Convergent Evolution and the Epigenome
Abstract
1. Introduction
1.1. Genetic Convergence as a Parsimonious Explanation for Trait Convergence
1.2. Epigenetic Priming of Convergent Evolution
1.3. Bias in De Novo Mutations and Recombination
1.4. Revisiting Mutational Bias Through an Epigenetic Lens
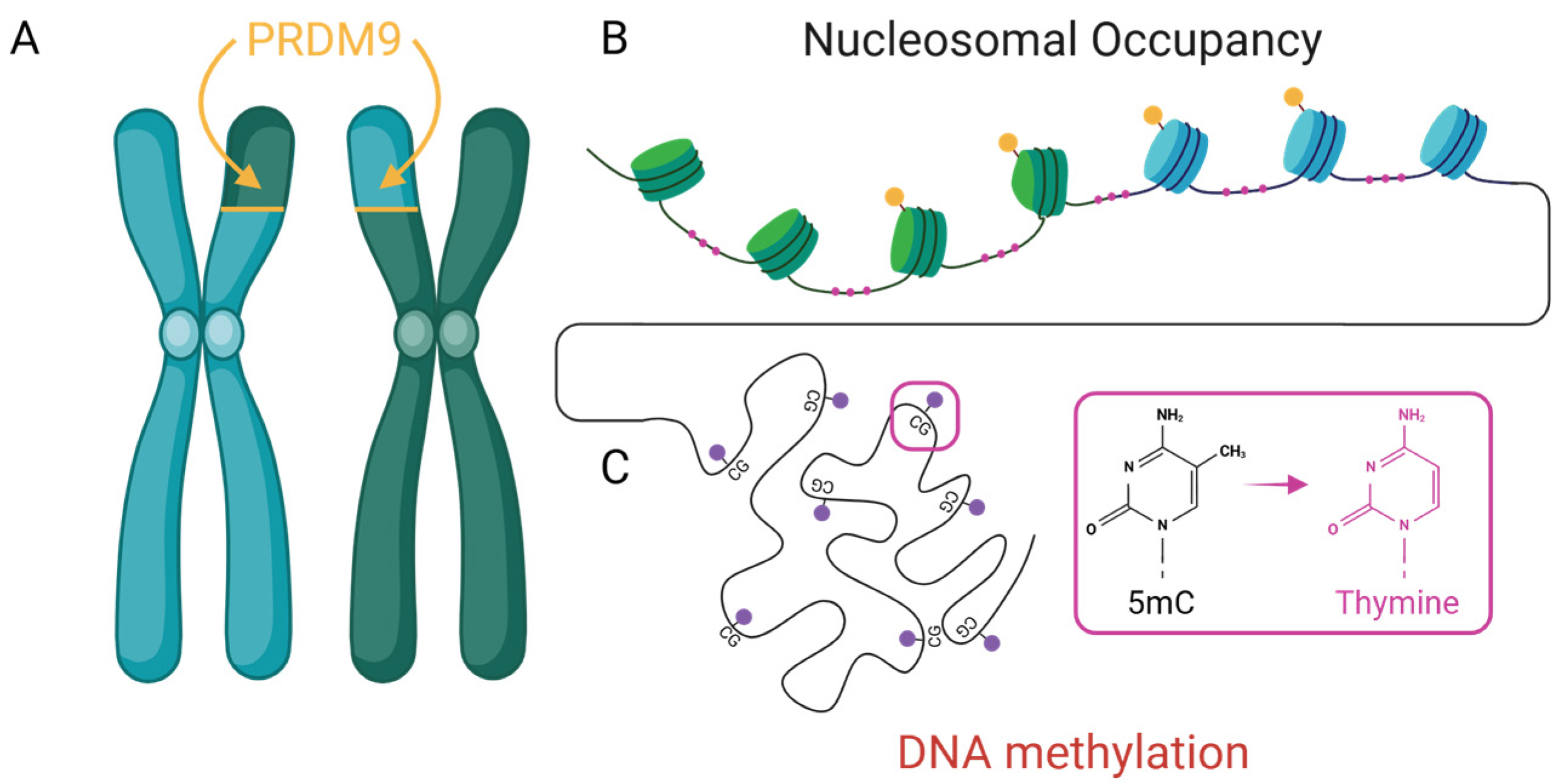
1.4.1. Silent Marks, Loud Consequences: DNA Methylation and Mutation Bias
1.4.2. PRDM9: The Cartographer of the Recombination Landscape
1.4.3. Tightly Wound, Lightly Wounded: Nucleosomal Armor in Mutation Bias
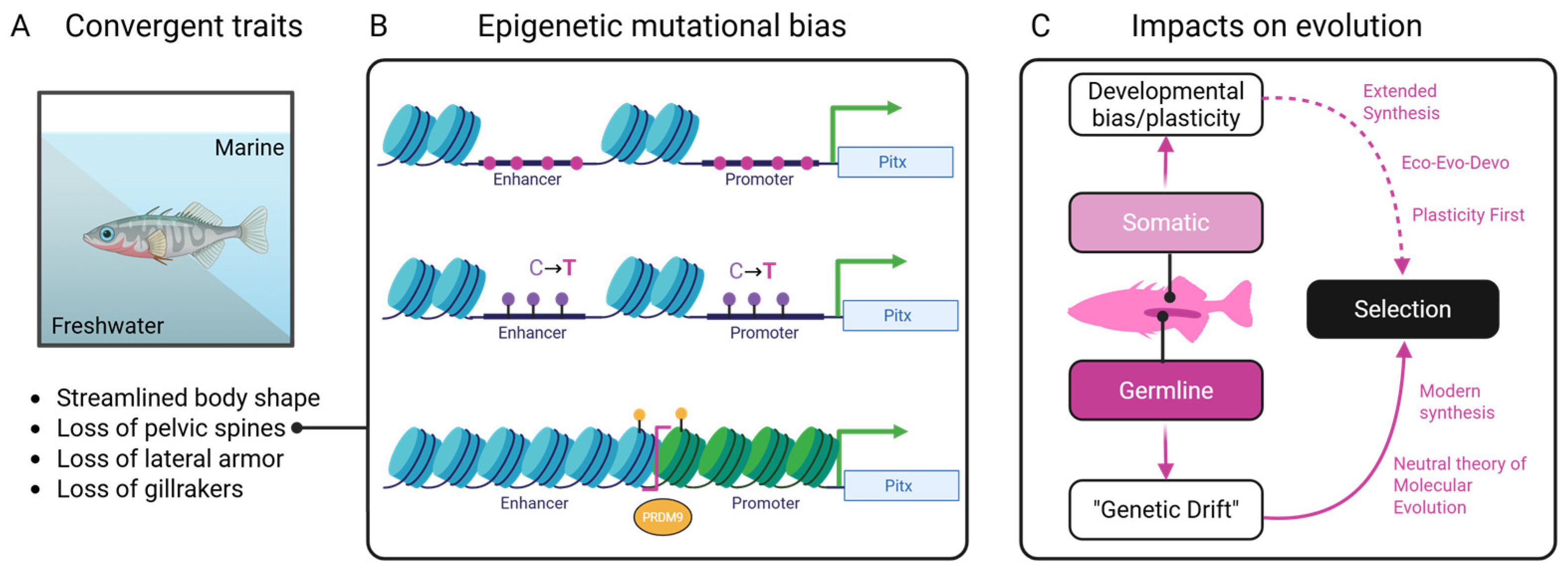
1.5. Epigenetic Bias and the Puzzle of Convergent Evolution
1.6. Agency in the Making: Signaling, Epigenetics, and the Future of Convergence
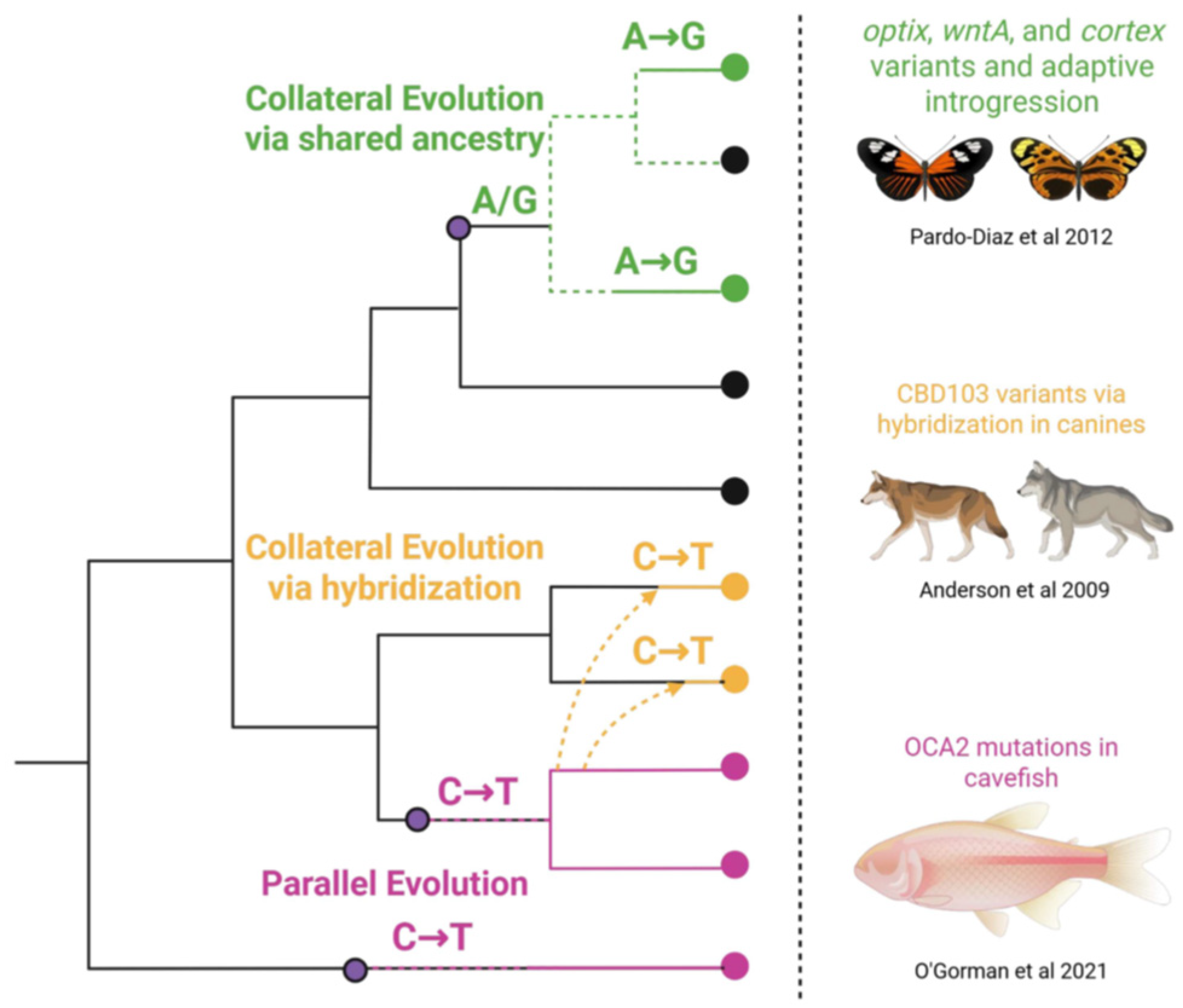
Prisms, Not Pipelines: A Spectrum of Convergent Mechanisms
2. Epigenomic Structure as a Substrate for Convergent Evolution
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arendt, J.; Reznick, D. Convergence and Parallelism Reconsidered: What Have We Learned about the Genetics of Adaptation? Trends Ecol. Evol. 2008, 23, 26–32. [Google Scholar] [CrossRef]
- Averof, M.; Cohen, S.M. Evolutionary Origin of Insect Wings from Ancestral Gills. Nature 1997, 385, 627–630. [Google Scholar] [CrossRef]
- Nath, A.; Chaube, R.; Subbiah, K. An Insight into the Molecular Basis for Convergent Evolution in Fish Antifreeze Proteins. Comput. Biol. Med. 2013, 43, 817–821. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, J.M.; Luque, J.; Bracken-Grissom, H.D. How to Become a Crab: Phenotypic Constraints on a Recurring Body Plan. BioEssays 2021, 43, e2100020. [Google Scholar] [CrossRef] [PubMed]
- Stern, D.L. The Genetic Causes of Convergent Evolution. Nat. Rev. Genet. 2013, 14, 751–764. [Google Scholar] [CrossRef]
- Guo, L.; Qiao, X.; Haji, D.; Zhou, T.; Liu, Z.; Whiteman, N.K.; Huang, J. Convergent Resistance to GABA Receptor Neurotoxins through Plant–Insect Coevolution. Nat. Ecol. Evol. 2023, 7, 1444–1456. [Google Scholar] [CrossRef]
- Morris, J.; Hanly, J.J.; Martin, S.H.; Belleghem, S.M.V.; Salazar, C.; Jiggins, C.D.; Dasmahapatra, K.K. Deep Convergence, Shared Ancestry, and Evolutionary Novelty in the Genetic Architecture of Heliconius Mimicry. Genetics 2020, 216, 765–780. [Google Scholar] [CrossRef]
- Schweizer, M.; Warmuth, V.; Kakhki, N.A.; Aliabadian, M.; Förschler, M.; Shirihai, H.; Suh, A.; Burri, R. Parallel Plumage Colour Evolution and Introgressive Hybridization in Wheatears. J. Evol. Biol. 2020, 32, 100–110. [Google Scholar] [CrossRef]
- Popovic, I.; Bierne, N.; Gaiti, F.; Tanurdžić, M.; Riginos, C. Pre-introduction Introgression Contributes to Parallel Differentiation and Contrasting Hybridization Outcomes between Invasive and Native Marine Mussels. J. Evol. Biol. 2021, 34, 175–192. [Google Scholar] [CrossRef]
- De-Kayne, R.; Selz, O.M.; Marques, D.A.; Frei, D.; Seehausen, O.; Feulner, P.G.D. Genomic Architecture of Adaptive Radiation and Hybridization in Alpine Whitefish. Nat. Commun. 2022, 13, 4479. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Diaz, C.; Salazar, C.; Baxter, S.W.; Merot, C.; Figueiredo-Ready, W.; Joron, M.; McMillan, W.O.; Jiggins, C.D. Adaptive introgression across species boundaries in Heliconius butterflies. PLoS Genet. 2012, 8, e1002752. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.M.; vonHoldt, B.M.; Candille, S.I.; Musiani, M.; Greco, C.; Stahler, D.R.; Smith, D.W.; Padhukasahasram, B.; Randi, E.; Leonard, J.A.; et al. Molecular and evolutionary history of melanism in North American gray wolves. Science 2009, 323, 1339–1343. [Google Scholar] [CrossRef]
- O’Gorman, M.; Thakur, S.; Imrie, G.; Moran, R.L.; Choy, S.; Sifuentes-Romero, I.; Bilandžija, H.; Renner, K.J.; Duboué, E.; Rohner, N.; et al. Pleiotropic function of the oca2 gene underlies the evolution of sleep loss and albinism in cavefish. Curr Biol. 2021, 31, 3694–3701.e4. [Google Scholar] [CrossRef]
- de Jong, M.J.; van Oosterhout, C.; Hoelzel, A.R.; Janke, A. Moderating the Neutralist–Selectionist Debate: Exactly Which Propositions Are We Debating, and Which Arguments Are Valid? Biol. Rev. 2024, 99, 23–55. [Google Scholar] [CrossRef]
- Zou, Z.; Zhang, J. Are Convergent and Parallel Amino Acid Substitutions in Protein Evolution More Prevalent Than Neutral Expectations? Mol. Biol. Evol. 2015, 32, 2085–2096. [Google Scholar] [CrossRef]
- Gilbert, S.F.; Bosch, T.C.G.; Ledón-Rettig, C. Eco-Evo-Devo: Developmental Symbiosis and Developmental Plasticity as Evolutionary Agents. Nat. Rev. Genet. 2015, 16, 611–622. [Google Scholar] [CrossRef]
- West-Eberhard, M.J. Developmental Plasticity and Evolution; Oxford University Press: Oxford, UK, 2003. [Google Scholar] [CrossRef]
- Ashe, A.; Colot, V.; Oldroyd, B.P. How Does Epigenetics Influence the Course of Evolution? Philos. Trans. R. Soc. B 2021, 376, 20200111. [Google Scholar] [CrossRef]
- Shapiro, J.A. Nothing in Evolution Makes Sense Except in the Light of Genomics: Read–Write Genome Evolution as an Active Biological Process. Biology 2016, 5, 27. [Google Scholar] [CrossRef]
- Chuong, E.B.; Elde, N.C.; Feschotte, C. Regulatory Activities of Transposable Elements: From Conflicts to Benefits. Nat. Rev. Genet. 2017, 18, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Feschotte, C. Transposable Elements and the Evolution of Regulatory Networks. Nat. Rev. Genet. 2008, 9, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Sommer, R.J. Phenotypic Plasticity: From Theory and Genetics to Current and Future Challenges. Genetics 2020, 215, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Nijhout, H.F. Genetic Assimilation, Robustness and Plasticity Are Key Processes in the Development and Evolution of Novel Traits. Dev. Biol. 2025, 523, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Peichel, C.L.; Marques, D.A. The Genetic and Molecular Architecture of Phenotypic Diversity in Sticklebacks. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20150486. [Google Scholar] [CrossRef]
- Hodgkinson, A.; Eyre-Walker, A. Variation in the Mutation Rate across Mammalian Genomes. Nat. Rev. Genet. 2011, 12, 756–766. [Google Scholar] [CrossRef]
- Makova, K.D.; Hardison, R.C. The Effects of Chromatin Organization on Variation in Mutation Rates in the Genome. Nat. Rev. Genet. 2015, 16, 213–223. [Google Scholar] [CrossRef]
- Sasaki, S.; Mello, C.C.; Shimada, A.; Nakatani, Y.; Hashimoto, S.; Ogawa, M.; Matsushima, K.; Gu, S.G.; Kasahara, M.; Ahsan, B.; et al. Chromatin-Associated Periodicity in Genetic Variation Downstream of Transcriptional Start Sites. Science 2009, 323, 401–404. [Google Scholar] [CrossRef]
- Cano, A.V.; Gitschlag, B.L.; Rozhoňová, H.; Stoltzfus, A.; McCandlish, D.M.; Payne, J.L. Mutation Bias and the Predictability of Evolution. Philos. Trans. R. Soc. B 2023, 378, 20220055. [Google Scholar] [CrossRef]
- Stoltzfus, A.; Norris, R.W. On the Causes of Evolutionary Transition:Transversion Bias. Mol. Biol. Evol. 2016, 33, 595–602. [Google Scholar] [CrossRef]
- Consortium, U.; Rahbari, R.; Wuster, A.; Lindsay, S.J.; Hardwick, R.J.; Alexandrov, L.B.; Turki, S.A.; Dominiczak, A.; Morris, A.; Porteous, D.; et al. Timing, Rates and Spectra of Human Germline Mutation. Nat. Genet. 2016, 48, 126–133. [Google Scholar] [CrossRef]
- Goriely, A.; Lord, H.; Lim, J.; Johnson, D.; Lester, T.; Firth, H.V.; Wilkie, A.O.M. Germline and Somatic Mosaicism for FGFR2 Mutation in the Mother of a Child with Crouzon Syndrome: Implications for Genetic Testing in “Paternal Age-effect” Syndromes. Am. J. Med. Genet. Part A 2010, 152, 2067–2073. [Google Scholar] [CrossRef] [PubMed]
- Geier, D.A.; Kern, J.K.; Sykes, L.K.; Geier, M.R. Examining Genotypic Variation in Autism Spectrum Disorder and Its Relationship to Parental Age and Phenotype. Appl. Clin. Genet. 2016, 9, 121–129. [Google Scholar] [CrossRef]
- Lawen, J.; Wang, E. Model for de Novo Mutation Propagation Depending on Paternal Age at Conception and Associated Neurological Disorders. F1000Research 2018, 7, 358. [Google Scholar] [CrossRef]
- Sayres, M.A.W.; Makova, K.D. Genome Analyses Substantiate Male Mutation Bias in Many Species. BioEssays 2011, 33, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Ellegren, H. Characteristics, Causes and Evolutionary Consequences of Male-Biased Mutation. Proc. R. Soc. B Biol. Sci. 2007, 274, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.L.; Strand, A.I.; Cox, L.A.; Ober, C.; Wall, J.D.; Moorjani, P.; Przeworski, M. A Comparison of Humans and Baboons Suggests Germline Mutation Rates Do Not Track Cell Divisions. PLoS Biol. 2020, 18, e3000838. [Google Scholar] [CrossRef] [PubMed]
- de Manuel, M.; Wu, F.L.; Przeworski, M. A Paternal Bias in Germline Mutation Is Widespread in Amniotes and Can Arise Independently of Cell Division Numbers. eLife 2022, 11, e80008. [Google Scholar] [CrossRef]
- Wang, R.J.; Peña-García, Y.; Raveendran, M.; Harris, R.A.; Nguyen, T.-T.; Gingras, M.-C.; Wu, Y.; Perez, L.; Yoder, A.D.; Simmons, J.H.; et al. Unprecedented Female Mutation Bias in the Aye-Aye, a Highly Unusual Lemur from Madagascar. PLoS Biol. 2025, 23, e3003015. [Google Scholar] [CrossRef]
- Agrawal, A.F.; Wang, A.D. Increased Transmission of Mutations by Low-Condition Females: Evidence for Condition-Dependent DNA Repair. PLoS Biol. 2008, 6, e30. [Google Scholar] [CrossRef]
- Chesi, A.; Staahl, B.T.; Jovičić, A.; Couthouis, J.; Fasolino, M.; Raphael, A.R.; Yamazaki, T.; Elias, L.; Polak, M.; Kelly, C.; et al. Exome Sequencing to Identify de Novo Mutations in Sporadic ALS Trios. Nat. Neurosci. 2013, 16, 851–855. [Google Scholar] [CrossRef]
- Samocha, K.E.; Robinson, E.B.; Sanders, S.J.; Stevens, C.; Sabo, A.; McGrath, L.M.; Kosmicki, J.A.; Rehnström, K.; Mallick, S.; Kirby, A.; et al. A Framework for the Interpretation of de Novo Mutation in Human Disease. Nat. Genet. 2014, 46, 944–950. [Google Scholar] [CrossRef]
- Melamed, D.; Nov, Y.; Malik, A.; Yakass, M.B.; Bolotin, E.; Shemer, R.; Hiadzi, E.K.; Skorecki, K.L.; Livnat, A. De Novo Mutation Rates at the Single-Mutation Resolution in a Human HBB Gene-Region Associated with Adaptation and Genetic Disease. Genome Res. 2022, 32, 488–498. [Google Scholar] [CrossRef]
- Melamed, D.; Shemer, R.; Bolotin, E.; Yakass, M.B.; Fink-Barkai, D.; Hiadzi, E.K.; Skorecki, K.L.; Livnat, A. De Novo Rates of a Trypanosoma-Resistant Mutation in Two Human Populations. bioRxiv 2024. bioRxiv:2024.10.10.617206. [Google Scholar] [CrossRef] [PubMed]
- Galen, S.C.; Natarajan, C.; Moriyama, H.; Weber, R.E.; Fago, A.; Benham, P.M.; Chavez, A.N.; Cheviron, Z.A.; Storz, J.F.; Witt, C.C. Contribution of a Mutational Hot Spot to Hemoglobin Adaptation in High-Altitude Andean House Wrens. Proc. Natl. Acad. Sci. USA 2015, 112, 13958–13963. [Google Scholar] [CrossRef] [PubMed]
- Livnat, A.; Melamed, D. Evolutionary Honing in and Mutational Replacement: How Long-Term Directed Mutational Responses to Specific Environmental Pressures Are Possible. Theory Biosci. 2023, 142, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Si, W.; Yuan, Y.; Huang, J.; Zhang, X.; Zhang, Y.; Zhang, Y.; Tian, D.; Wang, C.; Yang, Y.; Yang, S. Widely Distributed Hot and Cold Spots in Meiotic Recombination as Shown by the Sequencing of Rice F2 Plants. N. Phytol. 2015, 206, 1491–1502. [Google Scholar] [CrossRef]
- Paigen, K.; Petkov, P. Mammalian Recombination Hot Spots: Properties, Control and Evolution. Nat. Rev. Genet. 2010, 11, 221–233. [Google Scholar] [CrossRef]
- Smeds, L.; Mugal, C.F.; Qvarnström, A.; Ellegren, H. High-Resolution Mapping of Crossover and Non-Crossover Recombination Events by Whole-Genome Re-Sequencing of an Avian Pedigree. PLoS Genet. 2016, 12, e1006044. [Google Scholar] [CrossRef]
- Auton, A.; Li, Y.R.; Kidd, J.; Oliveira, K.; Nadel, J.; Holloway, J.K.; Hayward, J.J.; Cohen, P.E.; Greally, J.M.; Wang, J.; et al. Genetic Recombination Is Targeted towards Gene Promoter Regions in Dogs. PLoS Genet. 2013, 9, e1003984. [Google Scholar] [CrossRef]
- Hoge, C.; de Manuel, M.; Mahgoub, M.; Okami, N.; Fuller, Z.; Banerjee, S.; Baker, Z.; McNulty, M.; Andolfatto, P.; Macfarlan, T.S.; et al. Patterns of Recombination in Snakes Reveal a Tug-of-War between PRDM9 and Promoter-like Features. Science 2024, 383, eadj7026. [Google Scholar] [CrossRef]
- Fouks, B.; Miller, K.J.; Ross, C.; Jones, C.; Rueppell, O. Alternative Double Strand Break Repair Pathways Shape the Evolution of High Recombination in the Honey Bee, Apis Mellifera. Insect Mol. Biol. 2025, 34, 185–202. [Google Scholar] [CrossRef]
- Dreissig, S.; Mascher, M.; Heckmann, S. Variation in Recombination Rate Is Shaped by Domestication and Environmental Conditions in Barley. Mol. Biol. Evol. 2019, 36, 2029–2039. [Google Scholar] [CrossRef]
- Rodgers-Melnick, E.; Bradbury, P.J.; Elshire, R.J.; Glaubitz, J.C.; Acharya, C.B.; Mitchell, S.E.; Li, C.; Li, Y.; Buckler, E.S. Recombination in Diverse Maize Is Stable, Predictable, and Associated with Genetic Load. Proc. Natl. Acad. Sci. USA 2015, 112, 3823–3828. [Google Scholar] [CrossRef]
- Shen, B.; Freebern, E.; Jiang, J.; Maltecca, C.; Cole, J.B.; Liu, G.E.; Ma, L. Effect of Temperature and Maternal Age on Recombination Rate in Cattle. Front. Genet. 2021, 12, 682718. [Google Scholar] [CrossRef]
- Zhang, K.; Wu, X.-C.; Zheng, D.-Q.; Petes, T.D. Effects of Temperature on the Meiotic Recombination Landscape of the Yeast Saccharomyces Cerevisiae. mBio 2017, 8, 10–1128. [Google Scholar] [CrossRef]
- Winbush, A.; Singh, N.D. Genomics of Recombination Rate Variation in Temperature-Evolved Drosophila Melanogaster Populations. Genome Biol. Evol. 2020, 13, evaa252. [Google Scholar] [CrossRef]
- Dapper, A.L.; Payseur, B.A. Effects of Demographic History on the Detection of Recombination Hotspots from Linkage Disequilibrium. Mol. Biol. Evol. 2018, 35, 335–353. [Google Scholar] [CrossRef]
- Bogan, S.N.; Yi, S.V. Potential Role of DNA Methylation as a Driver of Plastic Responses to the Environment Across Cells, Organisms, and Populations. Genome Biol. Evol. 2024, 16, evae022. [Google Scholar] [CrossRef]
- Lokk, K.; Modhukur, V.; Rajashekar, B.; Märtens, K.; Mägi, R.; Kolde, R.; Koltšina, M.; Nilsson, T.K.; Vilo, J.; Salumets, A.; et al. DNA Methylome Profiling of Human Tissues Identifies Global and Tissue-Specific Methylation Patterns. Genome Biol. 2014, 15, 3248. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, S.; Rajakumar, R.; Abouheif, E.; Szyf, M. Epigenetic Variation in the Egfr Gene Generates Quantitative Variation in a Complex Trait in Ants. Nat. Commun. 2015, 6, 6513. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, C.A.; Chowdhury, A.; Quinn, K.A.; Jenkins, M.W.; Rollins, A.M.; Watanabe, M.; Ford, S.M. Fundamentals of DNA Methylation in Development. Pediatr. Res. 2024, 98, 458–469. [Google Scholar] [CrossRef]
- Metzger, D.C.H.; Schulte, P.M. Persistent and Plastic Effects of Temperature on DNA Methylation across the Genome of Threespine Stickleback (Gasterosteus aculeatus). Proc. R. Soc. B Biol. Sci. 2017, 284, 20171667. [Google Scholar] [CrossRef]
- Żemojtel, T.; kiełbasa, S.M.; Arndt, P.F.; Behrens, S.; Bourque, G.; Vingron, M. CpG Deamination Creates Transcription Factor–Binding Sites with High Efficiency. Genome Biol. Evol. 2011, 3, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, H.E. Genetics, Development and Evolution of Adaptive Pigmentation in Vertebrates. Heredity 2006, 97, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Manceau, M.; Domingues, V.S.; Linnen, C.R.; Rosenblum, E.B.; Hoekstra, H.E. Convergence in Pigmentation at Multiple Levels: Mutations, Genes and Function. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Luu, P.-L.; Stirzaker, C.; Clark, S.J. Methyl-CpG-Binding Domain Proteins: Readers of the Epigenome. Epigenomics 2015, 7, 1051–1073. [Google Scholar] [CrossRef]
- Alvarado, S.; Fernald, R.D.; Storey, K.B.; Szyf, M. The Dynamic Nature of DNA Methylation: A Role in Response to Social and Seasonal Variation. Integr. Comp. Biol. 2014, 54, 68–76. [Google Scholar] [CrossRef][Green Version]
- Klose, R.J.; Bird, A.P. Genomic DNA Methylation: The Mark and Its Mediators. Trends Biochem. Sci. 2006, 31, 89–97. [Google Scholar] [CrossRef]
- Roloff, T.C.; Ropers, H.H.; Nuber, U.A. Comparative Study of Methyl-CpG-Binding Domain Proteins. BMC Genom. 2003, 4, 1. [Google Scholar] [CrossRef]
- Zemach, A.; McDaniel, I.E.; Silva, P.; Zilberman, D. Genome-Wide Evolutionary Analysis of Eukaryotic DNA Methylation. Science 2010, 328, 916–919. [Google Scholar] [CrossRef]
- de Mendoza, A.; Lister, R.; Bogdanovic, O. Evolution of DNA Methylome Diversity in Eukaryotes. J. Mol. Biol. 2019, 432, 1687–1705. [Google Scholar] [CrossRef]
- Monroe, J.G.; Srikant, T.; Carbonell-Bejerano, P.; Becker, C.; Lensink, M.; Exposito-Alonso, M.; Klein, M.; Hildebrandt, J.; Neumann, M.; Kliebenstein, D.; et al. Mutation Bias Reflects Natural Selection in Arabidopsis Thaliana. Nature 2022, 602, 101–105. [Google Scholar] [CrossRef]
- Cooper, D.N.; Mort, M.; Stenson, P.D.; Ball, E.V.; Chuzhanova, N.A. Methylation-Mediated Deamination of 5-Methylcytosine Appears to Give Rise to Mutations Causing Human Inherited Disease in CpNpG Trinucleotides, as Well as in CpG Dinucleotides. Hum. Genom. 2010, 4, 406. [Google Scholar] [CrossRef] [PubMed]
- Ségurel, L.; Wyman, M.J.; Przeworski, M. Determinants of Mutation Rate Variation in the Human Germline. Annu. Rev. Genom. Hum. Genet. 2014, 15, 47–70. [Google Scholar] [CrossRef] [PubMed]
- Maurano, M.T.; Humbert, R.; Rynes, E.; Thurman, R.E.; Haugen, E.; Wang, H.; Reynolds, A.P.; Sandstrom, R.; Qu, H.; Brody, J.; et al. Systematic Localization of Common Disease-Associated Variation in Regulatory DNA. Science 2012, 337, 1190–1195. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.J.R.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Børresen-Dale, A.-L.; et al. Signatures of Mutational Processes in Human Cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef]
- Mihola, O.; Landa, V.; Pratto, F.; Brick, K.; Kobets, T.; Kusari, F.; Gasic, S.; Smagulova, F.; Grey, C.; Flachs, P.; et al. Rat PRDM9 Shapes Recombination Landscapes, Duration of Meiosis, Gametogenesis, and Age of Fertility. BMC Biol. 2021, 19, 86. [Google Scholar] [CrossRef]
- Baudat, F.; Imai, Y.; Massy, B. de Meiotic Recombination in Mammals: Localization and Regulation. Nat. Rev. Genet. 2013, 14, 794–806. [Google Scholar] [CrossRef]
- Singhal, S.; Leffler, E.M.; Sannareddy, K.; Turner, I.; Venn, O.; Hooper, D.M.; Strand, A.I.; Li, Q.; Raney, B.; Balakrishnan, C.N.; et al. Stable Recombination Hotspots in Birds. Science 2015, 350, 928–932. [Google Scholar] [CrossRef]
- Raynaud, M.; Sanna, P.; Joseph, J.; Clément, J.; Imai, Y.; Lareyre, J.-J.; Laurent, A.; Galtier, N.; Baudat, F.; Duret, L.; et al. PRDM9 Drives the Location and Rapid Evolution of Recombination Hotspots in Salmonid Fish. PLoS Biol. 2025, 23, e3002950. [Google Scholar] [CrossRef]
- Giannattasio, T.; Testa, E.; Palombo, R.; Chellini, L.; Franceschini, F.; Crevenna, Á.; Petkov, P.M.; Paronetto, M.P.; Barchi, M. The RNA-Binding Protein FUS/TLS Interacts with SPO11 and PRDM9 and Localize at Meiotic Recombination Hotspots. Cell. Mol. Life Sci. 2023, 80, 107. [Google Scholar] [CrossRef]
- Wu, M.; Kwoh, C.-K.; Przytycka, T.M.; Li, J.; Zheng, J. Epigenetic Functions Enriched in Transcription Factors Binding to Mouse Recombination Hotspots. Proteome Sci. 2012, 10, S11. [Google Scholar] [CrossRef]
- Smagulova, F.; Brick, K.; Pu, Y.; Camerini-Otero, R.D.; Petukhova, G.V. The Evolutionary Turnover of Recombination Hot Spots Contributes to Speciation in Mice. Genes Dev. 2016, 30, 266–280. [Google Scholar] [CrossRef]
- Berg, I.L.; Neumann, R.; Sarbajna, S.; Odenthal-Hesse, L.; Butler, N.J.; Jeffreys, A.J. Variants of the Protein PRDM9 Differentially Regulate a Set of Human Meiotic Recombination Hotspots Highly Active in African Populations. Proc. Natl. Acad. Sci. USA 2011, 108, 12378–12383. [Google Scholar] [CrossRef]
- Berg, I.L.; Neumann, R.; Lam, K.-W.G.; Sarbajna, S.; Odenthal-Hesse, L.; May, C.A.; Jeffreys, A.J. PRDM9 Variation Strongly Influences Recombination Hot-Spot Activity and Meiotic Instability in Humans. Nat. Genet. 2010, 42, 859–863. [Google Scholar] [CrossRef]
- Jeffreys, A.J.; Cotton, V.E.; Neumann, R.; Lam, K.-W.G. Recombination Regulator PRDM9 Influences the Instability of Its Own Coding Sequence in Humans. Proc. Natl. Acad. Sci. USA 2013, 110, 600–605. [Google Scholar] [CrossRef]
- Baker, C.L.; Kajita, S.; Walker, M.; Saxl, R.L.; Raghupathy, N.; Choi, K.; Petkov, P.M.; Paigen, K. PRDM9 Drives Evolutionary Erosion of Hotspots in Mus Musculus through Haplotype-Specific Initiation of Meiotic Recombination. PLoS Genet. 2015, 11, e1004916. [Google Scholar] [CrossRef] [PubMed]
- Tsompana, M.; Buck, M.J. Chromatin Accessibility: A Window into the Genome. Epigenetics Chromatin 2014, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liang, R.; Li, Y.; Jiang, L.; Ma, D.; Luo, Q.; Song, G. Chromatin Accessibility: Biological Functions, Molecular Mechanisms and Therapeutic Application. Signal Transduct. Target. Ther. 2024, 9, 340. [Google Scholar] [CrossRef] [PubMed]
- Pudelko, L.; Cabianca, D.S. The Influencers’ Era: How the Environment Shapes Chromatin in 3D. Curr. Opin. Genet. Dev. 2024, 85, 102173. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, W.; Jiang, J. Genome-Wide Nucleosome Occupancy and Positioning and Their Impact on Gene Expression and Evolution in Plants. Plant Physiol. 2015, 168, 1406–1416. [Google Scholar] [CrossRef]
- Washietl, S.; Machné, R.; Goldman, N. Evolutionary Footprints of Nucleosome Positions in Yeast. Trends Genet. 2008, 24, 583–587. [Google Scholar] [CrossRef]
- Gonzalez-Perez, A.; Sabarinathan, R.; Lopez-Bigas, N. Local Determinants of the Mutational Landscape of the Human Genome. Cell 2019, 177, 101–114. [Google Scholar] [CrossRef]
- Ljungman, M.; Hanawalt, P.C. Efficient Protection against Oxidative DNA Damage in Chromatin. Mol. Carcinog. 1992, 5, 264–269. [Google Scholar] [CrossRef]
- Brambilla, F.; Garcia-Manteiga, J.M.; Monteleone, E.; Hoelzen, L.; Zocchi, A.; Agresti, A.; Bianchi, M.E. Nucleosomes Effectively Shield DNA from Radiation Damage in Living Cells. Nucleic Acids Res. 2020, 48, gkaa613. [Google Scholar] [CrossRef]
- Li, C.; Luscombe, N.M. Nucleosome Positioning Stability Is a Modulator of Germline Mutation Rate Variation across the Human Genome. Nat. Commun. 2020, 11, 1363. [Google Scholar] [CrossRef] [PubMed]
- Gompel, N.; Prud’homme, B. The Causes of Repeated Genetic Evolution. Dev. Biol. 2009, 332, 36–47. [Google Scholar] [CrossRef]
- Chan, Y.F.; Marks, M.E.; Jones, F.C.; Villarreal, G., Jr.; Shapiro, M.D.; Brady, S.D.; Southwick, A.M.; Absher, D.M.; Grimwood, J.; Schmutz, J.; et al. Adaptive Evolution of Pelvic Reduction in Sticklebacks by Recurrent Deletion of a Pitx1 Enhancer. Science 2010, 327, 302–305. [Google Scholar] [CrossRef]
- Thompson, A.C.; Capellini, T.D.; Guenther, C.A.; Chan, Y.F.; Infante, C.R.; Menke, D.B.; Kingsley, D.M. A Novel Enhancer near the Pitx1 Gene Influences Development and Evolution of Pelvic Appendages in Vertebrates. eLife 2018, 7, e38555. [Google Scholar] [CrossRef]
- Xie, K.T.; Wang, G.; Thompson, A.C.; Wucherpfennig, J.I.; Reimchen, T.E.; MacColl, A.D.C.; Schluter, D.; Bell, M.A.; Vasquez, K.M.; Kingsley, D.M. DNA Fragility in the Parallel Evolution of Pelvic Reduction in Stickleback Fish. Science 2019, 363, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Cal, L.; Suarez-Bregua, P.; Cerdá-Reverter, J.M.; Braasch, I.; Rotllant, J. Fish Pigmentation and the Melanocortin System. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2017, 211, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Maan, M.E.; Sefc, K.M. Colour Variation in Cichlid Fish: Developmental Mechanisms, Selective Pressures and Evolutionary Consequences. Semin. Cell Dev. Biol. 2013, 24, 516–528. [Google Scholar] [CrossRef]
- Albertson, R.C.; Powder, K.E.; Hu, Y.; Coyle, K.P.; Roberts, R.B.; Parsons, K.J. Genetic Basis of Continuous Variation in the Levels and Modular Inheritance of Pigmentation in Cichlid Fishes. Mol. Ecol. 2014, 23, 5135–5150. [Google Scholar] [CrossRef]
- Fulgione, D.; Lega, C.; Trapanese, M.; Buglione, M. Genetic Factors Implied in Melanin-based Coloration of the Italian Wall Lizard. J. Zoöl. 2015, 296, 278–285. [Google Scholar] [CrossRef]
- Jin, Y.; Tong, H.; Shao, G.; Li, J.; Lv, Y.; Wo, Y.; Brown, R.P.; Fu, C. Dorsal Pigmentation and Its Association with Functional Variation in MC1R in a Lizard from Different Elevations on the Qinghai–Tibetan Plateau. Genome Biol. Evol. 2020, 12, 2303–2313. [Google Scholar] [CrossRef]
- Uy, J.A.C.; Cooper, E.A.; Cutie, S.; Concannon, M.R.; Poelstra, J.W.; Moyle, R.G.; Filardi, C.E. Mutations in Different Pigmentation Genes Are Associated with Parallel Melanism in Island Flycatchers. Proc. R. Soc. B Biol. Sci. 2016, 283, 20160731. [Google Scholar] [CrossRef]
- Mundy, N.I. A Window on the Genetics of Evolution: MC1R and Plumage Colouration in Birds. Proc. R. Soc. B Biol. Sci. 2005, 272, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Waterland, R.A.; Jirtle, R.L. Transposable Elements: Targets for Early Nutritional Effects on Epigenetic Gene Regulation. Mol. Cell Biol. 2003, 23, 5293–5300. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, W.R. Regressive Evolution in Astyanax Cavefish. Annu. Rev. Genet. 2009, 43, 25–47. [Google Scholar] [CrossRef] [PubMed]
- Strickler, A.G.; Yamamoto, Y.; Jeffery, W.R. Early and Late Changes in Pax6 Expression Accompany Eye Degeneration during Cavefish Development. Dev. Genes Evol. 2001, 211, 138–144. [Google Scholar] [CrossRef]
- Gore, A.V.; Tomins, K.A.; Iben, J.; Ma, L.; Castranova, D.; Davis, A.E.; Parkhurst, A.; Jeffery, W.R.; Weinstein, B.M. An Epigenetic Mechanism for Cavefish Eye Degeneration. Nat. Ecol. Evol. 2018, 2, 1155–1160. [Google Scholar] [CrossRef]
- Han, G.; Li, Y.; Yang, Z.; Wang, C.; Zhang, Y.; Wang, B. Molecular Mechanisms of Plant Trichome Development. Front. Plant Sci. 2022, 13, 910228. [Google Scholar] [CrossRef]
- Jabbour, F.; Nadot, S.; Damerval, C. Evolution of Floral Symmetry: A State of the Art. C. R. Biol. 2009, 332, 219–231. [Google Scholar] [CrossRef]
- Preston, J.C.; Hileman, L.C. Parallel Evolution of TCP and B-Class Genes in Commelinaceae Flower Bilateral Symmetry. EvoDevo 2012, 3, 6. [Google Scholar] [CrossRef]
- Damerval, C.; Guilloux, M.L.; Jager, M.; Charon, C. Diversity and Evolution of CYCLOIDEA-Like TCP Genes in Relation to Flower Development in Papaveraceae. Plant Physiol. 2006, 143, 759–772. [Google Scholar] [CrossRef]
- Egger, G.; Liang, G.; Aparicio, A.; Jones, P.A. Epigenetics in Human Disease and Prospects for Epigenetic Therapy. Nature 2004, 429, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Weaver, I.C.G.; Cervoni, N.; Champagne, F.A.; D’Alessio, A.C.; Sharma, S.; Seckl, J.R.; Dymov, S.; Szyf, M.; Meaney, M.J. Epigenetic Programming by Maternal Behavior. Nat. Neurosci. 2004, 7, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.S.; Liu, X.S.; Brodsky, A.S.; Li, W.; Meyer, C.A.; Szary, A.J.; Eeckhoute, J.; Shao, W.; Hestermann, E.V.; Geistlinger, T.R.; et al. Chromosome-Wide Mapping of Estrogen Receptor Binding Reveals Long-Range Regulation Requiring the Forkhead Protein FoxA1. Cell 2005, 122, 33–43. [Google Scholar] [CrossRef]
- Zannas, A.S.; Wiechmann, T.; Gassen, N.C.; Binder, E.B. Gene–Stress–Epigenetic Regulation of FKBP5: Clinical and Translational Implications. Neuropsychopharmacology 2016, 41, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Tsankova, N.; Renthal, W.; Kumar, A.; Nestler, E.J. Epigenetic Regulation in Psychiatric Disorders. Nat. Rev. Neurosci. 2007, 8, 355–367. [Google Scholar] [CrossRef]
- Nestler, E.J. Epigenetic Mechanisms of Drug Addiction. Neuropharmacology 2014, 76, 259–268. [Google Scholar] [CrossRef]
- Smale, S.T. Hierarchies of NF-κB Target-Gene Regulation. Nat. Immunol. 2011, 12, 689–694. [Google Scholar] [CrossRef]
- Vahedi, G.; Takahashi, H.; Nakayamada, S.; Sun, H.; Sartorelli, V.; Kanno, Y.; O’Shea, J.J. STATs Shape the Active Enhancer Landscape of T Cell Populations. Cell 2012, 151, 981–993. [Google Scholar] [CrossRef]
- Netea, M.G.; Joosten, L.A.B.; Latz, E.; Mills, K.H.G.; Natoli, G.; Stunnenberg, H.G.; O’Neill, L.A.J.; Xavier, R.J. Trained Immunity: A Program of Innate Immune Memory in Health and Disease. Science 2016, 352, aaf1098. [Google Scholar] [CrossRef]
- Wolff, G.L.; Kodell, R.L.; Moore, S.R.; Cooney, C.A. Maternal Epigenetics and Methyl Supplements Affect Agouti Gene Expression in Avy/a Mice. FASEB J. 1998, 12, 949–957. [Google Scholar] [CrossRef]
- Davie, J.R. Inhibition of Histone Deacetylase Activity by Butyrate. J. Nutr. 2003, 133, 2485S–2493S. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. Chapter Three The Role of Short-Chain Fatty Acids in Health and Disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef] [PubMed]
- Vecsey, C.G.; Hawk, J.D.; Lattal, K.M.; Stein, J.M.; Fabian, S.A.; Attner, M.A.; Cabrera, S.M.; McDonough, C.B.; Brindle, P.K.; Abel, T.; et al. Histone Deacetylase Inhibitors Enhance Memory and Synaptic Plasticity via CREB: CBP-Dependent Transcriptional Activation. J. Neurosci. 2007, 27, 6128–6140. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-F.; Mandel, E.M.; Thomson, J.M.; Wu, Q.; Callis, T.E.; Hammond, S.M.; Conlon, F.L.; Wang, D.-Z. The Role of microRNA-1 and microRNA-133 in Skeletal Muscle Proliferation and Differentiation. Nat. Genet. 2006, 38, 228–233. [Google Scholar] [CrossRef]
- Heimberg, A.M.; Sempere, L.F.; Moy, V.N.; Donoghue, P.C.J.; Peterson, K.J. MicroRNAs and the Advent of Vertebrate Morphological Complexity. Proc. Natl. Acad. Sci. USA 2008, 105, 2946–2950. [Google Scholar] [CrossRef]
- Kung, J.T.Y.; Colognori, D.; Lee, J.T. Long Noncoding RNAs: Past, Present, and Future. Genetics 2013, 193, 651–669. [Google Scholar] [CrossRef]
- Roundtree, I.A.; Evans, M.E.; Pan, T.; He, C. Dynamic RNA Modifications in Gene Expression Regulation. Cell 2017, 169, 1187–1200. [Google Scholar] [CrossRef]
- Lynch, V.J.; Leclerc, R.D.; May, G.; Wagner, G.P. Transposon-Mediated Rewiring of Gene Regulatory Networks Contributed to the Evolution of Pregnancy in Mammals. Nat. Genet. 2011, 43, 1154–1159. [Google Scholar] [CrossRef]
- Joron, M.; Frezal, L.; Jones, R.T.; Chamberlain, N.L.; Lee, S.F.; Haag, C.R.; Whibley, A.; Becuwe, M.; Baxter, S.W.; Ferguson, L.; et al. Chromosomal Rearrangements Maintain a Polymorphic Supergene Controlling Butterfly Mimicry. Nature 2011, 477, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, L.P.; Witzany, G. Viruses Are Essential Agents within the Roots and Stem of the Tree of Life. J. Theor. Biol. 2010, 262, 698–710. [Google Scholar] [CrossRef]
- Chuong, E.B.; Elde, N.C.; Feschotte, C. Regulatory Evolution of Innate Immunity through Co-Option of Endogenous Retroviruses. Science 2016, 351, 1083–1087. [Google Scholar] [CrossRef] [PubMed]
- Rebollo, R.; Romanish, M.T.; Mager, D.L. Transposable Elements: An Abundant and Natural Source of Regulatory Sequences for Host Genes. Annu. Rev. Genet. 2012, 46, 21–42. [Google Scholar] [CrossRef] [PubMed]
- Henry, L.P.; Bruijning, M.; Forsberg, S.K.G.; Ayroles, J.F. The Microbiome Extends Host Evolutionary Potential. Nat. Commun. 2021, 12, 5141. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarado, S.G.; Chang, A.; Tajerian, M. Convergent Evolution and the Epigenome. Epigenomes 2025, 9, 45. https://doi.org/10.3390/epigenomes9040045
Alvarado SG, Chang A, Tajerian M. Convergent Evolution and the Epigenome. Epigenomes. 2025; 9(4):45. https://doi.org/10.3390/epigenomes9040045
Chicago/Turabian StyleAlvarado, Sebastian Gaston, Annaliese Chang, and Maral Tajerian. 2025. "Convergent Evolution and the Epigenome" Epigenomes 9, no. 4: 45. https://doi.org/10.3390/epigenomes9040045
APA StyleAlvarado, S. G., Chang, A., & Tajerian, M. (2025). Convergent Evolution and the Epigenome. Epigenomes, 9(4), 45. https://doi.org/10.3390/epigenomes9040045





