Behavioral Epigenetics: Perspectives Based on Experience-Dependent Epigenetic Inheritance
Abstract
1. Background—Behavioral Epigenetics
2. Epigenetic Regulation Triggered by Environmental Enrichments
2.1. DNA Methylation
2.2. Histone Modification
2.3. Noncoding RNAs
3. Behavioral Epigenetic Inheritance Mechanisms
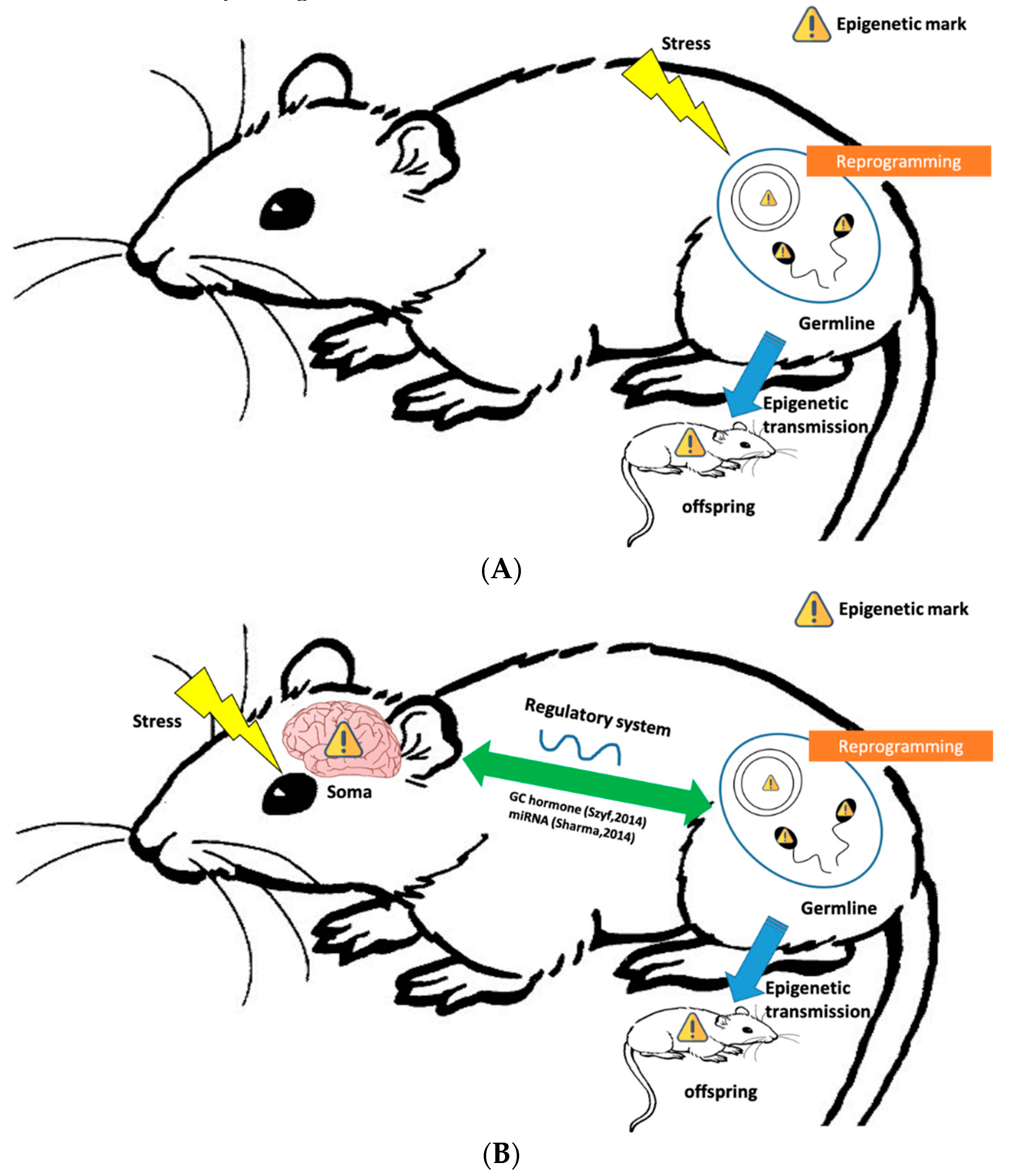
3.1. Germline-Mediated Epigenetic Inheritance
3.2. Integration between Germline and Somatic Cells in Epigenetic Inheritance
4. Considerations in the Establishment of Epigenetic Transgenerational Inheritance
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HPA | Hypothalamic-pituitary-adrenal |
References
- Slotkin, R.K.; Martienssen, R. Transposable elements and the epigenetic regulation of the genome. Nat. Rev. Genet. 2007, 8, 272. [Google Scholar] [CrossRef] [PubMed]
- Huypens, P.; Sass, S.; Wu, M.; Dyckhoff, D.; Tschöp, M.; Theis, F.; Marschall, S.; de Angelis, M.H.; Beckers, J. Epigenetic germline inheritance of diet-induced obesity and insulin resistance. Nat. Genet. 2016, 48, 497–499. [Google Scholar] [CrossRef] [PubMed]
- Anway, M.D.; Skinner, M.K. Epigenetic transgenerational actions of endocrine disruptors. Endocrinology 2006, 147, s43–s49. [Google Scholar] [CrossRef] [PubMed]
- Tracey, R.; Manikkam, M.; Guerrero-Bosagna, C.; Skinner, M.K. Hydrocarbons (jet fuel JP-8) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. Reprod. Toxicol. 2013, 36, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Barber, R.C.; Hickenbotham, P.; Hatch, T.; Kelly, D.; Topchiy, N.; Almeida, G.M.; Jones, G.D.D.; Johnson, G.E.; Parry, J.M.; Rothkamm, K.; et al. Radiation-induced transgenerational alterations in genome stability and DNA damage. Oncogene 2006, 25, 7336. [Google Scholar] [CrossRef] [PubMed]
- Powledge, T.M. Behavioral epigenetics: How nurture shapes nature. BioScience 2011, 61, 588–592. [Google Scholar] [CrossRef]
- McGowan, P.O.; Sasaki, A.; D’alessio, A.C.; Dymov, S.; Labonté, B.; Szyf, M.; Turecki, G.; Meaney, M.J. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat. Neurosci. 2009, 12, 342–348. [Google Scholar] [CrossRef]
- Franklin, T.B.; Russig, H.; Weiss, I.C.; Gräff, J.; Linder, N.; Michalon, A.; Vizi, S.; Mansuy, I.M. Epigenetic transmission of the impact of early stress across generations. Biol. Psychiatry 2010, 68, 408–415. [Google Scholar] [CrossRef]
- Mann, J.J.; Currier, D.M. Stress, genetics and epigenetic effects on the neurobiology of suicidal behavior and depression. Eur. Psychiatry 2010, 25, 268–271. [Google Scholar] [CrossRef]
- Maple, T.L.; Perdue, B.M. Environmental enrichment. In Zoo Animal Welfare; Springer: Heidelberg, Germany, 2013; pp. 95–117. [Google Scholar]
- Kinoshita, Y.; Saze, H.; Kinoshita, T.; Miura, A.; Soppe, W.J.; Koornneef, M.; Kakutaniet, T. Control of FWA gene silencing in Arabidopsis thaliana by SINE-related direct repeats. Plant J. 2007, 49, 38–45. [Google Scholar] [CrossRef]
- Chong, S.; Whitelaw, E. Epigenetic germline inheritance. Curr. Opin. Genet. Dev. 2004, 14, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.L.; Wang, F.F.; Shu, J.; Tian, S.; Jiang, Y.; Zhang, D.; Wang, N.; Luo, Q.; Zhang, Y.; Jin, F.; et al. Transgenerational glucose intolerance with Igf2/H19 epigenetic alterations in mouse islet induced by intrauterine hyperglycemia. Diabetes 2012, 61, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Rakyan, V.K.; Blewitt, M.E.; Druker, R.; Preis, J.I.; Whitelaw, E. Metastable epialleles in mammals. Trends Genet. 2002, 18, 348–351. [Google Scholar] [CrossRef]
- Halfmann, R.; Lindquist, S. Epigenetics in the extreme: Prions and the inheritance of environmentally acquired traits. Science 2010, 330, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Théodorou, V. Susceptibility to stress-induced visceral sensitivity: A bad legacy for next generations. Neurogastroenterol. Motil. 2013, 25, 927–930. [Google Scholar] [CrossRef]
- Witzmann, S.R.; Turner, J.D.; Mériaux, S.B.; Meijer, O.C.; Muller, C.P. Epigenetic regulation of the glucocorticoid receptor promoter 17 in adult rats. Epigenetics 2012, 7, 1290–1301. [Google Scholar] [CrossRef] [PubMed]
- Tsankova, N.M.; Berton, O.; Renthal, W.; Kumar, A.; Neve, R.L.; Nestler, E.J. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat. Neurosci. 2006, 9, 519. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, A.B.; Morgan, C.P.; Bronson, S.L.; Revello, S.; Bale, T.L. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J. Neurosci. 2013, 33, 9003–9012. [Google Scholar] [CrossRef]
- Roth, T.L.; Lubin, F.D.; Funk, A.J.; Sweatt, J.D. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol. Psychiatry 2009, 65, 760–769. [Google Scholar] [CrossRef]
- Dias, B.G.; Ressler, K.J. Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat. Neurosci. 2014, 17, 89–96. [Google Scholar] [CrossRef]
- Morgan, C.P.; Bale, T.L. Early prenatal stress epigenetically programs dysmasculinization in second-generation offspring via the paternal lineage. J. Neurosci. 2011, 31, 11748–11755. [Google Scholar] [CrossRef] [PubMed]
- Gapp, K.; Jawaid, A.; Sarkies, P.; Bohacek, J.; Pelczar, P.; Prados, J.; Farinelli, L.; Miska, E.; Mansuy, I.M. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat. Neurosci. 2014, 17, 667–669. [Google Scholar] [CrossRef] [PubMed]
- Peña, C.J.; Monk, C.; Champagne, F.A. Epigenetic effects of prenatal stress on 11β-hydroxysteroid dehydrogenase-2 in the placenta and fetal brain. PLoS ONE 2012, 7, e39791. [Google Scholar]
- Bird, A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002, 16, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Yong, W.S.; Hsu, F.M.; Chen, P.Y. Profiling genome-wide DNA methylation. Epigenetic Chromatin 2016, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Berton, O.; McClung, C.A.; DiLeone, R.J.; Krishnan, V.; Renthal, W.; Russo, S.J.; Graham, D.; Tsankova, N.M.; Bolanos, C.A.; Rioset, M.; et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 2006, 311, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef]
- Kouzarides, T. Chromatin modifications and their function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef]
- Vaissière, T.; Sawan, C.; Herceg, Z. Epigenetic interplay between histone modifications and DNA methylation in gene silencing. Mutat. Res. Rev. Mutat. Res. 2008, 659, 40–48. [Google Scholar] [CrossRef]
- Sato, F.; Tsuchiya, S.; Meltzer, S.J.; Shimizu, K. MicroRNAs and epigenetics. FEBS J. 2011, 278, 1598–1609. [Google Scholar] [CrossRef]
- Heard, E.; Martienssen, R.A. Transgenerational epigenetic inheritance: Myths and mechanisms. Cell 2014, 157, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.M.; Clark, A.; Chen, P.Y. Epigenetic reprogramming in the mammalian germline. Oncotarget 2015, 6, 35151–35152. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Santos, F.; Hendrich, B.; Reik, W.; Dean, W. Dynamic reprogramming of DNA methylation in the early mouse embryo. Dev. Biol. 2002, 241, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Gkountela, S.; Zhang, K.X.; Shafiq, T.A.; Liao, W.W.; Hargan-Calvopina, J.; Chen, P.Y.; Clark, A.T. DNA Demethylation Dynamics in the Human Prenatal Germline. Cell 2015, 161, 1425–1436. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, H.; Sun, Z. Lamarck rises from his grave: Parental environment-induced epigenetic inheritance in model organisms and humans. Biol. Rev. 2017, 92, 2084–2111. [Google Scholar] [CrossRef] [PubMed]
- Hargan-Calvopina, J.; Taylor, S.; Cook, H.; Hu, Z.X.; Lee, S.A.; Yen, M.R.; Chiang, Y.-S.; Chen, P.-Y.; Clark, A.T. Stage-Specific Demethylation in Primordial Germ Cells Safeguards against Precocious Differentiation. Dev. Cell 2016, 39, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.A.; Liang, G. Rethinking how DNA methylation patterns are maintained. Nat. Rev. Genet. 2009, 10, 805. [Google Scholar] [CrossRef] [PubMed]
- Tollefsbol, T.O. Transgenerational Epigenetics. Transgenerational Epigenetics; Academic Press: Birmingham, AL, USA, 2014; pp. 45–50. [Google Scholar]
- Zheng, H.; Huang, B.; Zhang, B.; Xiang, Y.; Du, Z.; Xu, Q.; Li, Y.; Wang, Q.; Ma, J.; Peng, X.; et al. Resetting epigenetic memory by reprogramming of histone modifications in mammals. Mol. Cell 2016, 63, 1066–1079. [Google Scholar] [CrossRef]
- Du, J.; Johnson, L.M.; Jacobsen, S.E.; Patel, D.J. DNA methylation pathways and their crosstalk with histone methylation. Nat. Rev. Mol. Cell Biol. 2015, 16, 519. [Google Scholar] [CrossRef]
- Zeybel, M.; Hardy, T.; Wong, Y.K.; Mathers, J.C.; Fox, C.R.; Gackowska, A.; Oakley, F.; Burt, A.D.; Wilson, C.L.; Anstee, Q.M.; et al. Multigenerational epigenetic adaptation of the hepatic wound-healing response. Nat. Med. 2012, 18, 1369–1377. [Google Scholar] [CrossRef]
- Sharma, A. Transgenerational epigenetic inheritance: Focus on soma to germline information transfer. Prog. Biophys. Mol. Biol. 2013, 113, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A. Transgenerational epigenetics: Integrating soma to germline communication with gametic inheritance. Mech. Ageing Dev. 2017, 163, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Matzke, M.A.; Mosher, R.A. RNA-directed DNA methylation: An epigenetic pathway of increasing complexity. Nat. Rev. Genet. 2014, 15, 394. [Google Scholar] [CrossRef] [PubMed]
- Morgan, H.D.; Sutherland, H.G.; Martin, D.I.; Whitelaw, E. Epigenetic inheritance at the agouti locus in the mouse. Nat. Genet. 1999, 23, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Rakyan, V.K.; Chong, S.; Champ, M.E.; Cuthbert, P.C.; Morgan, H.D.; Luu, K.V.; Whitelaw, E. Transgenerational inheritance of epigenetic states at the murine AxinFu allele occurs after maternal and paternal transmission. Proc. Natl. Acad. Sci. USA 2003, 100, 2538–2543. [Google Scholar] [CrossRef] [PubMed]
- Skinner, M.K. Environmental epigenetic transgenerational inheritance and somatic epigenetic mitotic stability. Epigenetics 2011, 6, 838–842. [Google Scholar] [PubMed]
- Francis, D.D.; Szegda, K.; Campbell, G.; Martin, W.D.; Insel, T.R. Epigenetic sources of behavioral differences in mice. Nat. Neurosci. 2003, 6, 445. [Google Scholar] [CrossRef]
- Pembrey, M.E.; Bygren, L.O.; Kaati, G.; Edvinsson, S.; Northstone, K.; Sjöström, M.; Golding, J. The ALSPAC Study Team. Sex-specific, male-line transgenerational responses in humans. Eur. J. Hum. Genet. EJHG 2006, 14, 159. [Google Scholar] [CrossRef]
- Chen, P.Y.; Chu, A.; Liao, W.W.; Rubbi, L.; Janzen, C.; Hsu, F.M.; Thamotharan, S.; Ganguly, A.; Lam, L.; Montoya, D.; et al. Prenatal Growth Patterns and Birthweight Are Associated with Differential DNA Methylation and Gene Expression of Cardiometabolic Risk Genes in Human Placentas: A Discovery-Based Approach. Reprod. Sci. 2018, 25, 523–539. [Google Scholar] [CrossRef]
- Bohacek, J.; Mansuy, I.M. A guide to designing germline-dependent epigenetic inheritance experiments in mammals. Nat. Meth. 2017, 14, 243–249. [Google Scholar] [CrossRef]
- Cantone, I.; Fisher, A.G. Epigenetic programming and reprogramming during development. Nat. Struct. Mol. Biol. 2013, 20, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Messerschmidt, D.M.; Knowles, B.B.; Solter, D. DNA methylation dynamics during epigenetic reprogramming in the germline and preimplantation embryos. Genes Dev. 2014, 28, 812–828. [Google Scholar] [CrossRef] [PubMed]
- Solberg, L.C.; Baum, A.E.; Ahmadiyeh, N.; Shimomura, K.; Li, R.; Turek, F.W.; Churchill, G.A.; Takahashi, J.S.; Redei, E.E. Sex-and lineage-specific inheritance of depression-like behavior in the rat. Mamm. Genome 2004, 15, 648–662. [Google Scholar] [CrossRef] [PubMed]
- Dean, W.; Santos, F.; Reik, W. Epigenetic reprogramming in early mammalian development and following somatic nuclear transfer. In Seminars in Cell & Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Weaver, I.C.; Cervoni, N.; Champagne, F.A.; D’Alessio, A.C.; Sharma, S.; Seckl, J.R.; Dymov, S.; Szyf, M.; Meaney, M.J. Epigenetic programming by maternal behavior. Nat. Neurosci. 2004, 7, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Calle, A.; Fernandez-Gonzalez, R.; Ramos-Ibeas, P.; Laguna-Barraza, R.; Perez-Cerezales, S.; Bermejo-Alvarez, P.; Ramirez, M.A.; Gutierrez-Adan, A. Long-term and transgenerational effects of in vitro culture on mouse embryos. Theriogenology 2012, 77, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Mahsoudi, B.; Li, A.; O’Neill, C. Assessment of the long-term and transgenerational consequences of perturbing preimplantation embryo development in mice. Biol. Reprod. 2007, 77, 889–896. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McRae, A.F.; Powell, J.E.; Henders, A.K.; Bowdler, L.; Hemani, G.; Shah, S.; Painter, J.N.; Martin, N.G.; Visscher, P.M.; Montgomery, G.W. Contribution of genetic variation to transgenerational inheritance of DNA methylation. Genome Biol. 2014, 15, R73. [Google Scholar] [CrossRef]
| Species | Inducing Conditions | Acquired Traits | Locus | Transmission Stability | Epigenetic Marks | Profiling Methods | References |
|---|---|---|---|---|---|---|---|
| Human | Childhood abuse (sexual contact, severe physical abuse, negligence) | Decreased expression of the glucocorticoid receptor 1F variant | NR3C1 promoter 1F | F1 | DNA methylation | Sodium bisulfite sequencing | McGowan et al., 2009 [7] |
| Mouse | Chronic stress (immobilization) | Alteration HPA axis that trigger long-term stress response | NR3C1 promoter 17 | F1 | DNA methylation | Pyrosequencing | Witzmann et al., 2012 [17] |
| Mouse | Unpredictable maternal separation | Depressive-like behavior | MeCP2, CB1, CRFR2 | F1-F3 | DNA methylation | Pyrosequencing | Franklin et al., 2010 [8] |
| Mouse | Chronic social defeat stress–social avoidance | Depressive-like behavior: less interactive in the population | BDNF gene | F1 | Post translation histone modifications | ChIP assays | Tsankova et al., 2006 [18] |
| Mouse | Paternal chronic stress exposure | Increased miRNA variants expression: HPA stress axis dysregulation | CRFr1, POMC, Mc2r, 11βHSD-1 | F1 | miRNAs | TaqMan Array microRNA | Rodgers et al., 2013 [19] |
| Rat | Adverse maternal care | Aberrant BDNF DNA methylation result in atypical adverse maternal behavior | BDNF gene | F1-F2 | DNA methylation | MeDIP-seq | Roth et al., 2009 [20] |
| Mouse | Parental olfactory experience | Hypomethylation: Increased behavioral sensitivity to acetophenone (odor) | Olfr151 gene | F1-F2 | DNA methylation | Illumina sequencer | Dias & Ressler, 2014 [21] |
| Mouse | Prenatal stress exposure | Dysmasculinization | F1-F2 | miRNAs | Morgan & Bale, 2011 [22] | ||
| Mouse | MSUS (unpredictable maternal separation & stress) | Altered metabolic response | F1-F3 | miRNAs & piRNAs | Gapp et al., 2014 [23] | ||
| Rat | Prenatal chronic restraint stress | Dysregulation of offspring gene expression and impact on offspring neurodevelopment system | HSD11B2 | F1 | DNA methylation | Pyrosequencing | Peña et al.,2012 [24] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, Y.-Y.; Lu, R.J.-H.; Chen, P.-Y. Behavioral Epigenetics: Perspectives Based on Experience-Dependent Epigenetic Inheritance. Epigenomes 2019, 3, 18. https://doi.org/10.3390/epigenomes3030018
Pang Y-Y, Lu RJ-H, Chen P-Y. Behavioral Epigenetics: Perspectives Based on Experience-Dependent Epigenetic Inheritance. Epigenomes. 2019; 3(3):18. https://doi.org/10.3390/epigenomes3030018
Chicago/Turabian StylePang, You-Yuan, Rita Jui-Hsien Lu, and Pao-Yang Chen. 2019. "Behavioral Epigenetics: Perspectives Based on Experience-Dependent Epigenetic Inheritance" Epigenomes 3, no. 3: 18. https://doi.org/10.3390/epigenomes3030018
APA StylePang, Y.-Y., Lu, R. J.-H., & Chen, P.-Y. (2019). Behavioral Epigenetics: Perspectives Based on Experience-Dependent Epigenetic Inheritance. Epigenomes, 3(3), 18. https://doi.org/10.3390/epigenomes3030018