Overcoming Obstacles: Perspective on How Mediterranean Oaks Defend Their Acorns from Insect Seed Predators
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Sampling
2.2. Larva Monitoring
2.3. Acorn Volume
2.4. Development Monitoring of Acorn Weevil Larvae
2.5. Statistical Analysis
3. Results
3.1. Infestation Rates of Acorns Among Oak Species and Between Maturation Periods
3.2. Differences in Tannin Concentration Between Species and Maturation Periods
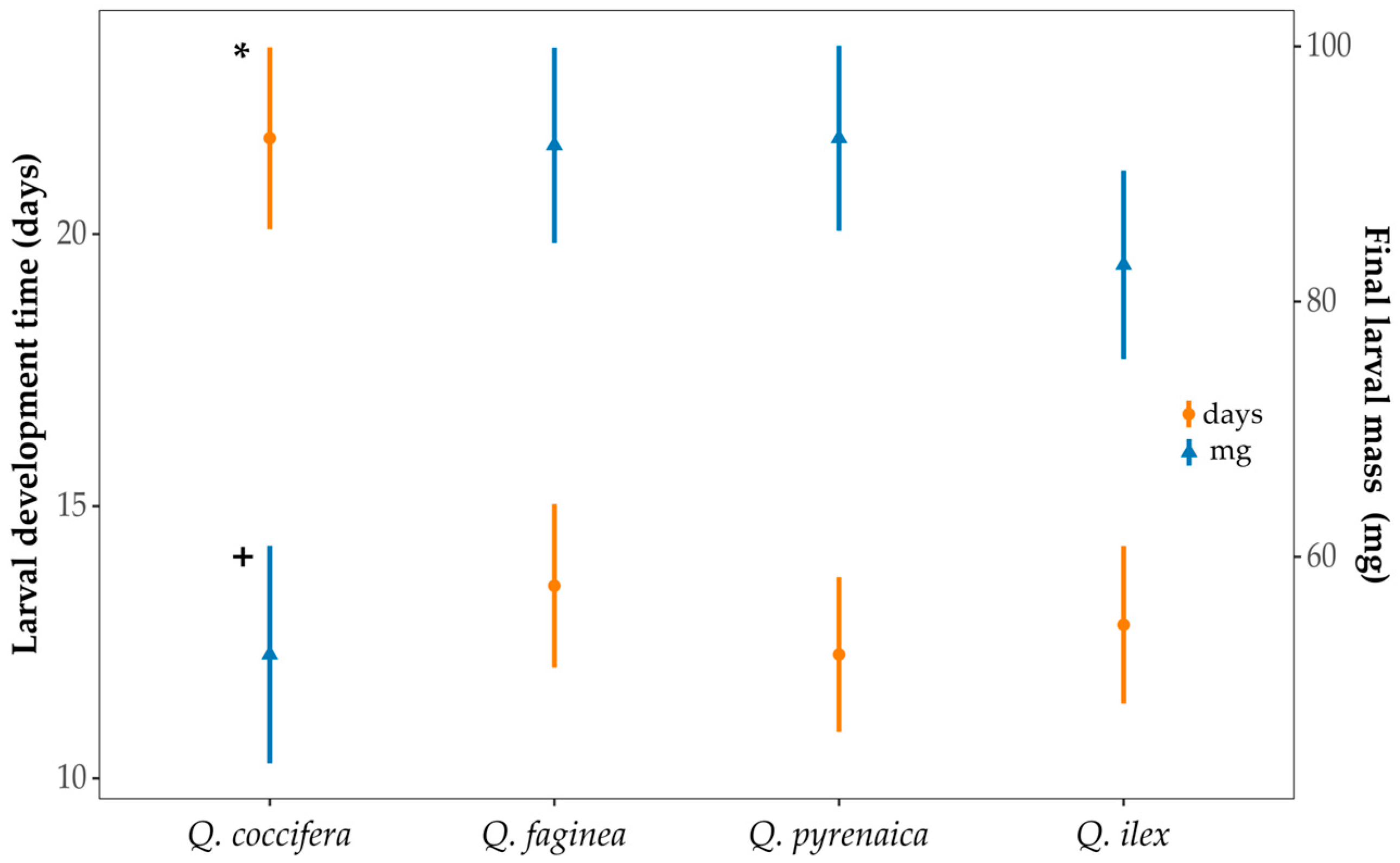
3.3. Larval Development in Acorns of Different Oak Species
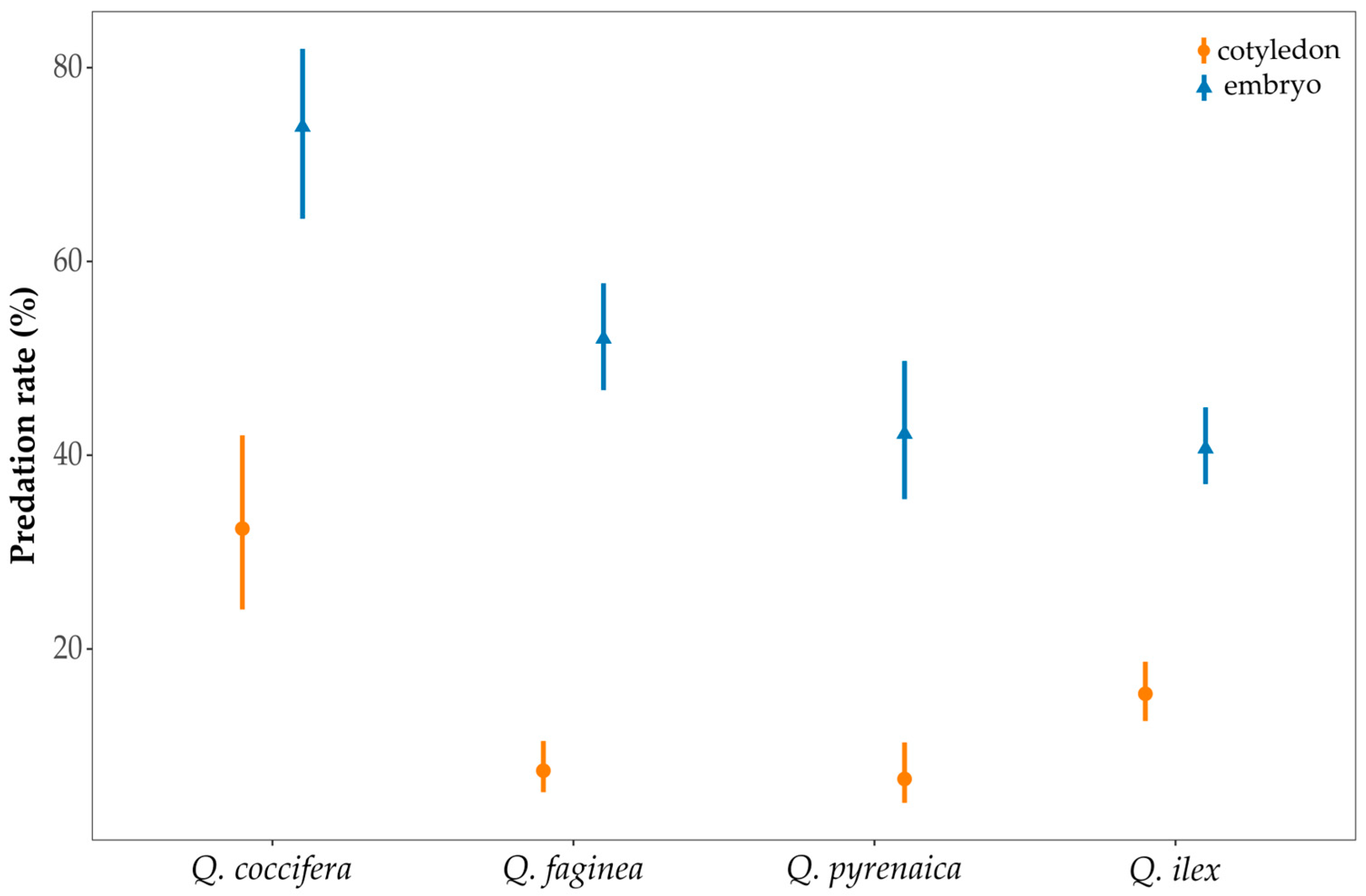
3.4. Acorn Volume and Predation Rate of Cotyledon and Embryo
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonal, R.; Muñoz, A.; Díaz, M. Satiation of Predispersal Seed Predators: The Importance of Considering Both Plant and Seed Levels. Evol. Ecol. 2007, 21, 367–380. [Google Scholar] [CrossRef]
- Kolb, A.; Ehrlén, J.; Eriksson, O. Ecological and Evolutionary Consequences of Spatial and Temporal Variation in Pre-Dispersal Seed Predation. Perspect. Plant Ecol. Evol. Syst. 2007, 9, 79–100. [Google Scholar] [CrossRef]
- Xia, K.; Harrower, W.L.; Turkington, R.; Tan, H.-Y.; Zhou, Z.-K. Pre-Dispersal Strategies by Quercus Schottkyana to Mitigate the Effects of Weevil Infestation of Acorns. Sci. Rep. 2016, 6, 37520. [Google Scholar] [CrossRef] [PubMed]
- Stachurska-Swakoń, A.; Barabasz-Krasny, B.; Klasa, A.; Palaczyk, A. Reduced Plant Fitness by Pre-Dispersal Seed Predation in the Threatened Plant Species Cirsium decussatum. Seed Sci. Res. 2018, 28, 123–130. [Google Scholar] [CrossRef]
- Linhart, Y.B.; Moreira, X.; Snyder, M.A.; Mooney, K.A. Variability in Seed Cone Production and Functional Response of Seed Predators to Seed Cone Availability: Support for the Predator Satiation Hypothesis. J. Ecol. 2014, 102, 576–583. [Google Scholar] [CrossRef]
- Vander Wall, S.B. The Evolutionary Ecology of Nut Dispersal. Bot. Rev. 2001, 67, 74–117. [Google Scholar] [CrossRef]
- Toju, H.; Sota, T. Imbalance of Predator and Prey Armament: Geographic Clines in Phenotypic Interface and Natural Selection. Am. Nat. 2006, 167, 105–117. [Google Scholar] [CrossRef]
- Díaz, M. Food Choice by Seed-Eating Birds in Relation to Seed Chemistry. Comp. Biochem. Physiol. A Physiol. 1996, 113, 239–246. [Google Scholar] [CrossRef]
- Herrera, C.M.; Pellmyr, O. Plant Animal Interactions: An Evolutionary Approach; John Wiley & Sons, Incorporated: Newark, UK, 2002; ISBN 978-1-4443-1229-4. [Google Scholar]
- Dev, M.; Shilpi, R.; Satya, K. Role of Secondary Metabolite in Plant Defense. In New Approach on Agriculture, Forestry, Horticulture and Fishery (Research and Applied); K.D. Publications: Delhi, India, 2016; Volume 1, pp. 115–118. ISBN 978-93-94570-42-9. [Google Scholar]
- Janzen, D.H. Seed Predation by Animals. Annu. Rev. Ecol. Syst. 1971, 2, 465–492. [Google Scholar] [CrossRef]
- Kelly, D.; Sork, V.L. Mast Seeding in Perennial Plants: Why, How, Where? Annu. Rev. Ecol. Syst. 2002, 33, 427. [Google Scholar] [CrossRef]
- Zhang, H.; Niu, H.; Steele, M.A.; Peng, L.; He, H.; Li, A.; Yi, X.; Li, H.; Zhang, Z. Masting Promotes Transformation from Predation to Mutualism in an Oak-Weevil-Rodent System. Sci. China Life Sci. 2024, 67, 1514–1524. [Google Scholar] [CrossRef]
- Espelta, J.M.; Cortés, P.; Molowny-Horas, R.; Retana, J. Acorn Crop Size and Pre-Dispersal Predation Determine Inter-Specific Differences in the Recruitment of Co-Occurring Oaks. Oecologia 2009, 161, 559–568. [Google Scholar] [CrossRef]
- Mack, A.L. An Advantage of Large Seed Size: Tolerating Rather than Succumbing to Seed Predators. Biotropica 1998, 30, 604–608. [Google Scholar] [CrossRef]
- Steele, M.A.; Dalgleish, H.J.; Marino, S.; Bartlow, A.W.; Curtis, R.; Stratford, J.A. Oak (Acorn)–Weevil Interactions across an Extensive Latitudinal Gradient in Eastern North America. Diversity 2021, 13, 303. [Google Scholar] [CrossRef]
- Boucher, D.H.; Sork, V.L. Early Drop of Nuts in Response to Insect Infestation. Oikos 1979, 33, 440–443. [Google Scholar] [CrossRef]
- Bonal, R.; Muñoz, A. Seed Growth Suppression Constrains the Growth of Seed Parasites: Premature Acorn Abscission Reduces Curculio elephas Larval Size. Ecol. Entomol. 2008, 33, 31–36. [Google Scholar] [CrossRef]
- Fürstenberg-Hägg, J.; Zagrobelny, M.; Bak, S. Plant Defense against Insect Herbivores. Int. J. Mol. Sci. 2013, 14, 10242–10297. [Google Scholar] [CrossRef]
- Reut, M.; Jakubczyk, E.; Chrabąszcz, M.; Moniuszko, H. Hard Nut to Crack. Acorn Hardness Implications on Oviposition of the Acorn Weevil Curculio glandium Marsham, 1802 (Coleoptera: Curculionidae). Diversity 2022, 14, 922. [Google Scholar] [CrossRef]
- Barat, M.; Tarayre, M.; Atlan, A. Plant Phenology and Seed Predation: Interactions between Gorses and Weevils in Brittany (France). Entomol. Exp. Appl. 2007, 124, 167–176. [Google Scholar] [CrossRef]
- Nishimon, M.; Hisano, M.; Matsuo, K.; Hirayama, K. Seasonal Patterns of Host Utilization in the Multivoltine Seed-Feeding Wasp, Macrodasyceras japonicum (Hymenoptera: Megastigmidae). Arthropod-Plant Interact. 2024, 18, 815–827. [Google Scholar] [CrossRef]
- Strong, W.B.; Mangini, A.C.; Candau, J.-N. Insects of Reproductive Structures. In Forest Entomology and Pathology: Entomology; Springer International Publishing: Cham, Switzerland, 2023; Volume 1, pp. 523–579. [Google Scholar]
- Jimenez-Pino, A.; Maistrello, L.; Lopez-Martinez, M.A.; Ocete-Rubio, M.E.; Soria-Iglesias, F.J. Spatial Distribution of Cydia Fagiglandana (Zeller) in an Exploited Holm Oak (Quercus Ilex L.) Forest. Span. J. Agric. Res. 2011, 9, 570–579. [Google Scholar] [CrossRef]
- Nieves-Aldrey, J.-L. Revisión de Las Especies Europeas Del Género Callirhytis (Hymenoptera, Cynipidae). Graellsia 1993, 48, 171–183. [Google Scholar]
- Jansen, M.A.; Niverty, S.; Chawla, N.; Franz, N.M. Reducing the Risk of Rostral Bending Failure in Curculio Linnaeus, 1758. Acta Biomater. 2021, 126, 350–371. [Google Scholar] [CrossRef]
- Ferraz de Oliveira, M.I.; Machado, M.; Cancela d’Abreu, M. Acorn Chemical Composition Depending on Shedding Date and Quercus Species; De Pedro, E.J., Cabezas, A.B., Eds.; CIHEAM: Zaragoza, Spain, 2012; pp. 229–234. [Google Scholar]
- Luczaj, L.; Adamczak, A.; Duda, M. Tannin Content in Acorns (Quercus spp.) from Poland. Dendrobiology 2014, 72, 103–111. [Google Scholar] [CrossRef]
- Fleck, D.C.; Layne, J.N. Variation in Tannin Activity of Acorns of Seven Species of Central Florida Oaks. J. Chem. Ecol. 1990, 16, 2925–2934. [Google Scholar] [CrossRef]
- Dordevic, D.; Zemancova, J.; Dordevic, S.; Kulawik, P.; Kushkevych, I. Quercus Acorns as a Component of Human Dietary Patterns. Open Agric. 2025, 10, 734–736. [Google Scholar] [CrossRef]
- Yi, X.F.; Yang, Y.Q. Large Acorns Benefit Seedling Recruitment by Satiating Weevil Larvae in Quercus aliena. Plant Ecol. 2010, 209, 291–300. [Google Scholar] [CrossRef]
- Espelta, J.M.; Bonal, R.; Sánchez-Humanes, B. Pre-Dispersal Acorn Predation in Mixed Oak Forests: Interspecific Differences Are Driven by the Interplay among Seed Phenology, Seed Size and Predator Size. J. Ecol. 2009, 97, 1416–1423. [Google Scholar] [CrossRef]
- Bonal, R.; Muñoz, A.; María Espelta, J. Mismatch between the Timing of Oviposition and the Seasonal Optimum. The Stochastic Phenology of Mediterranean Acorn Weevils. Ecol. Entomol. 2010, 35, 270–278. [Google Scholar] [CrossRef]
- Hernández-Agüero, J.A.; Ruiz-Tapiador, I.; Cayuela, L. What Feeds on Quercus Ilex L.? A Biogeographical Approach to Studying Trophic Interactions in a Mediterranean Keystone Species. Divers. Distrib. 2022, 28, 4–24. [Google Scholar] [CrossRef]
- Cruz-Alonso, V.; Pucher, C.; Ratcliffe, S.; Ruiz-Benito, P.; Astigarraga, J.; Neumann, M.; Hasenauer, H.; Rodríguez-Sánchez, F. The Easyclimate R Package: Easy Access to High-Resolution Daily Climate Data for Europe. Environ. Model. Softw. 2023, 161, 105627. [Google Scholar] [CrossRef]
- Pemán, J.; Navarro-Cerrillo, R.M.; Nicolás Peragón, J.; Prada Sáez, M.A.; Serrada, R. Producción y Manejo de Semillas y Plantas Forestales; Organismo Autónomo Parques Nacionales: Madrid, Spain, 2012; Volume 2, pp. 192–291. [Google Scholar]
- Goldstein, J.L.; Swain, T. Changes in Tannins in Ripening Fruits. Phytochemistry 1963, 2, 371–383. [Google Scholar] [CrossRef]
- Krebs, S.A.; Schummer, M.L. A Review of Plant Phenolics and Endozoochory. Ecol. Evol. 2024, 14, e70255. [Google Scholar] [CrossRef]
- Pulido, F.J.; Díaz, M. Regeneration of a Mediterranean Oak: A Whole-Cycle Approach. Écoscience 2005, 12, 92–102. [Google Scholar] [CrossRef]
- Canelo, T.; Gaytán, Á.; Pérez-Izquierdo, C.; Bonal, R. Effects of Longer Droughts on Holm Oak Quercus Ilex L. Acorn Pests: Consequences for Infestation Rates, Seed Biomass and Embryo Survival. Diversity 2021, 13, 110. [Google Scholar] [CrossRef]
- Bonal, R.; Muñoz, A. Multi-Trophic Effects of Ungulate Intraguild Predation on Acorn Weevils. Oecologia 2007, 152, 533–540. [Google Scholar] [CrossRef]
- Amorim, E.L.; Nascimento, J.E.; Monteiro, J.M.; Peixoto Sobrinho, T.J.S.; Araújo, T.A.; Albuquerque, U.P. A Simple and Accurate Procedure for the Determination of Tannin and Flavonoid Levels and Some Applications in Ethnobotany and Ethnopharmacology. Funct. Ecosyst. Communities 2008, 2, 88–94. [Google Scholar]
- Bonal, R.; Espelta, J.M.; Vogler, A.P. Complex Selection on Life-History Traits and the Maintenance of Variation in Exaggerated Rostrum Length in Acorn Weevils. Oecologia 2011, 167, 1053–1061. [Google Scholar] [CrossRef]
- Reut, M.; Bonal, R.; Chrabąszcz, M.; Moniuszko, H. Cannibalism as Competition Strategy in Larvae of the Acorn Weevil Curculio glandium (Coleoptera: Curculionidae). Diversity 2023, 15, 145. [Google Scholar] [CrossRef]
- Bonal, R.; Hernández, M.; Ortego, J.; Muñoz, A.; Espelta, J.M. Positive Cascade Effects of Forest Fragmentation on Acorn Weevils Mediated by Seed Size Enlargement. Insect Conserv Diversity 2012, 5, 381–388. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.10.6. 2024. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 17 June 2025).
- Feeny, P. Seasonal Changes in Oak Leaf Tannins and Nutrients as a Cause of Spring Feeding by Winter Moth Caterpillars. Ecology 1970, 51, 565–581. [Google Scholar] [CrossRef]
- VanderWeide, J.; Forte, A.; Peterlunger, E.; Sivilotti, P.; Medina-Meza, I.G.; Falchi, R.; Rustioni, L.; Sabbatini, P. Increase in Seed Tannin Extractability and Oxidation Using a Freeze-Thaw Treatment in Cool-Climate Grown Red (Vitis vinifera L.) Cultivars. Food Chem. 2020, 308, 125571. [Google Scholar] [CrossRef]
- Noman, A.; Aqeel, M.; Islam, W.; Khalid, N.; Akhtar, N.; Qasim, M.; Yasin, G.; Hashem, M.; Alamri, S.; Al-Zoubi, O.M.; et al. Insects–Plants-Pathogens: Toxicity, Dependence and Defense Dynamics. Toxicon 2021, 197, 87–98. [Google Scholar] [CrossRef]
- García-Saldaña, E.A.; Cerqueda-García, D.; Ibarra-Laclette, E.; Aluja, M. Insights into the Differences Related to the Resistance Mechanisms to the Highly Toxic Fruit Hippomane mancinella (Malpighiales: Euphorbiaceae) between the Larvae of the Sister Species Anastrepha acris and Anastrepha ludens (Diptera: Tephritidae) through Comparative Transcriptomics. Front. Physiol. 2024, 15, 1263475. [Google Scholar] [CrossRef]
- Desouhant, E.; Debouzie, D.; Ploye, H.; Menu, F. Clutch Size Manipulations in the Chestnut Weevil, Curculio elephas: Fitness of Oviposition Strategies. Oecologia 2000, 122, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.C.; Thomas, L.; Adkisson, C.S. Dietary Circumvention of Acorn Tannins by Blue Jays. Oecologia 1993, 94, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Saitoh, T.; Matsui, T. Does Acclimation Reduce the Negative Effects of Acorn Tannins in the Wood mouse Apodemus speciosus? Acta Theriol. 2004, 49, 203–214. [Google Scholar] [CrossRef]
- Chung-MacCoubrey, A.L.; Hagerman, A.E.; Kirkpatrick, R.L. Effects of Tannins on Digestion and Detoxification Activity in Gray Squirrels (Sciurus carolinensis). Physiol. Zool. 1997, 70, 270–277. [Google Scholar] [CrossRef]
- Koenig, W.D. The Effects of Tannins and Lipids on Digestion of Acorns by Acorn Woodpeckers. Auk 1991, 108, 79–88. [Google Scholar] [CrossRef]
- Ramírez-Valiente, J.A.; Valladares, F.; Gil, L.; Aranda, I. Population Differences in Juvenile Survival under Increasing Drought Are Mediated by Seed Size in Cork Oak (Quercus suber L.). For. Ecol. Manag. 2009, 257, 1676–1683. [Google Scholar] [CrossRef]
- Andivia, E.; Villar-Salvador, P.; Oliet, J.A.; Puértolas, J.; Dumroese, R.K.; Ivetić, V.; Molina-Venegas, R.; Arellano, E.C.; Li, G.; Ovalle, J.F. Climate and Species Stress Resistance Modulate the Higher Survival of Large Seedlings in Forest Restorations Worldwide. Ecol. Appl. 2021, 31, e02394. [Google Scholar] [CrossRef]
- Shackelford, N.; Paterno, G.B.; Winkler, D.E.; Erickson, T.E.; Leger, E.A.; Svejcar, L.N.; Breed, M.F.; Faist, A.M.; Harrison, P.A.; Curran, M.F.; et al. Drivers of Seedling Establishment Success in Dryland Restoration Efforts. Nat. Ecol. Evol. 2021, 5, 1283–1290. [Google Scholar] [CrossRef]
- Muñoz, A.; Bonal, R.; Díaz, M. Ungulates, Rodents, Shrubs: Interactions in a Diverse Mediterranean Ecosystem. Basic Appl. Ecol. 2009, 10, 151–160. [Google Scholar] [CrossRef]
- Bossema, I. Jays and Oaks: An Eco-Ethological Study of a Symbiosis. Behaviour 1979, 70, 1–117. [Google Scholar] [CrossRef]
- Muñoz, A.; Bonal, R. Rodents Change Acorn Dispersal Behaviour in Response to Ungulate Presence. Oikos 2007, 116, 1631–1638. [Google Scholar] [CrossRef]
- Andivia, E.; Villar-Salvador, P.; Tovar, L.; Rabasa, S.; Rey Benayas, J.M. Multiscale Assessment of Woody Species Recruitment in Mediterranean Shrublands: Facilitation and Beyond. J. Veg. Sci. 2017, 28, 639–648. [Google Scholar] [CrossRef]
- Martínez-Baroja, L.; Pérez-Camacho, L.; Villar-Salvador, P.; Rebollo, S.; Quiles, P.; Gómez-Sánchez, D.; Molina-Morales, M.; Leverkus, A.B.; Castro, J.; Rey-Benayas, J.M. Massive and Effective Acorn Dispersal into Agroforestry Systems by an Overlooked Vector, the Eurasian Magpie (Pica pica). Ecosphere 2019, 10, e02989. [Google Scholar] [CrossRef]
- Bonacchi, A.; Bartolommei, P.; Gasperini, S.; Manzo, E.; Cozzolino, R. Acorn Choice by Small Mammals in a Mediterranean Deciduous Oak Forest. Ethol. Ecol. Evol. 2017, 29, 105–118. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhang, Z.; Wang, Y. Dispersal and Germination of Big and Small Nuts of Quercus serrata in a Subtropical Broad-Leaved Evergreen Forest. For. Ecol. Manag. 2004, 195, 141–150. [Google Scholar] [CrossRef]
- Muñoz, A.; Bonal, R. Are You Strong Enough to Carry That Seed? Seed Size/Body Size Ratios Influence Seed Choices by Rodents. Anim. Behav. 2008, 76, 709–715. [Google Scholar] [CrossRef]
- Pons, J.; Pausas, J.G. Oak Regeneration in Heterogeneous Landscapes: The Case of Fragmented Quercus suber Forests in the Eastern Iberian Peninsula. For. Ecol. Manag. 2006, 231, 196–204. [Google Scholar] [CrossRef]
- Coley, P.D. Costs and Benefits of Defense by Tannins in a Neotropical Tree. Oecologia 1986, 70, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yin, W.; Ding, J. Trade-Offs between Chemical Resistance to Herbivory and Responses to Abiotic Stresses in Invasive Plants. J. Plant Ecol. 2024, 17, rtae007. [Google Scholar] [CrossRef]



| Locality | Longitude | Latitude | Altitude | Tm | TM | aP | Species |
|---|---|---|---|---|---|---|---|
| Huecas | 4°12′60″ W | 39°59′60″ N | 543 | 9.57 | 21.59 | 343.59 | Q. ilex Q. coccifera |
| Castrejón | 4°14′9″ W | 39°49′49″ N | 521 | 9.63 | 21.47 | 348.32 | Q. ilex Q. coccifera |
| Sevilla la Nueva | 4°2′57″ W | 40°21′51″ N | 639 | 9.12 | 20.53 | 376.27 | Q. ilex Q. faginea |
| Robledillo | 4°55′19″ W | 40°33′11″ N | 1113 | 5.51 | 16.81 | 380.79 | Q. ilex, Q. faginea Q. pyrenaica |
| Zarzalejo | 4°10′36″ W | 40°33′5″ N | 1146 | 6.67 | 16.62 | 534.67 | Q. ilex Q.pyrenaica |
| Insect Taxa | Cur | Cyd | Call | Cur + Cyd * | Cur + Call * | Cyd + Call * |
|---|---|---|---|---|---|---|
| Period | ||||||
| All season long | 31 | 48 | 12 | 6 | 2 | 2 |
| T1 | 38 | 41 | 10 | 8 | 3 | 0 |
| T2 | 21 | 58 | 14 | 3 | 1 | 4 |
| Concentration of Tannins (%) | |||
|---|---|---|---|
| Locality | Species | T1 | T2 |
| Huecas | Q. coccifera | 28.18 | 12.22 |
| Huecas | Q. ilex | 5.3 | 3.43 |
| Castrejón | Q. coccifera | 21.57 | 17.16 |
| Castrejón | Q. ilex | 6.19 | 5.01 |
| Sevilla la Nueva | Q. faginea | 4.58 | 4.50 |
| Sevilla la Nueva | Q. ilex | 2.26 | 1.07 |
| Robledillo | Q. faginea | 7.91 | 5.24 |
| Robledillo | Q. pyrenaica | 8.42 | 3.2 |
| Zarzalejo | Q. pyrenaica | 3.61 | 3.41 |
| Zarzalejo | Q. ilex | 2.24 | 0.98 |
| Q. coccifera | Q. faginea | Q. ilex | Q. pyrenaica | |
|---|---|---|---|---|
| T1 | 1764 ± 261 | 1853 ± 256 | 2164 ± 188 | 2346 ± 335 |
| T2 | 3419 ± 609 | 2066 ± 276 | 2588 ± 237 | 6119 ± 874 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oropesa-Olmedo, D.A.; Andivia, E.; Reut, M.; Cisneros, P.; Bonal, R. Overcoming Obstacles: Perspective on How Mediterranean Oaks Defend Their Acorns from Insect Seed Predators. Insects 2025, 16, 990. https://doi.org/10.3390/insects16090990
Oropesa-Olmedo DA, Andivia E, Reut M, Cisneros P, Bonal R. Overcoming Obstacles: Perspective on How Mediterranean Oaks Defend Their Acorns from Insect Seed Predators. Insects. 2025; 16(9):990. https://doi.org/10.3390/insects16090990
Chicago/Turabian StyleOropesa-Olmedo, David A., Enrique Andivia, Michał Reut, Pablo Cisneros, and Raúl Bonal. 2025. "Overcoming Obstacles: Perspective on How Mediterranean Oaks Defend Their Acorns from Insect Seed Predators" Insects 16, no. 9: 990. https://doi.org/10.3390/insects16090990
APA StyleOropesa-Olmedo, D. A., Andivia, E., Reut, M., Cisneros, P., & Bonal, R. (2025). Overcoming Obstacles: Perspective on How Mediterranean Oaks Defend Their Acorns from Insect Seed Predators. Insects, 16(9), 990. https://doi.org/10.3390/insects16090990






