Continued Susceptibility of the wMel Wolbachia Infection in Aedes aegypti to Heat Stress Following Field Deployment and Selection
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Mosquito Strains and Colony Maintenance
2.3. Selection Regime
2.4. Cytoplasmic Incompatibility and Restoration of Compatibility
2.5. Wolbachia Quantification
2.6. Statistical Analysis
3. Results
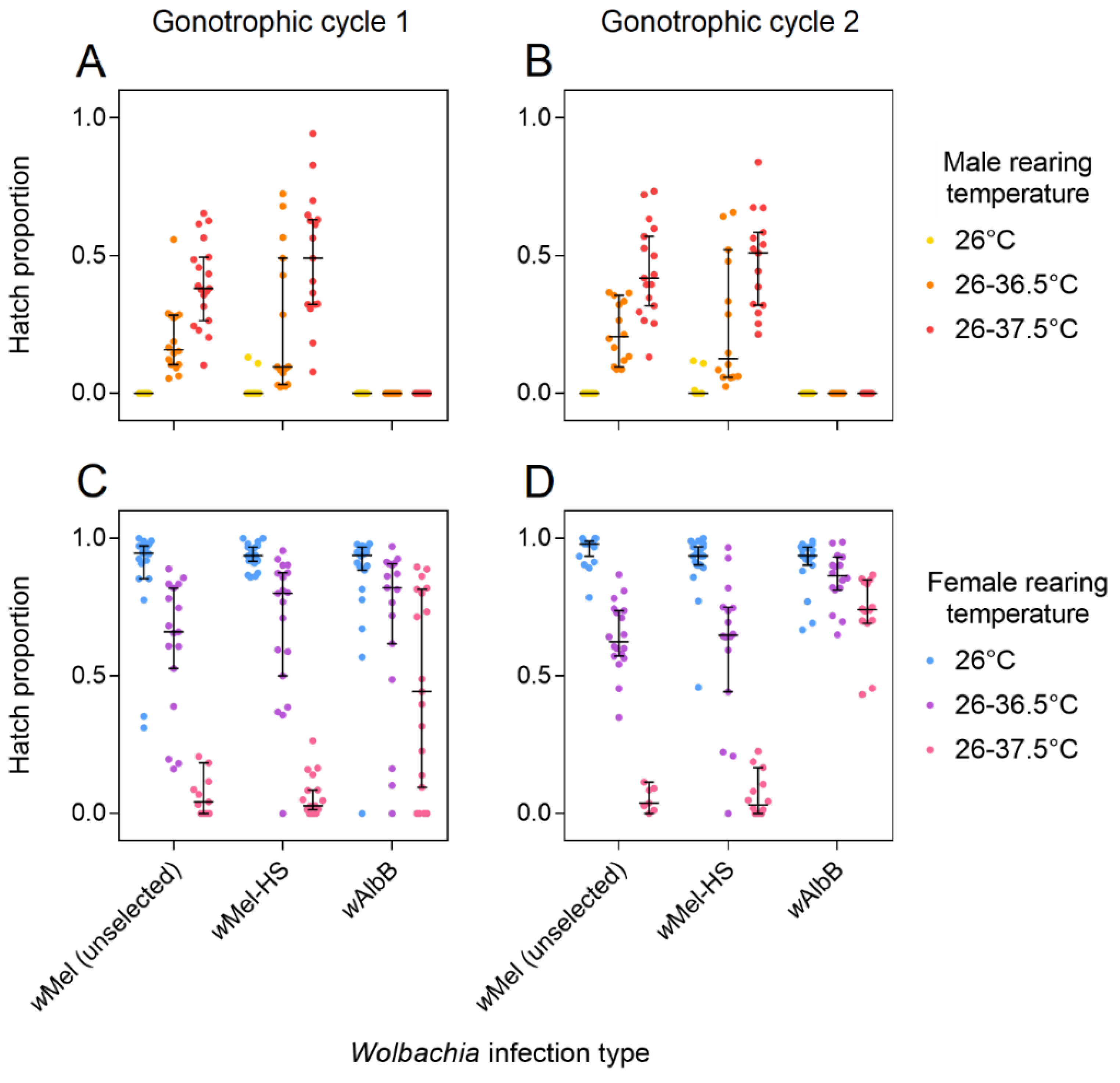
3.1. Egg Hatch Proportions during Selection
3.2. Cytoplasmic Incompatibility and Restoration of Compatibility Following Selection
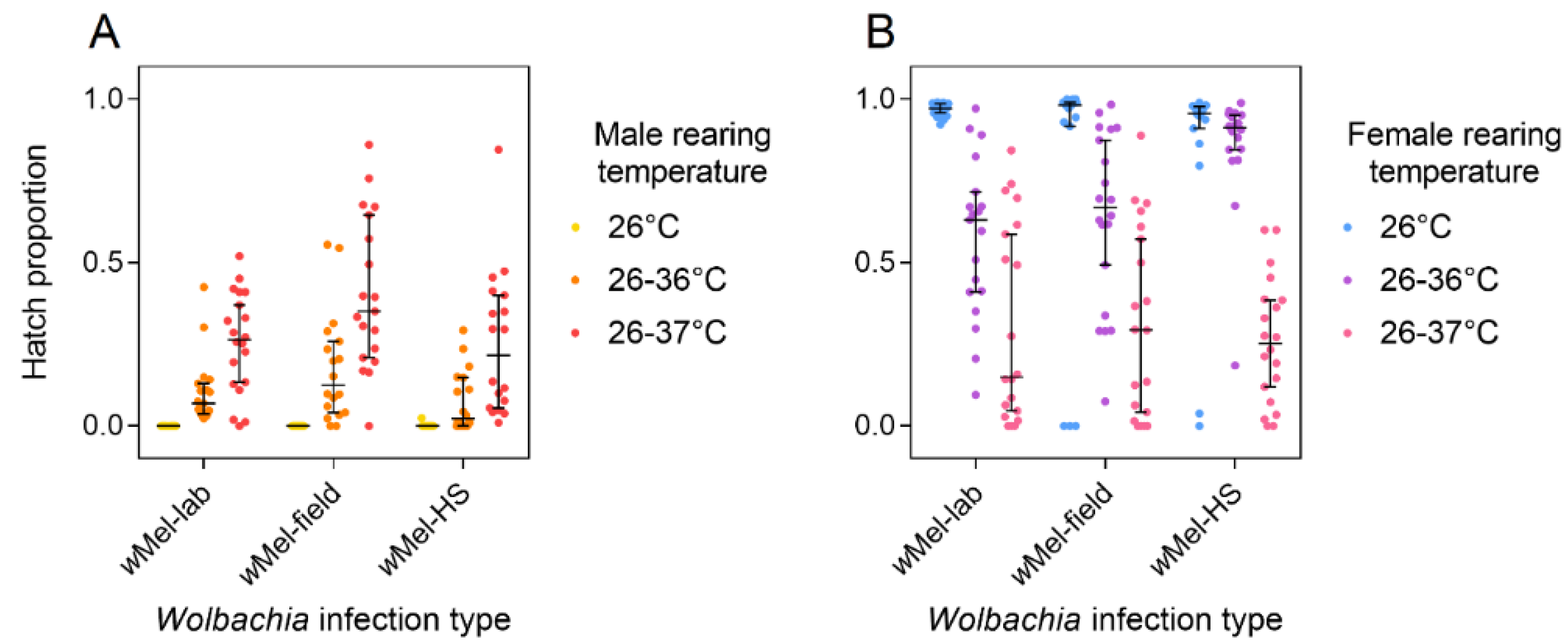
3.3. Response of Laboratory and Field wMel Infections to Cyclical Heat Stress
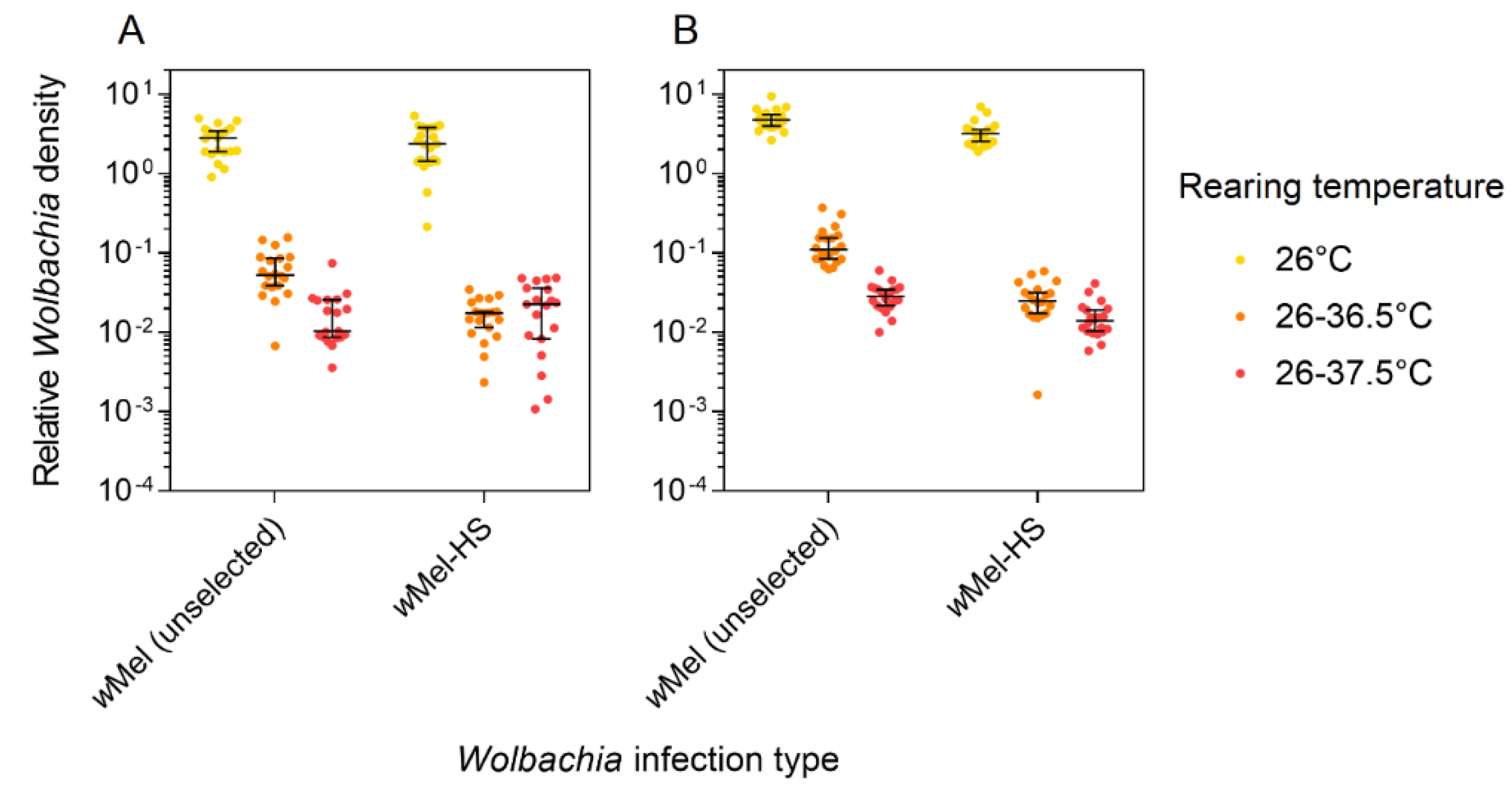
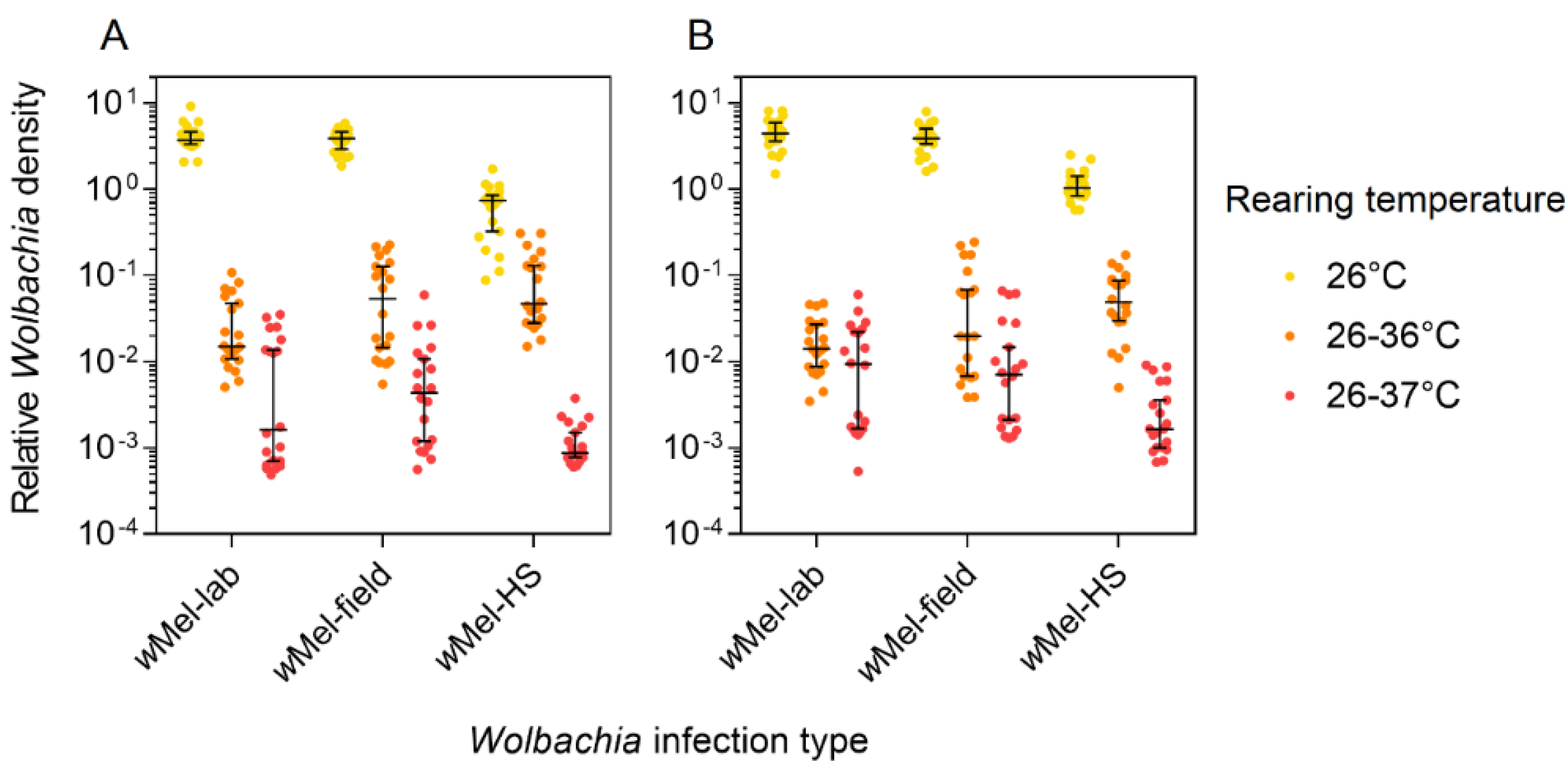
3.4. Wolbachia Density
4. Discussion
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Hoffmann, A.A.; Montgomery, B.L.; Popovici, J.; Iturbe-Ormaetxe, I.; Johnson, P.H.; Muzzi, F.; Greenfield, M.; Durkan, M.; Leong, Y.S.; Dong, Y.; et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 2011, 476, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.L.; Barton, N.H.; Rasic, G.; Turley, A.P.; Montgomery, B.L.; Iturbe-Ormaetxe, I.; Cook, P.E.; Ryan, P.A.; Ritchie, S.A.; Hoffmann, A.A.; et al. Local introduction and heterogeneous spatial spread of dengue-suppressing Wolbachia through an urban population of Aedes aegypti. PLoS Biol. 2017, 15. [Google Scholar] [CrossRef] [PubMed]
- Flores, H.A.; O’Neill, S.L. Controlling vector-borne diseases by releasing modified mosquitoes. Nat. Rev. Microbiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.A.; Iturbe-Ormaetxe, I.; Jeffery, J.A.; Lu, G.; Pyke, A.T.; Hedges, L.M.; Rocha, B.C.; Hall-Mendelin, S.; Day, A.; Riegler, M.; et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, chikungunya, and plasmodium. Cell 2009, 139, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Bian, G.; Xu, Y.; Lu, P.; Xie, Y.; Xi, Z. The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog. 2010, 6, e1000833. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.; Khoo, C.C.; Dobson, S.L. Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science 2005, 310, 326–328. [Google Scholar] [CrossRef] [PubMed]
- Ruang-Areerate, T.; Kittayapong, P. Wolbachia transinfection in Aedes aegypti: A potential gene driver of dengue vectors. Proc. Natl. Acad. Sci. USA 2006, 103, 12534–12539. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.; Johnson, P.H.; Moreira, L.A.; Iturbe-Ormaetxe, I.; Frentiu, F.D.; McMeniman, C.J.; Leong, Y.S.; Dong, Y.; Axford, J.; Kriesner, P.; et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 2011, 476, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Joubert, D.A.; Walker, T.; Carrington, L.B.; De Bruyne, J.T.; Kien, D.H.; Hoang Nle, T.; Chau, N.V.; Iturbe-Ormaetxe, I.; Simmons, C.P.; O’Neill, S.L. Establishment of a Wolbachia superinfection in Aedes aegypti mosquitoes as a potential approach for future resistance management. PLoS Pathog. 2016, 12, e1005434. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.E.; De Bruyne, J.T.; Iturbe-Ormaetxe, I.; Stepnell, J.; Burns, R.L.; Flores, H.A.; O’Neill, S.L. Novel Wolbachia-transinfected Aedes aegypti mosquitoes possess diverse fitness and vector competence phenotypes. PLoS Pathog. 2017, 13, e1006751. [Google Scholar] [CrossRef] [PubMed]
- Ant, T.H.; Herd, C.S.; Geoghegan, V.; Hoffmann, A.A.; Sinkins, S.P. The Wolbachia strain wAu provides highly efficient virus transmission blocking in Aedes aegypti. PLoS Pathog. 2018, 14, e1006815. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.A.; Ross, P.A.; Rašić, G. Wolbachia strains for disease control: Ecological and evolutionary considerations. Evol. Appl. 2015, 8, 751–768. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.A.; Iturbe-Ormaetxe, I.; Callahan, A.G.; Phillips, B.L.; Billington, K.; Axford, J.K.; Montgomery, B.; Turley, A.P.; O’Neill, S.L. Stability of the wMel Wolbachia infection following invasion into Aedes aegypti populations. PLoS Negl. Trop. Dis. 2014, 8, e3115. [Google Scholar] [CrossRef] [PubMed]
- Frentiu, F.D.; Zakir, T.; Walker, T.; Popovici, J.; Pyke, A.T.; van den Hurk, A.; McGraw, E.A.; O’Neill, S.L. Limited dengue virus replication in field-collected Aedes aegypti mosquitoes infected with Wolbachia. PLoS Negl. Trop. Dis. 2014, 8, e2688. [Google Scholar] [CrossRef] [PubMed]
- Carrington, L.B.; Tran, B.C.N.; Le, N.T.H.; Luong, T.T.H.; Nguyen, T.T.; Nguyen, P.T.; Nguyen, C.V.V.; Nguyen, H.T.C.; Vu, T.T.; Vo, L.T.; et al. Field- and clinically derived estimates of Wolbachia-mediated blocking of dengue virus transmission potential in Aedes aegypti mosquitoes. Proc. Natl. Acad. Sci. USA 2017, 115, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.A.; Endersby, N.M.; Yeap, H.L.; Hoffmann, A.A. Larval competition extends developmental time and decreases adult size of wMelPop Wolbachia-infected Aedes aegypti. Am. J. Trop. Med. Hyg. 2014, 91, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.A.; Endersby, N.M.; Hoffmann, A.A. Costs of three Wolbachia infections on the survival of Aedes aegypti larvae under starvation conditions. PLoS Negl. Trop. Dis. 2016, 10, e0004320. [Google Scholar] [CrossRef] [PubMed]
- Kho, E.A.; Hugo, L.E.; Lu, G.; Smith, D.D.; Kay, B.H. Effects of larval nutrition on Wolbachia-based dengue virus interference in Aedes aegypti (diptera: Culicidae). J. Med. Entomol. 2016, 53, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Dutra, H.L.; Lopes da Silva, V.; da Rocha Fernandes, M.; Logullo, C.; Maciel-de-Freitas, R.; Moreira, L.A. The influence of larval competition on Brazilian Wolbachia-infected Aedes aegypti mosquitoes. Parasit Vectors 2016, 9, 282. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, S.; Villela, D.A.M.; Dias, F.B.S.; Moreira, L.A.; Maciel de Freitas, R. How does competition among wild type mosquitoes influence the performance of Aedes aegypti and dissemination of Wolbachia pipientis? PLoS Negl. Trop. Dis. 2017, 11, e0005947. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, N.M.; Hue Kien, D.T.; Clapham, H.; Aguas, R.; Trung, V.T.; Bich Chau, T.N.; Popovici, J.; Ryan, P.A.; O’Neill, S.L.; McGraw, E.A.; et al. Modeling the impact on virus transmission of Wolbachia-mediated blocking of dengue virus infection of Aedes aegypti. Sci. Transl. Med. 2015, 7, 279ra237. [Google Scholar] [CrossRef] [PubMed]
- King, J.G.; Souto-Maior, C.; Sartori, L.M.; Maciel-de-Freitas, R.; Gomes, M.G.M. Variation in Wolbachia effects on Aedes mosquitoes as a determinant of invasiveness and vectorial capacity. Nat. Commun. 2018, 9, 1483. [Google Scholar] [CrossRef] [PubMed]
- McMeniman, C.J.; Lane, R.V.; Cass, B.N.; Fong, A.W.; Sidhu, M.; Wang, Y.-F.; O’Neill, S.L. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science 2009, 323, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Corbin, C.; Heyworth, E.R.; Ferrari, J.; Hurst, G.D.D. Heritable symbionts in a world of varying temperature. Heredity 2017, 118, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Zhukova, M.V.; Voronin, D.A.; Kiseleva, E.V. High temperature initiates changes in Wolbachia ultrastructure in ovaries and early embryos of Drosophila melanogaster. Cell Tissue Biol. 2008, 2, 546–556. [Google Scholar] [CrossRef]
- Strunov, A.; Kiseleva, E.; Gottlieb, Y. Spatial and temporal distribution of pathogenic Wolbachia strain wMelPop in Drosophila melanogaster central nervous system under different temperature conditions. J. Invertebr. Pathol. 2013, 114, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, J.N.; Beier, J.C.; Devine, G.J.; Hugo, L.E. Heat sensitivity of wMel Wolbachia during Aedes aegypti development. PLoS Negl. Trop. Dis. 2016, 10, e0004873. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.A.; Wiwatanaratanabutr, I.; Axford, J.K.; White, V.L.; Endersby-Harshman, N.M.; Hoffmann, A.A. Wolbachia infections in Aedes aegypti differ markedly in their response to cyclical heat stress. PLoS Pathog. 2017, 13, e1006006. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.L.; Filipovic, I.; Hoffmann, A.A.; Rasic, G. Fine-scale landscape genomics helps explain the slow spatial spread of Wolbachia through the Aedes aegypti population in Cairns, Australia. Heredity 2018, 120, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, N.N.; Thorshauge, P.M.; Kristensen, T.N.; de Jonge, N.; Bahrndorff, S.; Kjeldal, H.; Nielsen, J.L. Strong responses of Drosophila melanogaster microbiota to developmental temperature. Fly (Austin) 2017, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.L.; Cavanaugh, C.M. Intrahost genetic diversity of bacterial symbionts exhibits evidence of mixed infections and recombinant haplotypes. Mol. Biol. Evol. 2017, 34, 2747–2761. [Google Scholar] [CrossRef] [PubMed]
- Chrostek, E.; Teixeira, L. Within host selection for faster replicating bacterial symbionts. PLoS ONE 2018, 13, e0191530. [Google Scholar] [CrossRef] [PubMed]
- Le Clec’h, W.; Dittmer, J.; Raimond, M.; Bouchon, D.; Sicard, M. Phenotypic shift in Wolbachia virulence towards its native host across serial horizontal passages. Proc. R. Soc. B Biol. Sci. 2017, 284. [Google Scholar] [CrossRef] [PubMed]
- McGraw, E.A.; Merritt, D.J.; Droller, J.N.; O’Neill, S.L. Wolbachia density and virulence attenuation after transfer into a novel host. Proc. Natl. Acad. Sci. USA 2002, 99, 2918–2923. [Google Scholar] [CrossRef] [PubMed]
- McMeniman, C.J.; Lane, A.M.; Fong, A.W.; Voronin, D.A.; Iturbe-Ormaetxe, I.; Yamada, R.; McGraw, E.A.; O’Neill, S.L. Host adaptation of a Wolbachia strain after long-term serial passage in mosquito cell lines. Appl. Environ. Microbiol. 2008, 74, 6963–6969. [Google Scholar] [CrossRef] [PubMed]
- Carrington, L.B.; Hoffmann, A.A.; Weeks, A.R. Monitoring long-term evolutionary changes following Wolbachia introduction into a novel host: The Wolbachia popcorn infection in Drosophila simulans. Proc. R. Soc. B Biol. Sci. 2010, 277, 2059–2068. [Google Scholar] [CrossRef] [PubMed]
- Faria, V.G.; Martins, N.E.; Magalhaes, S.; Paulo, T.F.; Nolte, V.; Schlotterer, C.; Sucena, E.; Teixeira, L. Drosophila adaptation to viral infection through defensive symbiont evolution. PLoS Genet. 2016, 12, e1006297. [Google Scholar] [CrossRef] [PubMed]
- Chrostek, E.; Teixeira, L. Mutualism breakdown by amplification of Wolbachia genes. PLoS Biol. 2015, 13, e1002065. [Google Scholar] [CrossRef] [PubMed]
- Pintureau, B.; Chapelle, L.; Delobel, B. Effects of repeated thermic and antibiotic treatments on a Trichogramma (Hym., Trichogrammatidae) symbiont. J. Appl. Entomol. 1999, 123, 473–483. [Google Scholar] [CrossRef]
- Versace, E.; Nolte, V.; Pandey, R.V.; Tobler, R.; Schlotterer, C. Experimental evolution reveals habitat-specific fitness dynamics among Wolbachia clades in Drosophila melanogaster. Mol. Ecol. 2014, 23, 802–814. [Google Scholar] [CrossRef] [PubMed]
- Brownlie, J.C.; Adamski, M.; Slatko, B.; McGraw, E.A. Diversifying selection and host adaptation in two endosymbiont genomes. BMC Evol. Biol. 2007, 7, 68. [Google Scholar] [CrossRef] [PubMed]
- Axford, J.K.; Ross, P.A.; Yeap, H.L.; Callahan, A.G.; Hoffmann, A.A. Fitness of wAlbB Wolbachia infection in Aedes aegypti: Parameter estimates in an outcrossed background and potential for population invasion. Am. J. Trop. Med. Hyg. 2016, 94, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.A.; Axford, J.K.; Richardson, K.M.; Endersby-Harshman, N.M.; Hoffmann, A.A. Maintaining Aedes aegypti mosquitoes infected with Wolbachia. J. Vis. Exp. 2017, 126, e526124. [Google Scholar]
- Lee, S.F.; White, V.L.; Weeks, A.R.; Hoffmann, A.A.; Endersby, N.M. High-throughput PCR assays to monitor Wolbachia infection in the dengue mosquito (Aedes aegypti) and Drosophila simulans. Appl. Environ. Microbiol. 2012, 78, 4740–4743. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.A.; Chown, S.L.; Clusella-Trullas, S.; Fox, C. Upper thermal limits in terrestrial ectotherms: How constrained are they? Funct. Ecol. 2013, 27, 934–949. [Google Scholar] [CrossRef]
- Ritchie, S.A.; van den Hurk, A.F.; Smout, M.J.; Staunton, K.M.; Hoffmann, A.A. Mission accomplished? We need a guide to the ‘post release’ world of Wolbachia for Aedes-borne disease control. Trends Parasitol. 2018, 34, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Murdock, C.C.; Evans, M.V.; McClanahan, T.D.; Miazgowicz, K.L.; Tesla, B. Fine-scale variation in microclimate across an urban landscape shapes variation in mosquito population dynamics and the potential of Aedes albopictus to transmit arboviral disease. PLoS Negl. Trop. Dis. 2017, 11, e0005640. [Google Scholar] [CrossRef] [PubMed]
| Generation | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Mean maximum daily temperature | 37.2 °C | 37.3 °C | 26 °C | 36.9 °C | 36.9 °C | 37.0 °C | 36.8 °C | 36.8 °C |
| Egg hatch (wMel-HS ♀ × wMel 26 °C ♂) | 30.02% | 2.64% | 69.88% | 2.12% | 2.87% | 2.90% | 3.67% | 0% |
| Eggs scored | 991 | 4040 | 934 | 2113 | 2115 | 8396 | 4741 | 4183 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ross, P.A.; Hoffmann, A.A. Continued Susceptibility of the wMel Wolbachia Infection in Aedes aegypti to Heat Stress Following Field Deployment and Selection. Insects 2018, 9, 78. https://doi.org/10.3390/insects9030078
Ross PA, Hoffmann AA. Continued Susceptibility of the wMel Wolbachia Infection in Aedes aegypti to Heat Stress Following Field Deployment and Selection. Insects. 2018; 9(3):78. https://doi.org/10.3390/insects9030078
Chicago/Turabian StyleRoss, Perran A., and Ary A. Hoffmann. 2018. "Continued Susceptibility of the wMel Wolbachia Infection in Aedes aegypti to Heat Stress Following Field Deployment and Selection" Insects 9, no. 3: 78. https://doi.org/10.3390/insects9030078
APA StyleRoss, P. A., & Hoffmann, A. A. (2018). Continued Susceptibility of the wMel Wolbachia Infection in Aedes aegypti to Heat Stress Following Field Deployment and Selection. Insects, 9(3), 78. https://doi.org/10.3390/insects9030078






