Effects of Forest Age and Invasive Shrubs on Mycophilous Coleoptera Communities in a Temperate Deciduous Woodland
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
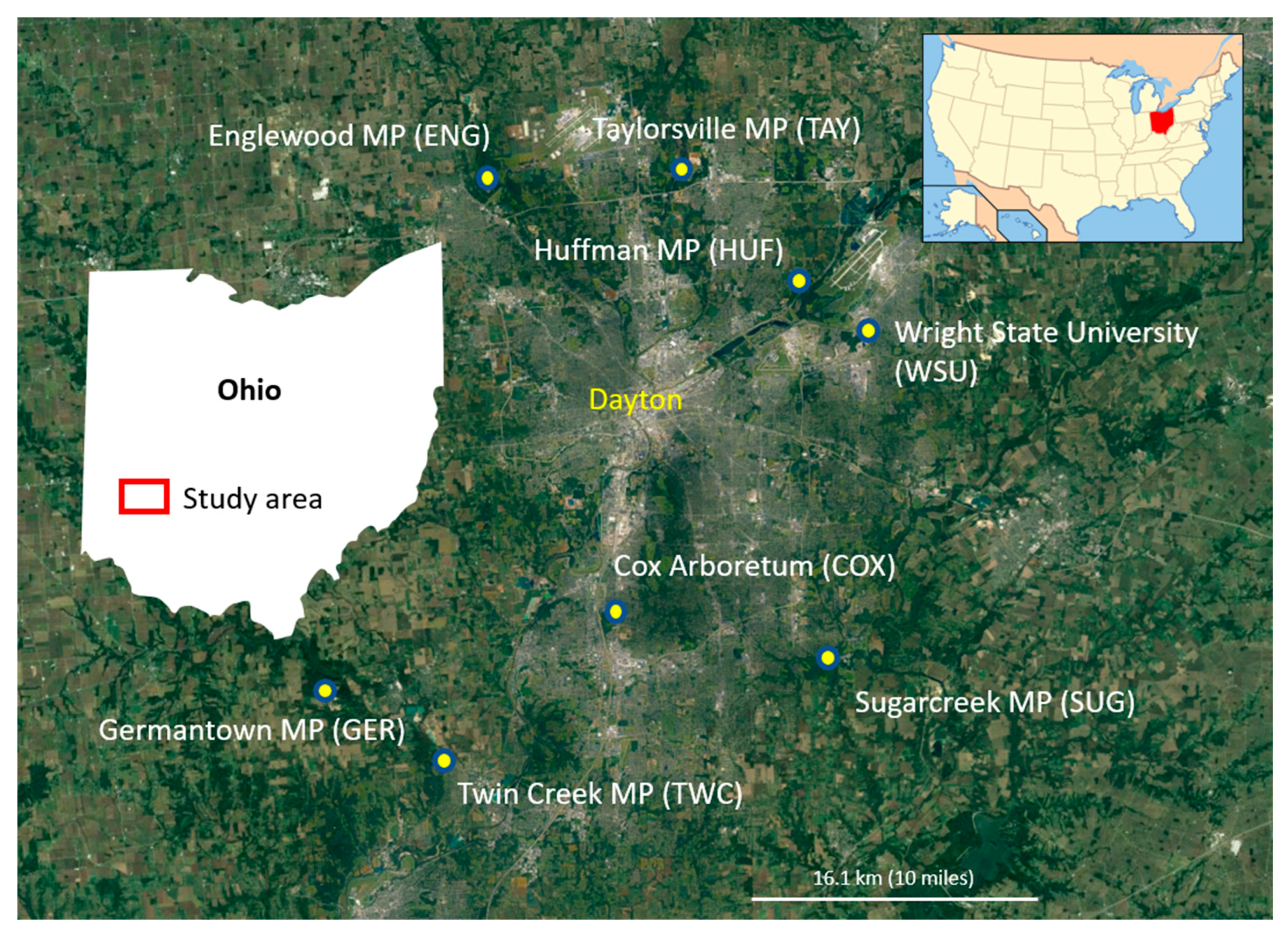
2.1. Study Area
2.2. Beetle Surveys
2.3. Statistical Analysis
3. Results
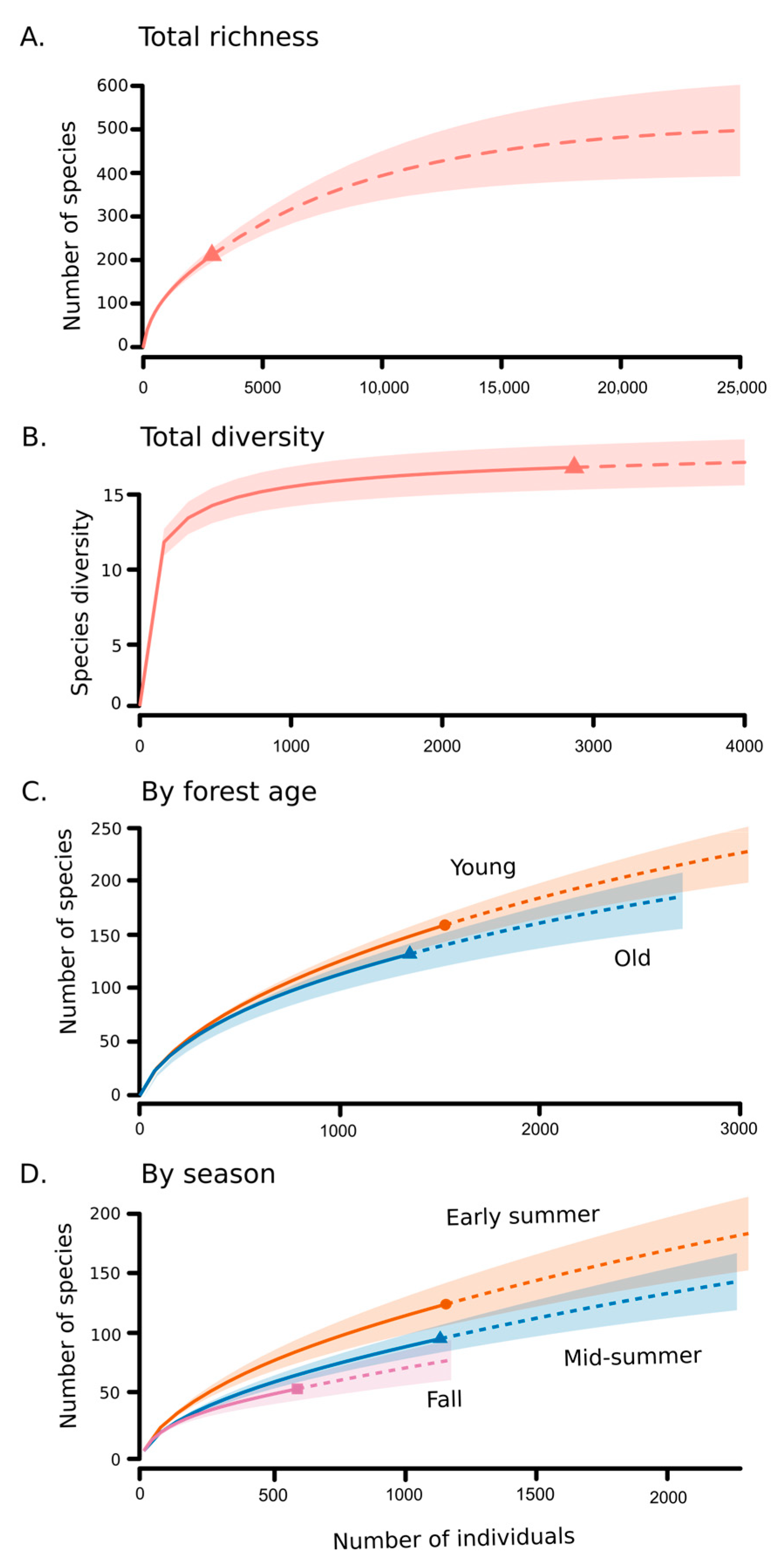
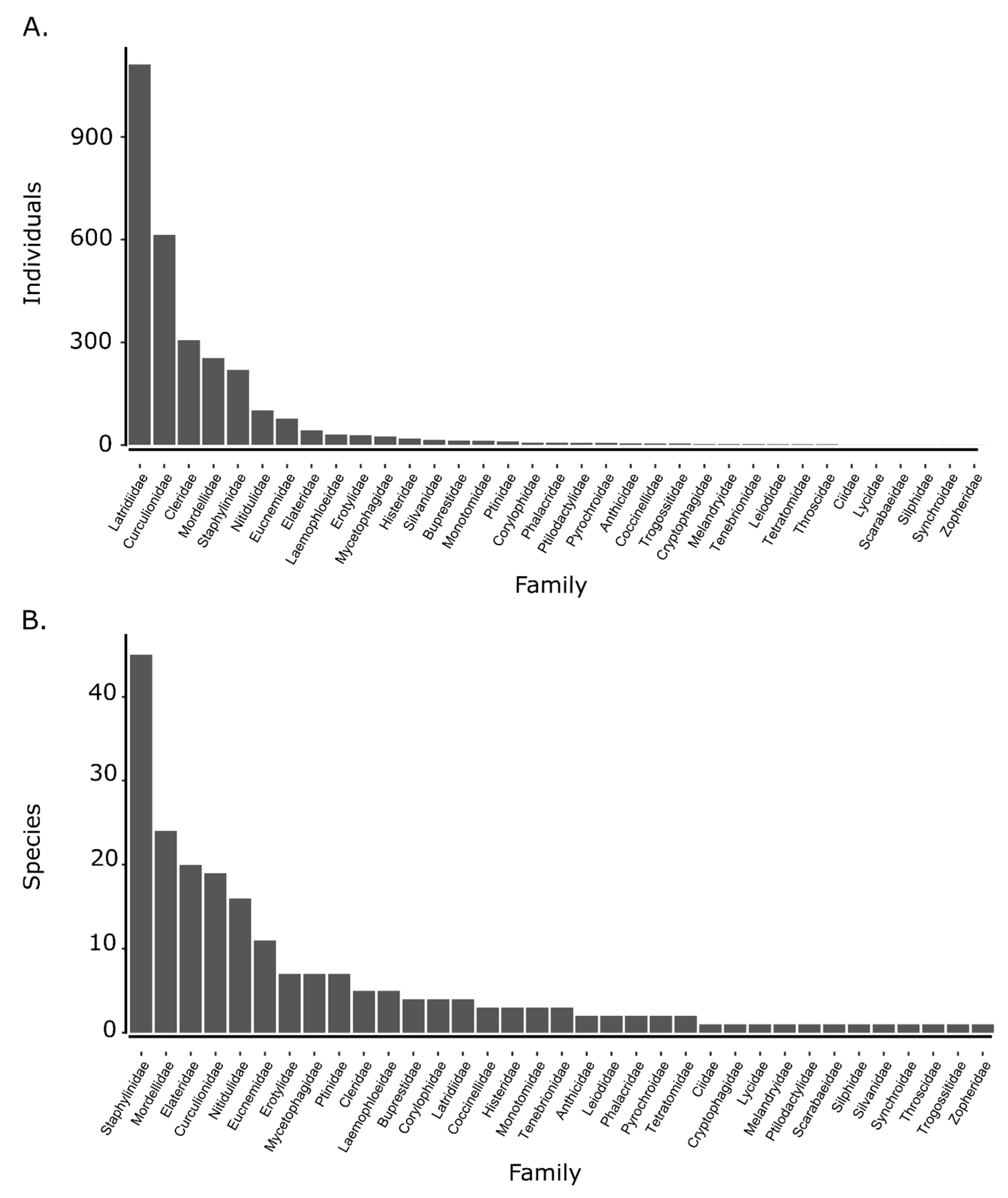
3.1. Beetle Abundance and Richness
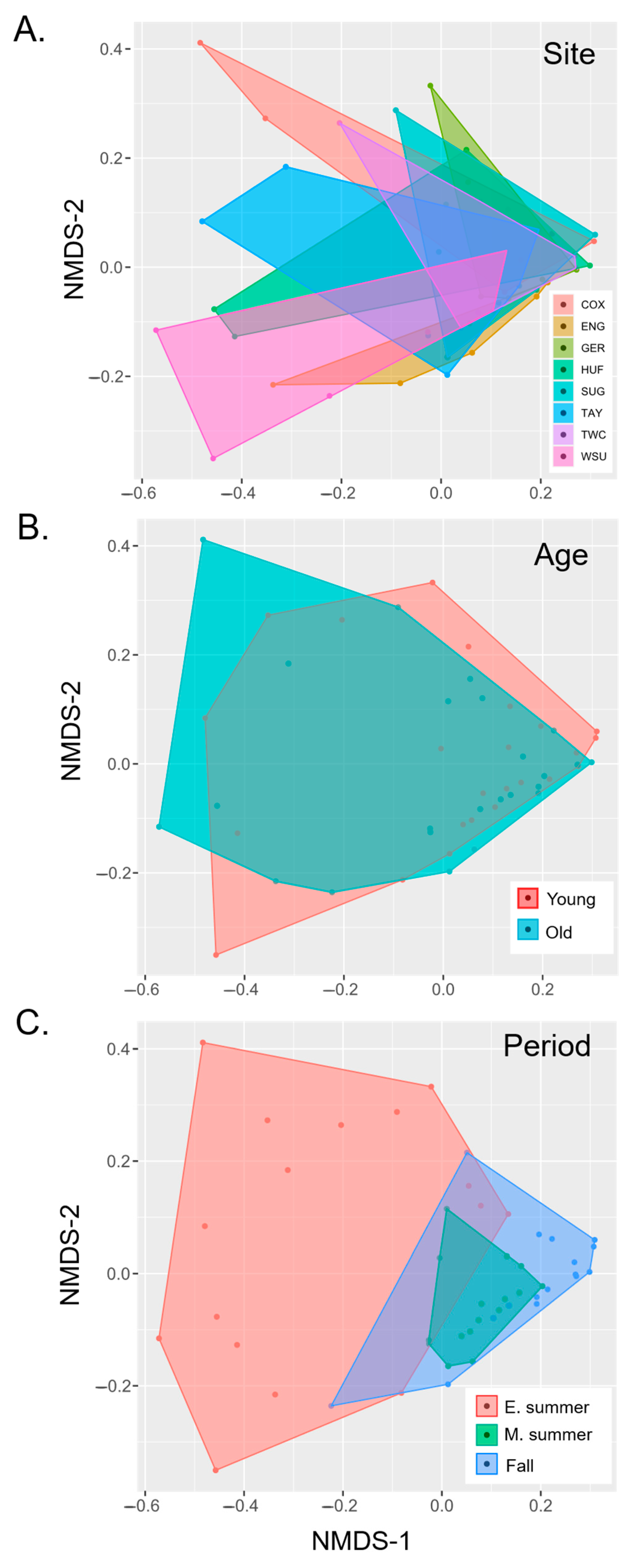
3.2. Effects of Forest Traits
4. Discussion
4.1. Fungus-Associated Beetle Communities in Southwest Ohio Woodlands
4.2. Effects of Forest Variables
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meier, A.; Bratton, S.P.; Duffy, D.C. Possible ecological mechanisms for loss of vernal-herb diversity in logged eastern deciduous forests. Ecol. Appl. 1995, 5, 935–946. [Google Scholar] [CrossRef]
- Schowalter, T. Arthropod diversity and functional importance in old-growth forests of North America. Forests 2017, 8, 97. [Google Scholar] [CrossRef]
- Davis, M.D. Eastern Old-Growth Forests: Prospects for Rediscovery and Recovery; Island Press: Washington, DC, USA, 1996; 383p. [Google Scholar]
- DeMars, B.G.; Runkle, J.R. Groundlayer vegetation ordination and site-factor analysis of the Wright State University Woods (Greene County, Ohio). Ohio J. Sci. 1992, 92, 98–106. [Google Scholar]
- Olivero, A.M.; Hix, D.M. Influence of aspect and stand age on ground flora of southeastern Ohio forest ecosystems. Plant Ecol. 1998, 139, 177–187. [Google Scholar] [CrossRef]
- Giese, E.E.G.; Howe, R.W.; Wolf, A.T.; Miller, N.A.; Walton, N.G. Sensitivity of breeding birds to the “human footprint” in western Great Lakes forest landscapes. Ecosphere 2015, 6, 90. [Google Scholar] [CrossRef]
- Summerville, K.S.; Crist, T.O. Determinants of lepidopteran community composition and species diversity in eastern deciduous forests: Roles of season, eco-region and patch size. Oikos 2003, 100, 134–148. [Google Scholar] [CrossRef]
- Lindner, D.L.; Burdsall, H.H.; Stanosz, G.R. Species diversity of polyporoid and corticioid fungi in northern hardwood forests with differing management histories. Mycologia 2006, 98, 195–217. [Google Scholar] [CrossRef] [PubMed]
- Jeffries, J.M.; Marquis, R.J.; Forkner, R.E. Forest age influences oak insect herbivore community structure, richness, and density. Ecol. Appl. 2006, 16, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Attiwill, P.M.; Adams, M.A. Nutrient cycling in forests. New Phytol. 1993, 124, 561–582. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, B. Fungi: Ecological importance and impact on humans. ELS 2011, 1–5. [Google Scholar] [CrossRef]
- Nair, D.N.; Padmavathy, S. Impact of endophytic microorganisms on plants, environment and humans. Sci. World J. 2014, 2014, 250693. [Google Scholar] [CrossRef] [PubMed]
- Větrovský, T.; Voříšková, J.; Šnajdr, J.; Gabriel, J.; Baldrian, P. Ecology of coarse wood decomposition by the saprotrophic fungus. Biodegradation 2011, 22, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, K.; Morschhauser, T.; Pál-Fám, F.; Botta-Dukát, Z. Exploring the relationship between macrofungi diversity, abundance, and vascular plant diversity in semi-natural and managed forests in north-east Hungary. Ecol. Res. 2013, 28, 543–552. [Google Scholar] [CrossRef]
- Zhiguang, H.; Xin, S.; Mengsha, L. Effects of forest age on soil fungal community in a northern temperate ecosystem. Indian J. Microbiol. 2016, 56, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Zelinka, S.L.; Kirker, G.T.; Bishell, A.B.; Glass, S.V. Effects of wood moisture content and the level of acetylation on brown rot decay. Forests 2020, 11, 299. [Google Scholar] [CrossRef]
- Rousk, J.; Brookes, P.C.; BååtH, E. Contrasting soil pH effects on fungal and bacterial growth suggest functional redundancy in carbon mineralization. Appl. Environ. Microbiol. 2009, 75, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- McKenna, D.D.; Shin, S.; Ahrens, D.; Balke, M.; Beza-Beza, C.; Clarke, D.J.; Donath, A.; Escalona, H.E.; Friedrich, F.; Letsch, H.; et al. The evolution and genomic basis of beetle diversity. Proc. Natl. Acad. Sci. USA 2019, 116, 24729–24737. [Google Scholar] [CrossRef] [PubMed]
- Wertheim, B.; Sevenster, J.G.; Eijs, I.E.M.; Van Alphen, J.J.M. Species diversity in a mycophagous insect community: The case of spatial aggregation vs. resource partitioning. J. Anim. Ecol. 2000, 69, 335–351. [Google Scholar] [CrossRef]
- Ashe, J.S. Studies of the life history and habits of Phanerota fasciata Say (Coleoptera: Staphylinidae: Aleocharinae) with notes on the mushroom as a habitat and descriptions of the immature stages. Coleopt. Bull. 1981, 35, 83–96. [Google Scholar]
- Vega, F.E.; Hoftstetter, R.W. Bark Beetles: Biology and Ecology of Native and Invasive Species; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar] [CrossRef]
- Lilleskov, E.A.; Bruns, T.D. Spore dispersal of a resupinate ectomycorrhizal fungus, Tomentella sublilacina, via soil food webs. Mycologia 2005, 97, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Fong, J.J.; Lee, H.; Shin, S.; Lee, S.; Lee, N.; Lim, Y.W. Determination of coleopteran insects associated with spore dispersal of Cryptoporus volvatus (Polyporaceae: Basidiomycota) in Korea. J. Asia-Pac. Èntomol. 2014, 17, 647–651. [Google Scholar] [CrossRef]
- Põldmaa, K.; Kaasik, A.; Tammaru, T.; Kurina, O.; Jürgenstein, S.; Teder, T. Polyphagy on unpredictable resources does not exclude host specialization: Insects feeding on mushrooms. Ecology 2016, 97, 2824–2833. [Google Scholar] [CrossRef] [PubMed]
- Hanley, R.S.; Goodrich, M.A. Review of mycophagy, host relationships and behavior in the new world Oxyporinae (Coleoptera: Staphylinidae). Coleopt. Bull. 1995, 49, 267–280. [Google Scholar]
- Epps, M.J.; Arnold, E. Diversity, abundance and community network structure in sporocarp-associated beetle communities of the central Appalachian Mountains. Mycologia 2010, 102, 785–802. [Google Scholar] [CrossRef] [PubMed]
- Brazee, N.J.; Lindner, D.L.; D’aMato, A.W.; Fraver, S.; Forrester, J.A.; Mladenoff, D.J. Disturbance and diversity of wood-inhabiting fungi: Effects of canopy gaps and downed woody debris. Biodivers. Conserv. 2014, 23, 2155–2172. [Google Scholar] [CrossRef]
- Dove, N.C.; Keeton, W.S. Structural complexity enhancement increases fungal species richness in northern hardwood forests. Fungal Ecol. 2014, 13, 181–192. [Google Scholar] [CrossRef]
- Müller, J.; Ulyshen, M.; Seibold, S.; Cadotte, M.; Chao, A.; Bässler, C.; Vogel, S.; Hagge, J.; Weiß, I.; Baldrian, P.; et al. Primary determinants of communities in deadwood vary among taxa but are regionally consistent. Oikos 2020, 129, 1579–1588. [Google Scholar] [CrossRef]
- Klimaszewski, J.; Peck, S.B. Succession and phenology of beetle faunas (Coleoptera) in the fungus Polyporellus squamosus (Huds.:Fr.) Karst. (Polyporaceae) in Silesia, Poland. Can. J. Zool. 1987, 65, 542–550. [Google Scholar] [CrossRef]
- Cline, A.R.; Leschen, R.A.B. Coleoptera associated with the oyster mushroom, Pleurotus ostreatus Fries, in North America. Southeast. Nat. 2005, 4, 409–420. [Google Scholar] [CrossRef]
- Johansson, T.; Olsson, J.; Hjältén, J.; Jonsson, B.G.; Ericson, L. Beetle attraction to sporocarps and wood infected with mycelia of decay fungi in old-growth spruce forests of northern Sweden. For. Ecol. Manag. 2006, 237, 335–341. [Google Scholar] [CrossRef]
- Thunes, K.H.; Midtgaard, F.; Gjerde, I. Diversity of coleoptera of the bracket fungus Fomitopsis pinicola in a Norwegian spruce forest. Biodivers. Conserv. 2000, 9, 833–852. [Google Scholar] [CrossRef]
- Lassauce, A.; Paillet, Y.; Jactel, H.; Bouget, C. Deadwood as a surrogate for forest biodiversity: Meta-analysis of correlations between deadwood volume and species richness of saproxylic organisms. Ecol. Indic. 2011, 11, 1027–1039. [Google Scholar] [CrossRef]
- Epps, M.J.; Arnold, A.E. Quantifying beetle-macrofungal associations in a temperate biodiversity hot spot. Mycologia 2018, 110, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, B.; Wu, J.; Hu, S.; Seabloom, E. Invasive plants differentially affect soil biota through litter and rhizosphere pathways: A meta-analysis. Ecol. Lett. 2019, 22, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Gorchov, D.L.; Trisel, D.E. Competitive effects of the invasive shrub, Lonicera maackii (Rupr.) Herder (Caprifoliaceae), on the growth and survival of native tree seedlings. Plant Ecol. 2003, 166, 13–24. [Google Scholar] [CrossRef]
- Poulette, M.M.; Arthur, M.A. The impact of the invasive shrub Lonicera maackii on the decomposition dynamics of a native plant community. Ecol. Appl. 2012, 22, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Arthur, M.A.; Bray, S.R.; Kuchle, C.R.; McEwan, R.W. The influence of the invasive shrub, Lonicera maackii, on leaf decomposition and microbial community dynamics. Plant Ecol. 2012, 213, 1571–1582. [Google Scholar] [CrossRef]
- North, M.; Trappe, J.; Franklin, J. Standing crop and animal consumption of fungal sporocarps in pacific northwest forests. Ecology 1997, 78, 1543–1554. [Google Scholar] [CrossRef]
- Arnett, R.; Thomas, M. American Beetles, Volumes 1 and 2, 1st ed.; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Evans, A. Beetles of Eastern North America; Princeton University Press: Princeton, NJ, USA, 2014. [Google Scholar]
- Cottam, G.; Curtis, J.T. The use of distance measures in phytosociological sampling. Ecology 1956, 35, 451–460. [Google Scholar] [CrossRef]
- Purcell, L. How Old Is My Tree? 2019. Available online: https://purduelandscapereport.org/article/how-old-is-my-tree/ (accessed on 15 August 2020).
- Morton Arboretum. Trees and Plants. 2025. Available online: https://mortonarb.org/plant-and-protect/trees-and-plants/ (accessed on 14 July 2025).
- Angers, V.A.; Messier, C.; Beaudet, M.; Leduc, A. Comparing composition and structure in old-growth and harvested (selection and diameter-limit cuts) northern hardwood stands in Quebec. For. Ecol. Manag. 2005, 217, 275–293. [Google Scholar] [CrossRef]
- Yuan, J.; Zheng, X.; Cheng, F.; Zhu, X.; Hou, L.; Li, J.; Zhang, S. Fungal community structure of fallen pine and oak wood at different stages of decomposition in the Qinling Mountains, China. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: iNterpolation and EXTrapolation for Species Diversity. 2020. Available online: http://chao.stat.nthu.edu.tw/wordpress/software-download/08/2022 (accessed on 3 January 2025).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 48. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package. R Package Version 2.6-4. Available online: https://CRAN.R-project.org/package=vegan (accessed on 15 August 2020).
- Bock, C.E.; Jones, Z.F.; Bock, J.H. Relationships between species richness, evenness, and abundance in a southwestern savanna. Ecology 2007, 88, 1322–1327. [Google Scholar] [CrossRef] [PubMed]
- Kodada, J.; Selnekovič, D.; Goffová, K. Hydnobius punctulatus Hampe, 1861 (Coleoptera: Leiodidae): Redescription of species, comments on variability and new distributional data for Central Europe. Biologia 2019, 74, 975–980. [Google Scholar] [CrossRef]
- Tian, J.; Qiao, Y.; Wu, B.; Chen, H.; Li, W.; Jiang, N.; Zhang, X.; Liu, X. Ecological succession pattern of fungal community in soil along a retreating glacier. Front. Microbiol. 2017, 8, 1028. [Google Scholar] [CrossRef] [PubMed]
- Spake, R.; Ezard, T.H.G.; Martin, P.A.; Newton, A.C.; Doncaster, C.P. A meta-analysis of functional group responses to forest recovery outside of the tropics. Conserv. Biol. 2015, 29, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Prado e Castro, C.; García, M.D.; Martins da Silva, P.; Faria e Silva, I.; Serrano, A. Coleoptera of forensic interest: A study of seasonal community composition and succession in Lisbon, Portugal. Forensic Sci. Int. 2013, 232, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Genevieve, L.; Pierre-Luc, C.; Roxanne, G.-T.; Amélie, M.; Danny, B.; Vincent, M.; Hugo, G. Estimation of fungal diversity and identification of major abiotic drivers influencing fungal richness and communities in northern temperate and boreal Quebec forests. Forests 2019, 10, 1096. [Google Scholar] [CrossRef]
- Straatsmaa, G.; Ayer, F.; Eglib, S. Species richness, abundance, and phenology of fungal fruit bodies over 21 years in a Swiss forest plot. Mycol. Res. 2001, 105, 515–523. [Google Scholar] [CrossRef]
- Dorning, M.; Cipollini, D. Leaf and root extracts of the invasive shrub, Lonicera maackii, inhibit seed germination of three herbs with no autotoxic effects. Plant Ecol. 2006, 184, 287–296. [Google Scholar] [CrossRef]
- Shannon, S.M.; Bauer, J.T.; Anderson, W.E.; Reynolds, H.L. Plant-soil feedbacks between invasive shrubs and native forest understory species lead to shifts in the abundance of mycorrhizal fungi. Plant Soil 2014, 382, 317–328. [Google Scholar] [CrossRef]
- Kauserud, H.; Stige, L.C.; Vik, J.O.; Økland, R.H.; Høiland, K.; Stenseth, N.C. Mushroom fruiting and climate change. Proc. Natl. Acad. Sci. USA 2008, 105, 3811–3814. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |





| Metric | Age Class | COX | ENG | GER | HUF | SUG | TAY | TWC | WSU | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| Richness | Young | 39 | 22 | 45 | 47 | 35 | 42 | 30 | 23 | 154 |
| Old | 43 | 36 | 22 | 34 | 25 | 36 | 19 | 38 | 128 | |
| Total | 61 | 50 | 55 | 64 | 52 | 60 | 41 | 53 | 211 | |
| Abundance | Young | 282 | 74 | 302 | 307 | 119 | 147 | 169 | 124 | 1524 |
| Old | 231 | 172 | 140 | 315 | 106 | 128 | 130 | 127 | 1349 | |
| Total | 513 | 246 | 442 | 622 | 225 | 275 | 299 | 251 | 2873 |
| Site | Age Class | Average Max. Age | Average Age |
|---|---|---|---|
| COX | Old | 109 | 63 |
| Young | 69 | 47 | |
| ENG | Old | 98 * | 58 * |
| Young | 126 | 65 | |
| GER | Old | 91 | 81 |
| Young | 80 | 55 | |
| HUF | Old | 171 | 92 |
| Young | 140 | 57 | |
| SUG | Old | 104 | 47 * |
| Young | 71 | 66 | |
| TAY | Old | 122 | 69 |
| Young | 76 | 48 | |
| TWC | Old | 114 | 75 |
| Young | 100 | 64 | |
| WSU | Old | 118 | 80 |
| Young | 73 | 52 |
| Abundance | Estimate | df | t Value | Pr(>|t|) |
|---|---|---|---|---|
| Age | −0.2951 | 38 | −1.215 | 0.2318 |
| Mid-summer | 0.0200 | 36 | 0.070 | 0.9442 |
| Fall | −1.2547 | 36 | −4.393 | <0.0001 |
| Honeysuckle | −0.0004 | 38 | −2.857 | 0.0069 |
| Log(CWD) * | 0.0619 | 39 | 0.391 | 0.6978 |
| Richness | ||||
| Age | −2.2102 | 38 | −1.954 | 0.0582 |
| Mid-summer | −3.7500 | 36 | −2.828 | 0.0076 |
| Fall | −8.8750 | 36 | −6.692 | <0.0001 |
| Honeysuckle | −0.0021 | 41 | −2.936 | 0.0054 |
| Log(CWD)* | 0.8196 | 39 | 1.099 | 0.2787 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, J.M.; Stireman, J.O., III. Effects of Forest Age and Invasive Shrubs on Mycophilous Coleoptera Communities in a Temperate Deciduous Woodland. Insects 2025, 16, 735. https://doi.org/10.3390/insects16070735
Brown JM, Stireman JO III. Effects of Forest Age and Invasive Shrubs on Mycophilous Coleoptera Communities in a Temperate Deciduous Woodland. Insects. 2025; 16(7):735. https://doi.org/10.3390/insects16070735
Chicago/Turabian StyleBrown, Jeffrey M., and John O. Stireman, III. 2025. "Effects of Forest Age and Invasive Shrubs on Mycophilous Coleoptera Communities in a Temperate Deciduous Woodland" Insects 16, no. 7: 735. https://doi.org/10.3390/insects16070735
APA StyleBrown, J. M., & Stireman, J. O., III. (2025). Effects of Forest Age and Invasive Shrubs on Mycophilous Coleoptera Communities in a Temperate Deciduous Woodland. Insects, 16(7), 735. https://doi.org/10.3390/insects16070735







