Ups. J. Med. Sci. 2024, 129, e10603. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Heim, C.; Newport, D.J.; Mletzko, T.; Miller, A.H.; Nemeroff, C.B. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology 2008, 33, 693–710. [Google Scholar] [CrossRef]
- Heim, C.; Bradley, B.; Mletzko, T.C.; Deveau, T.C.; Musselman, D.L.; Nemeroff, C.B.; Ressler, K.J.; Binder, E.B. Effect of childhood trauma on adult depression and neuroendocrine function: Sex-specific moderation by CRH receptor 1 gene. Front. Behav. Neurosci. 2009, 3, 41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Perroud, N.; Paoloni-Giacobino, A.; Prada, P.; Olié, E.; Salzmann, A.; Nicastro, R.; Guillaume, S.; Mouthon, D.; Stouder, C.; Dieben, K.; et al. Increased methylation of glucocorticoid receptor gene (NR3C1) in adults with a history of childhood maltreatment: A link with the severity and type of trauma. Transl. Psychiatry 2011, 1, e59. [Google Scholar] [CrossRef]
- Radtke, K.M.; Ruf, M.; Gunter, H.M.; Dohrmann, K.; Schauer, M.; Meyer, A.; Elbert, T. Transgenerational impact of intimate partner violence on methylation in the promoter of the glucocorticoid receptor. Transl. Psychiatry 2011, 1, e21. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, A.C.; Aiello, A.E.; Galea, S.; Ratanatharathorn, A.; Noronha, C.; Wildman, D.E.; Uddin, M. Glucocorticoid receptor DNA methylation, childhood maltreatment and major depression. J. Affect. Disord. 2016, 206, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Palma-Gudiel, H.; Cordova-Palomera, A.; Leza, J.C.; Fananas, L. Glucocorticoid receptor gene (NR3C1) methylation processes as mediators of early adversity in stress-related disorders causality: A critical review. Neurosci. Biobehav. Rev. 2015, 55, 520–535. [Google Scholar] [CrossRef] [PubMed]
- Melas, P.A.; Wei, Y.; Wong, C.C.; Sjöholm, L.K.; Åberg, E.; Mill, J.; Schalling, M.; Forsell, Y.; Lavebratt, C. Genetic and epigenetic associations of MAOA and NR3C1 with depression and childhood adversities. Int. J. Neuropsychopharmacol. 2013, 16, 1513–1528. [Google Scholar] [CrossRef] [PubMed]
- Weder, N.; Zhang, H.; Jensen, K.; Yang, B.Z.; Simen, A.; Jackowski, A.; Lipschitz, D.; Douglas-Palumberi, H.; Ge, M.; Perepletchikova, F.; et al. Child abuse, depression, and methylation in genes involved with stress, neural plasticity, and brain circuitry. J. Am. Acad. Child Adolescemt Psychiatry 2014, 53, 417–424.e415. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Irizarry, R.A.; Ladd-Acosta, C.; Wen, B.; Wu, Z.; Montano, C.; Onyango, P.; Cui, H.; Gabo, K.; Rongione, M.; Webster, M.; et al. The human colon cancer methylome shows similar hypo- and hypermethylation at conserved tissue-specific CpG island shores. Nat. Genet. 2009, 41, 178–186. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shields, A.E. Epigenetic signals of how social disadvantage “gets under the skin”: A challenge to the public health community. Epigenomics 2017, 9, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, K.L.; Moore, S.R.; Davis, E.G.; MacIsaac, J.L.; Lin, D.T.S.; Kobor, M.S.; Gotlib, I.H. DNA methylation of HPA-axis genes and the onset of major depressive disorder in adolescent girls: A prospective analysis. Transl. Psychiatry 2019, 9, 245. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dionisio-Garcia, D.M.; Genis-Mendoza, A.D.; Gonzalez-Castro, T.B.; Tovilla-Zarate, C.A.; Juarez-Rojop, I.E.; Lopez-Narvaez, M.L.; Hernandez-Diaz, Y.; Nicolini, H.; Olvera-Hernandez, V. DNA Methylation of Genes Involved in the HPA Axis in Presence of Suicide Behavior: A Systematic Review. Brain Sci. 2023, 13, 584. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Centers for Disease Control and Prevention (CDC). About the CDC-Kaiser ACE Study: U.S. Department of Health & Human Services. 2021. Available online: https://www.cdc.gov/violenceprevention/aces/about.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fviolenceprevention%2Facestudy%2Fabout.html (accessed on 13 October 2024).
- Ghosh Ippen, C.; Harris, W.W.; Van Horn, P.; Lieberman, A.F. Traumatic and stressful events in early childhood: Can treatment help those at highest risk? Child Abus. Negl. 2011, 35, 504–513. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brown, D.W.; Anda, R.F.; Tiemeier, H.; Felitti, V.J.; Edwards, V.J.; Croft, J.B.; Giles, W.H. Adverse childhood experiences and the risk of premature mortality. Am. J. Prev. Med. 2009, 37, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Radloff, L.S. The CES-D scale: A self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Li, D.; Liu, L.; Odouli, R. Presence of depressive symptoms during early pregnancy and the risk of preterm delivery: A prospective cohort study. Hum. Reprod. 2009, 24, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Carey, C.; Xie, R.; Davis, J.W.; LaManna, J.B.; Misra, D.; Giurgescu, C. Racial discrimination, social support, and psychological distress among Black pregnant women. West. J. Nurs. Res. 2024, 46, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Giurgescu, C.; Adaji, R.; Hyer, S.; Wheeler, J.; Misra, D.P. Neighborhood environment and perceived stress before and during the COVID-19 pandemic among childbearing Black women. J. Perinat. Neonatal Nurs. 2024, 38, 334–341. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nowak, A.L.; Anderson, C.M.; Ford, J.L.; Mackos, A.; Ohm, J.; Saadat, N.; Tan, A.; Zhao, Y.; Misra, D.P.; Giurgescu, C. DNA methylation patterns of glucocorticoid pathway genes in preterm birth among Black women. Biol. Res. Nurs. 2022, 24, 493–502. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tingley, D.; Yamamoto, T.; Hirose, K.; Keele, L.; Imai, K. Mediation: R package for causal mediation analysis. J. Stat. Softw. 2014, 59, 1–38. [Google Scholar] [CrossRef]
| Variable | N | Mean (SD) (Range) | Scores | Number (%) |
|---|---|---|---|---|
| Maternal Age (years) | 61 | 27.8 (5.5) (18–41) | ||
| GA at Data | ||||
| Collection (weeks) | 60 | 13.6 (3.3) (8.3–22.2) | ||
| ACEs * | 49 | 2.4 (2.6) | 0 | 8 (16.3) |
| (0–4) | 1–3 | 30 (61.2) | ||
| 4+ | 11 (22.5) | |||
| CES-D + | 59 | 17.2 (12.0) | <16 | 33 (55.9) |
| (0–60) | ≥16 | 26 (44.1) | ||
| <23 | 42 (71.2) | |||
| ≥23 | 17 (28.8) | |||
| Marital Status | 59 | 20 (34.0) | ||
| Married or Living w/Partner | 20 (34.0) | |||
| Divorced/Separated | 4 (6.7) | |||
| Never Married | 35 (59.3) | |||
| Employment | 61 | |||
| Working | 32 (52.4) | |||
| Temporarily Laid-Off | 4 (6.6) | |||
| Not Working | 25 (41.0) | |||
| Annual Household Income | 61 | |||
| <$10,000 | 21 (34.4) | |||
| $10,000–$30,000 | 31 (50.8) | |||
| $30,000–$39,999 | 3 (4.9) | |||
| $40,000–$59,999 | 4 (6.6) | |||
| >$60,000 | 2 (3.3) |
| Gene | CpG a | CpG Location | Chromosomal Position | Relation to CpG Island | Total Effect | ME | DE | PME | PDE |
|---|---|---|---|---|---|---|---|---|---|
| CRHR1 | cg03238273 | 5′UTR | Ch.17:43825672 | Ukn | 2.287 | −0.27 | 2.56 | <0.001 | 2 × 10−16 |
| NR3C1 | cg08845721 | 5′UTR | Ch.5:142780693 | N. Shore | 2.287 | −0.25 | 2.54 | 0.02 | 2 × 10−16 |
| NR3C1 | cg16594263 | Body | Ch.5:142768048 | Ukn | 2.287 | −0.26 | 2.54 | 0.04 | 2 × 10−16 |
| NR3C1 | cg19820298 | Body | Ch.5:142770782 | Ukn | 2.287 | 0.47 | 1.81 | <0.001 | 0.04 |
| NR3C1 | cg23430507 | 5′UTR | Ch.5:142798375 | Ukn | 2.287 | −0.22 | 2.51 | 0.04 | 2 × 10−16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak, A.L.; Schilt-Solberg, M.A.; Liang, X.; Magaña, F.; Misra, D.P.; Giurgescu, C. Adverse Childhood Experiences, DNA Methylation, and Depressive Symptoms in Black Pregnant Women. Epigenomes 2025, 9, 48. https://doi.org/10.3390/epigenomes9040048
Nowak AL, Schilt-Solberg MA, Liang X, Magaña F, Misra DP, Giurgescu C. Adverse Childhood Experiences, DNA Methylation, and Depressive Symptoms in Black Pregnant Women. Epigenomes. 2025; 9(4):48. https://doi.org/10.3390/epigenomes9040048
Chicago/Turabian StyleNowak, Alexandra L., Marvin A. Schilt-Solberg, Xiaoyu Liang, Fabiola Magaña, Dawn P. Misra, and Carmen Giurgescu. 2025. "Adverse Childhood Experiences, DNA Methylation, and Depressive Symptoms in Black Pregnant Women" Epigenomes 9, no. 4: 48. https://doi.org/10.3390/epigenomes9040048
APA StyleNowak, A. L., Schilt-Solberg, M. A., Liang, X., Magaña, F., Misra, D. P., & Giurgescu, C. (2025). Adverse Childhood Experiences, DNA Methylation, and Depressive Symptoms in Black Pregnant Women. Epigenomes, 9(4), 48. https://doi.org/10.3390/epigenomes9040048







