The Roles of the Histone Protein Modifier EZH2 in the Uterus and Placenta
Abstract
1. Introduction
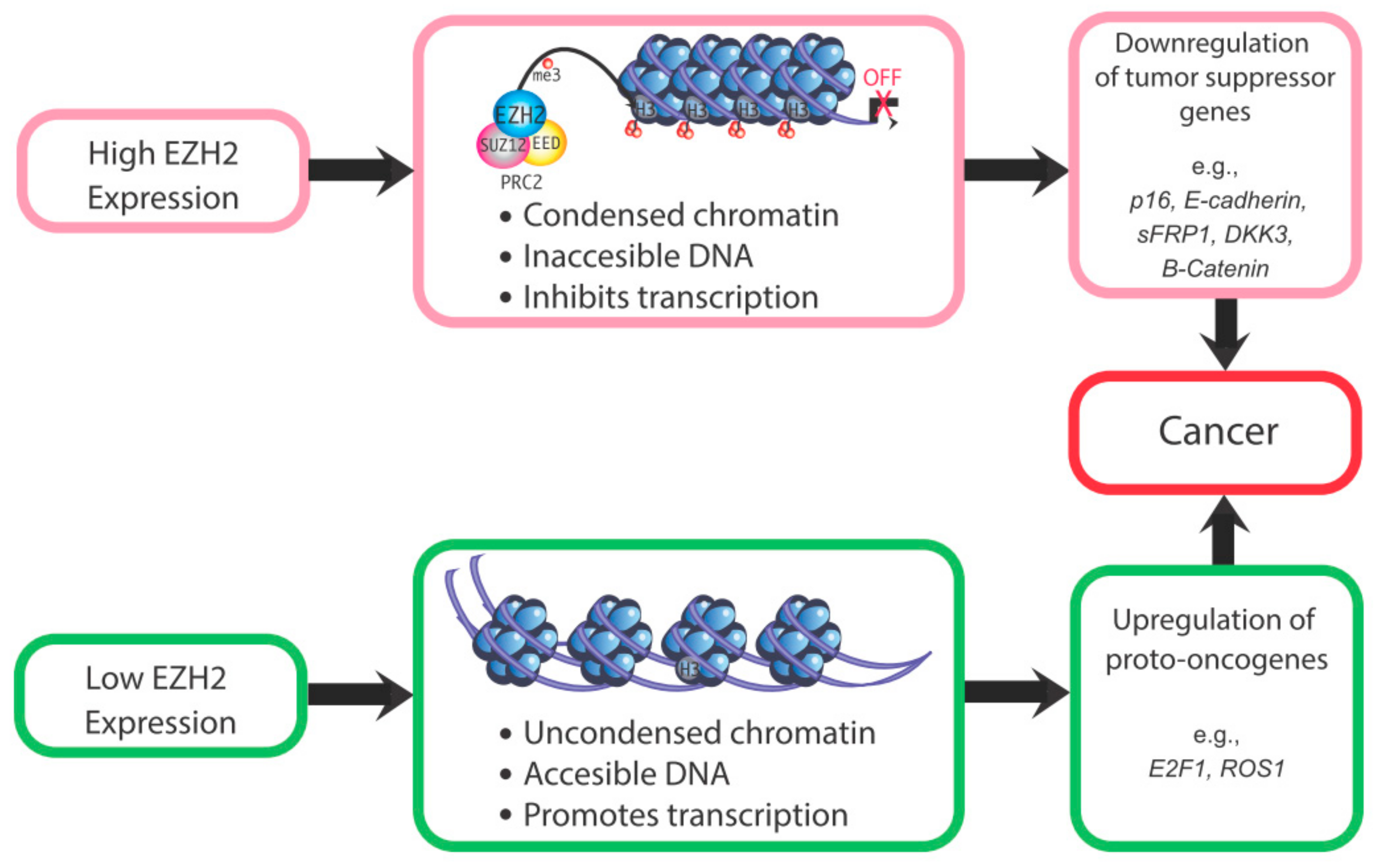
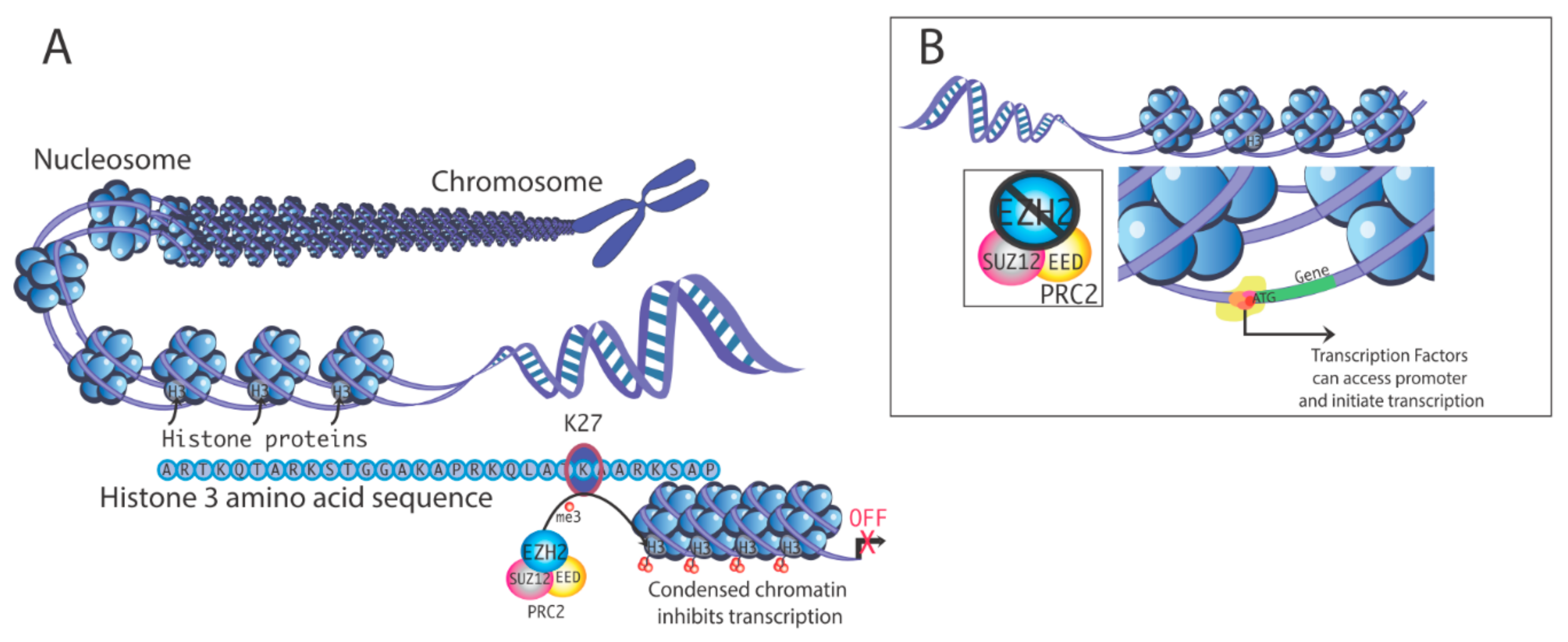
2. Actions of EZH2
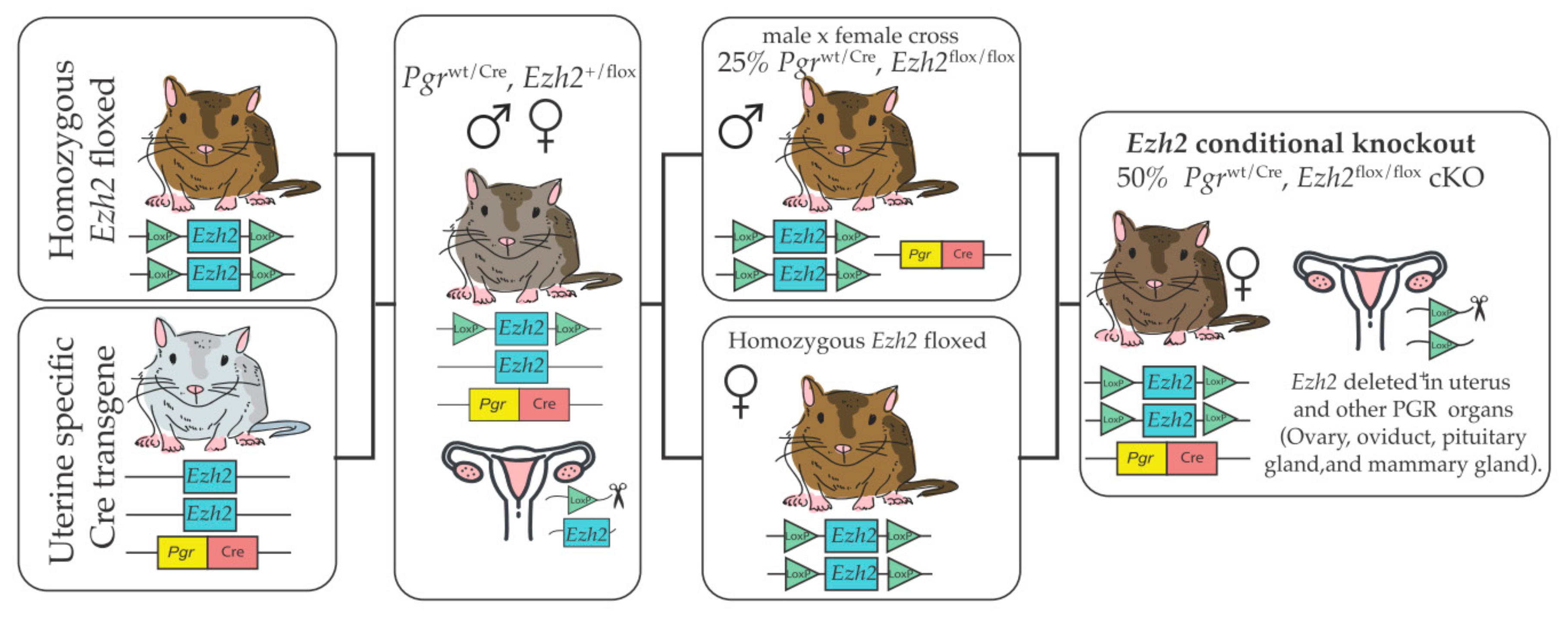
3. Transgenic Models to Study Global and Uterine Roles of EZH2
4. Roles of EZH2 in the Normal Uterus and Uterine Pathologies
5. Role of EZH2 in the Placenta
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AEBP2 | AE Binding Protein 2 |
| AKT | protein kinase B |
| CBX | chromobox-containing protein |
| CYP19 | aromatase P450 |
| 11ß-HSD2 | 11ß-hydroxysteroid dehydrogenase 2 |
| Cdca5 | cell division cycle associated 5 |
| CDX1 | caudal type homeobox 1 |
| E2 | 17β-estradiol |
| EED | embryonic ectoderm development |
| EMT | epithelial mesenchymal transition |
| EREs | estrogen response elements |
| ESR1 | estrogen receptor 1 |
| EZH2 | enhancer of zeste homolog 2 |
| Ezh2cKO | Ezh2 conditional knockout |
| GATA4 | GATA Binding Protein 4 |
| GD | gestation day |
| HDACs | histone deacetylases |
| HDMTs | histone demethylases |
| HATs | histone acetylases |
| JARID | jumonji, AT rich interactive domain 2 |
| Krt5/Krt15/Krt6A/Krt14 | keratin 5, 15, 6A and 14 |
| Lgr5 | leucine-rich repeat-containing G-protein-coupled receptor 5 |
| lncRNAs | long non-coding RNA |
| miRNA | microRNA |
| ncRNA | non-coding RNAs |
| PGR | progesterone receptor |
| piRNA | piwi-interacting RNA |
| PCL | polycomb-like |
| PND | postnatal day |
| PRC1 and PRC2 | polycomb repressive complex 1 and 2 |
| PTM | post-translational modifications |
| RbAp46/48 | retinoblastoma (Rb)-associated protein 46/48 |
| RORα | RAR-related orphan receptor alpha |
| siRNA | small interfering RNA |
| STAT3 | signal transducer and activator of transcription 3 |
| SUZ12 | suppressor of zeste 12 |
| TNM | tumor necrosis and metastasis (method to classify tumors) |
| V | vehicle |
| WGCNA | weighted gene co-expression network analysis |
| YY1 | Yin Yang 1 |
References
- Richards, E.J. Inherited epigenetic variation—Revisiting soft inheritance. Nat. Rev. Genet. 2006, 7, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Miska, E.A.; Ferguson-Smith, A.C. Transgenerational inheritance: Models and mechanisms of non–DNA sequence–based inheritance. Science 2016, 354, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Deaton, A.M.; Bird, A. CpG islands and the regulation of transcription. Genes Dev. 2011, 25, 1010–1022. [Google Scholar] [CrossRef]
- Clapier, C.R.; Cairns, B.R. The biology of chromatin remodeling complexes. Annu. Rev. Biochem. 2009, 78, 273–304. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and its basic function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef]
- Hernández-Romero, I.A.; Guerra-Calderas, L.; Salgado-Albarrán, M.; Maldonado-Huerta, T.; Soto-Reyes, E. The regulatory roles of non-coding RNAs in angiogenesis and neovascularization from anepigenetic perspective. Front. Oncol. 2019, 9, 1091. [Google Scholar] [CrossRef]
- Fernandes, J.C.R.; Acuña, S.M.; Aoki, J.I.; Floeter-Winter, L.M.; Muxel, S.M. Long non-coding RNAs in the regulation of gene expression: Physiology and disease. Non-Codin. RNA 2019, 5, 17. [Google Scholar] [CrossRef]
- Jenuwein, T.; Allis, C.D. Translating the histone code. Science 2001, 293, 1074–1080. [Google Scholar] [CrossRef]
- Hammond, C.M.; Strømme, C.B.; Huang, H.; Patel, D.J.; Groth, A. Histone chaperone networks shaping chromatin function. Nat. Rev. Mol. Cell Biol. 2017, 18, 141. [Google Scholar] [CrossRef]
- Sadakierska-Chudy, A.; Filip, M. A comprehensive view of the epigenetic landscape. Part II: Histone post-translational modification, nucleosome level, and chromatin regulation by ncRNAs. Neurotox. Res. 2015, 27, 172–197. [Google Scholar] [CrossRef]
- Hyun, K.; Jeon, J.; Park, K.; Kim, J. Writing, erasing and reading histone lysine methylations. Exp. Mol. Med. 2017, 49, e324. [Google Scholar] [CrossRef] [PubMed]
- Black, J.C.; Van Rechem, C.; Whetstine, J.R. Histone lysine methylation dynamics: Establishment, regulation, and biological impact. Mol. Cell 2012, 48, 491–507. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.-W.; Huang, K.; Yang, C.; Kang, C.-S. Non-coding RNAs as regulators in epigenetics. Oncol. Rep. 2017, 37, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Treviño, L.S.; Wang, Q.; Walker, C.L. Phosphorylation of epigenetic “readers, writers and erasers”: Implications for developmental reprogramming and the epigenetic basis for health and disease. Prog. Biophys. Mol. Biol. 2015, 118, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Gillette, T.G.; Hill, J.A. Readers, writers, and erasers: Chromatin as the whiteboard of heart disease. Circ. Res. 2015, 116, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Aranda, S.; Mas, G.; Di Croce, L. Regulation of gene transcription by Polycomb proteins. Sci. Adv. 2015, 1, e1500737. [Google Scholar] [CrossRef]
- Pirrotta, V. Polycombing the genome: PcG, trxG, and chromatin silencing. Cell 1998, 93, 333–336. [Google Scholar] [CrossRef]
- Cao, R.; Wang, L.; Wang, H.; Xia, L.; Erdjument-Bromage, H.; Tempst, P.; Jones, R.S.; Zhang, Y. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 2002, 298, 1039–1043. [Google Scholar] [CrossRef]
- Bracken, A.P.; Pasini, D.; Capra, M.; Prosperini, E.; Colli, E.; Helin, K. EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer. EMBO J. 2003, 22, 5323–5335. [Google Scholar] [CrossRef]
- Colon-Caraballo, M.; Monteiro, J.B.; Flores, I. H3K27me3 is an epigenetic mark of relevance in endometriosis. Reprod. Sci. 2015, 22, 1134–1142. [Google Scholar] [CrossRef]
- Eskander, R.N.; Ji, T.; Huynh, B.; Wardeh, R.; Randall, L.M.; Hoang, B. Inhibition of enhancer of zeste homolog 2 (EZH2) expression is associated with decreased tumor cell proliferation, migration, and invasion in endometrial cancer cell lines. Int. J. Gynecol. Cancer 2013, 23, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Fluge, Ø.; Gravdal, K.; Carlsen, E.; Vonen, B.; Kjellevold, K.; Refsum, S.; Lilleng, R.; Eide, T.; Halvorsen, T.; Tveit, K.M. Expression of EZH2 and Ki-67 in colorectal cancer and associations with treatment response and prognosis. Br. J. Cancer 2009, 101, 1282–1289. [Google Scholar] [CrossRef]
- Kleer, C.G.; Cao, Q.; Varambally, S.; Shen, R.; Ota, I.; Tomlins, S.A.; Ghosh, D.; Sewalt, R.G.; Otte, A.P.; Hayes, D.F. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc. Natl. Acad. Sci. USA 2003, 100, 11606–11611. [Google Scholar] [CrossRef] [PubMed]
- Clair, J.M.-S.; Soydaner-Azeloglu, R.; Lee, K.E.; Taylor, L.; Livanos, A.; Pylayeva-Gupta, Y.; Miller, G.; Margueron, R.; Reinberg, D.; Bar-Sagi, D. EZH2 couples pancreatic regeneration to neoplastic progression. Genes Dev. 2012, 26, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Gil, J.; O'Loghlen, A. PRC1 complex diversity: Where is it taking us? Trends. Cell Biol. 2014, 24, 632–641. [Google Scholar] [CrossRef]
- Kahn, T.G.; Dorafshan, E.; Schultheis, D.; Zare, A.; Stenberg, P.; Reim, I.; Pirrotta, V.; Schwartz, Y.B. Interdependence of PRC1 and PRC2 for recruitment to polycomb response elements. Nucleic Acids Res. 2016, 44, 10132–10149. [Google Scholar] [CrossRef]
- Tan, J.-Z.; Yan, Y.; Wang, X.-X.; Jiang, Y.; Xu, H.E. EZH2: Biology, disease, and structure-based drug discovery. Acta Pharmacol. Sin. 2014, 35, 161–174. [Google Scholar] [CrossRef]
- Bredfeldt, T.G.; Greathouse, K.L.; Safe, S.H.; Hung, M.-C.; Bedford, M.T.; Walker, C.L. Xenoestrogen-induced regulation of EZH2 and histone methylation via estrogen receptor signaling to PI3K/AKT. J. Mol. Endocrinol. 2010, 24, 993–1006. [Google Scholar] [CrossRef]
- Wassef, M.; Luscan, A.; Aflaki, S.; Zielinski, D.; Jansen, P.W.T.C.; Baymaz, H.I.; Battistella, A.; Kersouani, C.; Servant, N.; Wallace, M.R.; et al. EZH1/2 function mostly within canonical PRC2 and exhibit proliferation-dependent redundancy that shapes mutational signatures in cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 6075–6080. [Google Scholar] [CrossRef]
- Han, Z.; Xing, X.; Hu, M.; Zhang, Y.; Liu, P.; Chai, J. Structural basis of EZH2 recognition by EED. Structure 2007, 15, 1306–1315. [Google Scholar] [CrossRef][Green Version]
- Margueron, R.; Reinberg, D. The Polycomb complex PRC2 and its mark in life. Nature 2011, 469, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Hung, M.-C. Regulation and role of EZH2 in cancer. Cancer Res. Treat. 2014, 46, 209. [Google Scholar] [CrossRef]
- Cha, T.-L.; Zhou, B.P.; Xia, W.; Wu, Y.; Yang, C.-C.; Chen, C.-T.; Ping, B.; Otte, A.P.; Hung, M.-C. Akt-mediated phosphorylation of EZH2 suppresses methylation of lysine 27 in histone H3. Science 2005, 310, 306–310. [Google Scholar] [CrossRef]
- Kaneko, S.; Li, G.; Son, J.; Xu, C.-F.; Margueron, R.; Neubert, T.A.; Reinberg, D. Phosphorylation of the PRC2 component Ezh2 is cell cycle-regulated and up-regulates its binding to ncRNA. Genes Dev. 2010, 24, 2615–2620. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Yang, Y.; Li, Q.; Feng, Y.; Liu, T.; Guo, W. Epigenetic regulation of cancer progression by EZH2: From biological insights to therapeutic potential. Biomark. Res. 2018, 6, 10. [Google Scholar] [CrossRef]
- O’Carroll, D.; Erhardt, S.; Pagani, M.; Barton, S.C.; Surani, M.A.; Jenuwein, T. The polycomb-group gene Ezh2 is required for early mouse development. Mol. Cell Biol. 2001, 21, 4330–4336. [Google Scholar] [CrossRef]
- Soyal, S.M.; Mukherjee, A.; Lee, K.Y.S.; Li, J.; Li, H.; DeMayo, F.J.; Lydon, J.P. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis 2005, 41, 58–66. [Google Scholar] [CrossRef]
- Shen, X.; Liu, Y.; Hsu, Y.-J.; Fujiwara, Y.; Kim, J.; Mao, X.; Yuan, G.-C.; Orkin, S.H. EZH1 mediates methylation on histone H3 lysine 27 and complements EZH2 in maintaining stem cell identity and executing pluripotency. Mol. Cell 2008, 32, 491–502. [Google Scholar] [CrossRef]
- Mesa, A.M.; Mao, J.; Nanjappa, M.K.; Medrano, T.I.; Tevosian, S.; Yu, F.; Kinkade, J.; Lyu, Z.; Liu, Y.; Joshi, T.; et al. Mice lacking uterine enhancer of zeste homolog 2 have transcriptomic changes associated with uterine epithelial proliferation. Physiol. Genom. 2020, 52, 81–95. [Google Scholar] [CrossRef]
- Fang, X.; Ni, N.; Lydon, J.P.; Ivanov, I.; Bayless, K.J.; Rijnkels, M.; Li, Q. Enhancer of zeste 2 polycomb repressive complex 2 subunit is required for uterine epithelial integrity. Am. J. Pathol. 2019, 189, 1212–1225. [Google Scholar] [CrossRef]
- Greathouse, K.L.; Bredfeldt, T.; Everitt, J.I.; Lin, K.; Berry, T.; Kannan, K.; Mittelstadt, M.L.; Ho, S.M.; Walker, C.L. Environmental estrogens differentially engage the histone methyltransferase EZH2 to increase risk of uterine tumorigenesis. Mol. Cancer Res. 2012, 10, 546–557. [Google Scholar] [CrossRef]
- Yang, Q.; Laknaur, A.; Elam, L.; Ismail, N.; Gavrilova-Jordan, L.; Lue, J.; Diamond, M.P.; Al-Hendy, A. Identification of polycomb group protein EZH2-mediated DNA mismatch repair gene MSH2 in human uterine fibroids. Reprod. Sci. 2016, 23, 1314–1325. [Google Scholar] [CrossRef][Green Version]
- Yang, Q.; Diamond, M.P.; Al-Hendy, A. Early life adverse environmental exposures increase the risk of uterine fibroid development: Role of epigenetic regulation. Front. Pharmacol. 2016, 7, 40. [Google Scholar] [CrossRef]
- Yang, Q.; Nair, S.; Laknaur, A.; Ismail, N.; Diamond, M.P.; Al-Hendy, A. The polycomb group protein EZH2 impairs DNA damage repair gene expression in hman uterine fibroids. Biol. Reprod. 2016, 94, 69. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Q.; Guo, S.W. Histological and immunohistochemical characterization of the similarity and difference between ovarian endometriomas and deep infiltrating endometriosis. Reprod. Sci. 2018, 25, 329–340. [Google Scholar] [CrossRef]
- Nanjappa, M.K.; Mesa, A.M.; Medrano, T.I.; Jefferson, W.N.; DeMayo, F.J.; Williams, C.J.; Lydon, J.P.; Levin, E.R.; Cooke, P.S. The histone methyltransferase EZH2 is required for normal uterine development and function in mice. Biol. Reprod. 2019, 101, 306–317. [Google Scholar] [CrossRef]
- Oki, S.; Sone, K.; Oda, K.; Hamamoto, R.; Ikemura, M.; Maeda, D.; Takeuchi, M.; Tanikawa, M.; Mori-Uchino, M.; Nagasaka, K.; et al. Oncogenic histone methyltransferase EZH2: A novel prognostic marker with therapeutic potential in endometrial cancer. Oncotarget 2017, 8, 40402–40411. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, C.; Wang, H. HOXA5 inhibits the proliferation and induces the apoptosis of cervical cancer cells via regulation of protein kinase B and p27. Oncol. Rep. 2019, 41, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, S.; Li, Q. Uterine epithelial cell proliferation and endometrial hyperplasia: Evidence from a mouse model. Mol. Hum. Reprod. 2014, 20, 776–786. [Google Scholar] [CrossRef]
- Varambally, S.; Dhanasekaran, S.M.; Zhou, M.; Barrette, T.R.; Kumar-Sinha, C.; Sanda, M.G.; Ghosh, D.; Pienta, K.J.; Sewalt, R.G.A.B.; Otte, A.P. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 2002, 419, 624–629. [Google Scholar] [CrossRef]
- Zhang, Q.; Dong, P.; Liu, X.; Sakuragi, N.; Guo, S.-W. Enhancer of Zeste homolog 2 (EZH2) induces epithelial-mesenchymal transition in endometriosis. Sci. Rep. 2017, 7, 6804. [Google Scholar] [CrossRef]
- Kalluri, R.; Neilson, E.G. Epithelial-mesenchymal transition and its implications for fibrosis. J. Clin. Investig. 2003, 112, 1776–1784. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.J.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Colón-Caraballo, M.; Torres-Reverón, A.; Soto-Vargas, J.L.; Young, S.L.; Lessey, B.; Mendoza, A.; Urrutia, R.; Flores, I. Effects of histone methyltransferase inhibition in endometriosis†. Biol. Reprod. 2018, 99, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zhang, J.; Guan, H. Expression of EZH2 in endometrial carcinoma and its effects on proliferation and invasion of endometrial carcinoma cells. Oncol. Lett. 2017, 14, 7191–7196. [Google Scholar] [CrossRef][Green Version]
- Alldredge, J.K.; Eskander, R.N. EZH2 inhibition in ARID1A mutated clear cell and endometrioid ovarian and endometrioid endometrial cancers. Gynecol. Oncol. Res. Pract. 2017, 4, 17. [Google Scholar] [CrossRef]
- Kim, K.H.; Roberts, C.W. Targeting EZH2 in cancer. Nat. Med. 2016, 22, 128–134. [Google Scholar] [CrossRef]
- Shih, C.H.; Chang, Y.J.; Huang, W.C.; Jang, T.H.; Kung, H.J.; Wang, W.C.; Yang, M.H.; Lin, M.C.; Huang, S.F.; Chou, S.W.; et al. EZH2-mediated upregulation of ROS1 oncogene promotes oral cancer metastasis. Oncogene 2017, 36, 6542–6554. [Google Scholar] [CrossRef]
- Wu, Z.L.; Zheng, S.S.; Li, Z.M.; Qiao, Y.Y.; Aau, M.Y.; Yu, Q. Polycomb protein EZH2 regulates E2F1-dependent apoptosis through epigenetically modulating Bim expression. Cell Death Differ. 2010, 17, 801–810. [Google Scholar] [CrossRef]
- Kumari, K.; Das, B.; Adhya, A.K.; Rath, A.K.; Mishra, S.K. Genome-wide expression analysis reveals six contravened targets of EZH2 associated with breast cancer patient survival. Sci. Rep. 2019, 9, 1974. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.; Lu, X.; Song, B.; Fong, K.W.; Cao, Q.; Licht, J.D.; Zhao, J.C.; Yu, J. Polycomb- and methylation-independent roles of EZH2 as a transcription activator. Cell Rep. 2018, 25, 2808–2820. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Yan, Y.; Bai, Y.; Yang, Y.; Pan, Y.; Gang, X.; Karnes, R.J.; Zhang, J.; Lv, Q.; Wu, Q.; et al. Overcoming EZH2 inhibitor resistance by taxane in PTEN-mutated cancer. Theranostics 2019, 9, 5020–5034. [Google Scholar] [CrossRef] [PubMed]
- Brosens, I.; Pijnenborg, R.; Vercruysse, L.; Romero, R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am. J. Obstet. Gynecol. 2011, 204, 193–201. [Google Scholar] [CrossRef]
- Woods, L.; Perez-Garcia, V.; Hemberger, M. Regulation of placental development and its impact on fetal growth-new insights from mouse models. Front. Endocrinol. 2018, 9, 570. [Google Scholar] [CrossRef] [PubMed]
- Turco, M.Y.; Moffett, A. Development of the human placenta. Development 2019, 146, dev163428. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.J.; Varberg, K.M.; Iqbal, K. Hemochorial placentation: Development, function, and adaptations. Biol. Reprod. 2018, 99, 196–211. [Google Scholar] [CrossRef]
- Rossant, J.; Cross, J.C. Placental development: Lessons from mouse mutants. Nat. Rev. Genet. 2001, 2, 538–548. [Google Scholar] [CrossRef]
- Nugent, B.M.; O’Donnell, C.M.; Epperson, C.N.; Bale, T.L. Placental H3K27me3 establishes female resilience to prenatal insults. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Howerton, C.L.; Morgan, C.P.; Fischer, D.B.; Bale, T.L. O-GlcNAc transferase (OGT) as a placental biomarker of maternal stress and reprogramming of CNS gene transcription in development. Proc. Natl. Acad. Sci. USA 2013, 110, 5169–5174. [Google Scholar] [CrossRef]
- Wenzel, P.L.; Leone, G. Expression of Cre recombinase in early diploid trophoblast cells of the mouse placenta. Genesis 2007, 45, 129–134. [Google Scholar] [CrossRef]
- Cottrell, E.C.; Seckl, J. Prenatal stress, glucocorticoids and the programming of adult disease. Front. Behav. Neurosci. 2009, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Wyrwoll, C.S.; Seckl, J.R.; Holmes, M.C. Altered placental function of 11beta-hydroxysteroid dehydrogenase 2 knockout mice. Endocrinology 2009, 150, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Cottrell, E.C.; Seckl, J.R.; Holmes, M.C.; Wyrwoll, C.S. Foetal and placental 11β-HSD2: A hub for developmental programming. Acta Physiol. 2014, 210, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Zuo, R.; Liu, X.; Wang, W.; Li, W.; Ying, H.; Sun, K. A repressive role of enhancer of zeste homolog 2 in 11β-hydroxysteroid dehydrogenase type 2 expression in the human placenta. J. Biol. Chem. 2017, 292, 7578–7587. [Google Scholar] [CrossRef]
- Xu, Y.; Ge, Z.; Zhang, E.; Zuo, Q.; Huang, S.; Yang, N.; Wu, D.; Zhang, Y.; Chen, Y.; Xu, H.; et al. The lncRNA TUG1 modulates proliferation in trophoblast cells via epigenetic suppression of RND3. Cell Death Dis. 2017, 8, e3104. [Google Scholar] [CrossRef]
- Xu, Y.; Lian, Y.; Zhang, Y.; Huang, S.; Zuo, Q.; Yang, N.; Chen, Y.; Wu, D.; Sun, L. The long non-coding RNA PVT1 represses ANGPTL4 transcription through binding with EZH2 in trophoblast cell. J. Cell Mol. Med. 2018, 22, 1272–1282. [Google Scholar] [CrossRef]
- Lv, S.; Wang, N.; Lv, H.; Yang, J.; Liu, J.; Li, W.-P.; Zhang, C.; Chen, Z.-J. The attenuation of trophoblast invasion caused by the downregulation of EZH2 is involved in the pathogenesis of human recurrent miscarriage. Mol. Ther. Nucleic Acids 2019, 14, 377–387. [Google Scholar] [CrossRef]
- Huppertz, B. Traditional and new routes of trophoblast invasion and their implications for pregnancy diseases. Int. J. Mol. Sci. 2019, 21, 289. [Google Scholar] [CrossRef]
- Okae, H.; Toh, H.; Sato, T.; Hiura, H.; Takahashi, S.; Shirane, K.; Kabayama, Y.; Suyama, M.; Sasaki, H.; Arima, T. Derivation of human trophoblast stem cells. Cell Stem Cell 2018, 22, 50–63. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mesa, A.M.; Rosenfeld, C.S.; Tuteja, G.; Medrano, T.I.; Cooke, P.S. The Roles of the Histone Protein Modifier EZH2 in the Uterus and Placenta. Epigenomes 2020, 4, 20. https://doi.org/10.3390/epigenomes4030020
Mesa AM, Rosenfeld CS, Tuteja G, Medrano TI, Cooke PS. The Roles of the Histone Protein Modifier EZH2 in the Uterus and Placenta. Epigenomes. 2020; 4(3):20. https://doi.org/10.3390/epigenomes4030020
Chicago/Turabian StyleMesa, Ana M., Cheryl S. Rosenfeld, Geetu Tuteja, Theresa I. Medrano, and Paul S. Cooke. 2020. "The Roles of the Histone Protein Modifier EZH2 in the Uterus and Placenta" Epigenomes 4, no. 3: 20. https://doi.org/10.3390/epigenomes4030020
APA StyleMesa, A. M., Rosenfeld, C. S., Tuteja, G., Medrano, T. I., & Cooke, P. S. (2020). The Roles of the Histone Protein Modifier EZH2 in the Uterus and Placenta. Epigenomes, 4(3), 20. https://doi.org/10.3390/epigenomes4030020