Egg Allocation on Anastrepha ludens Larvae by Mass-Reared Diachasmimorpha longicaudata Females
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing Procedures
2.2. Host Exposure and Female Parasitoid Ages Evaluated
2.3. Experimental Setup
2.3.1. Parasitized Host Mortality, Superparasitism, Parasitoid Emergence and Offspring Sex Ratio
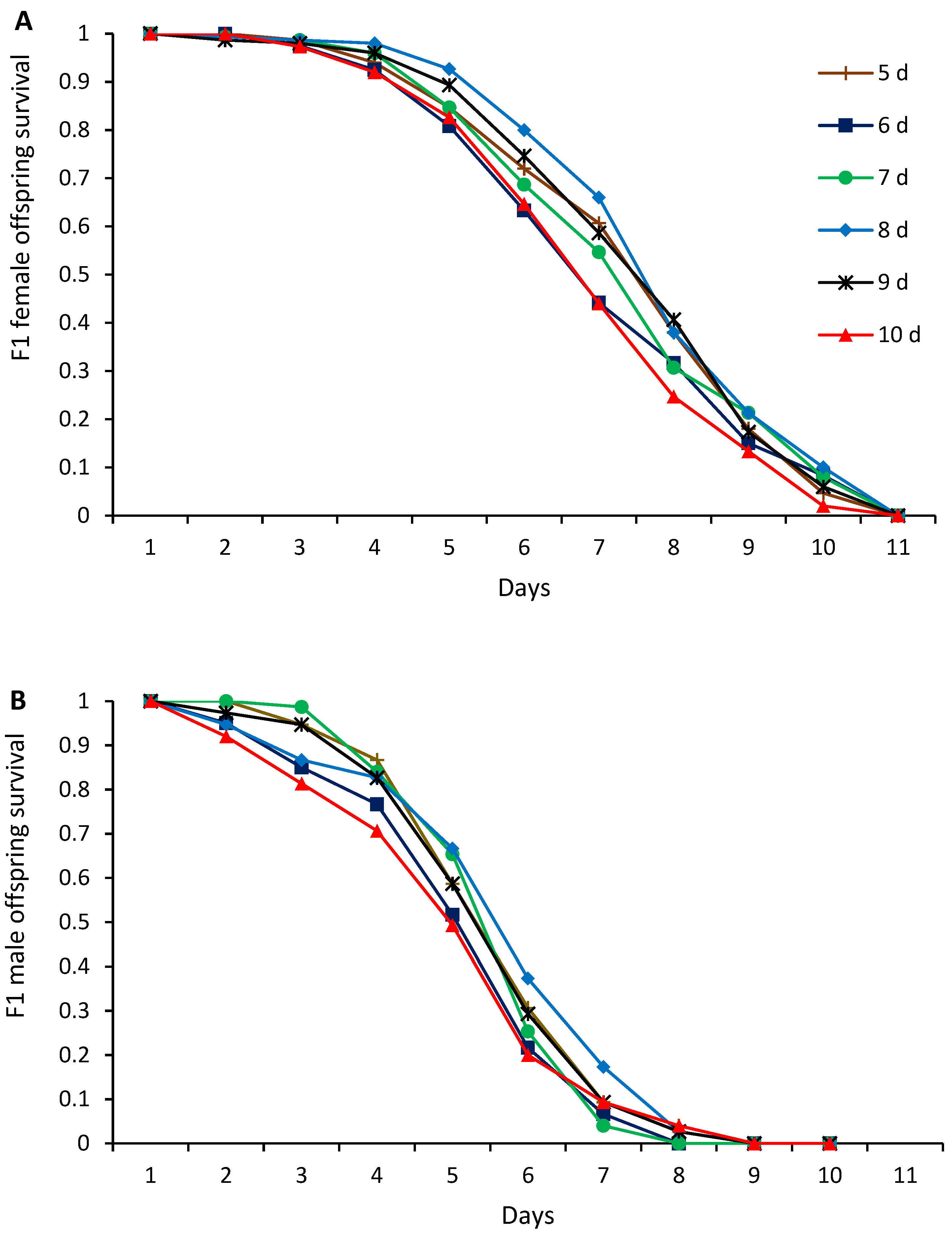
2.3.2. Fitness-Related Parameters of Parasitoid Offspring
2.4. Data Analysis
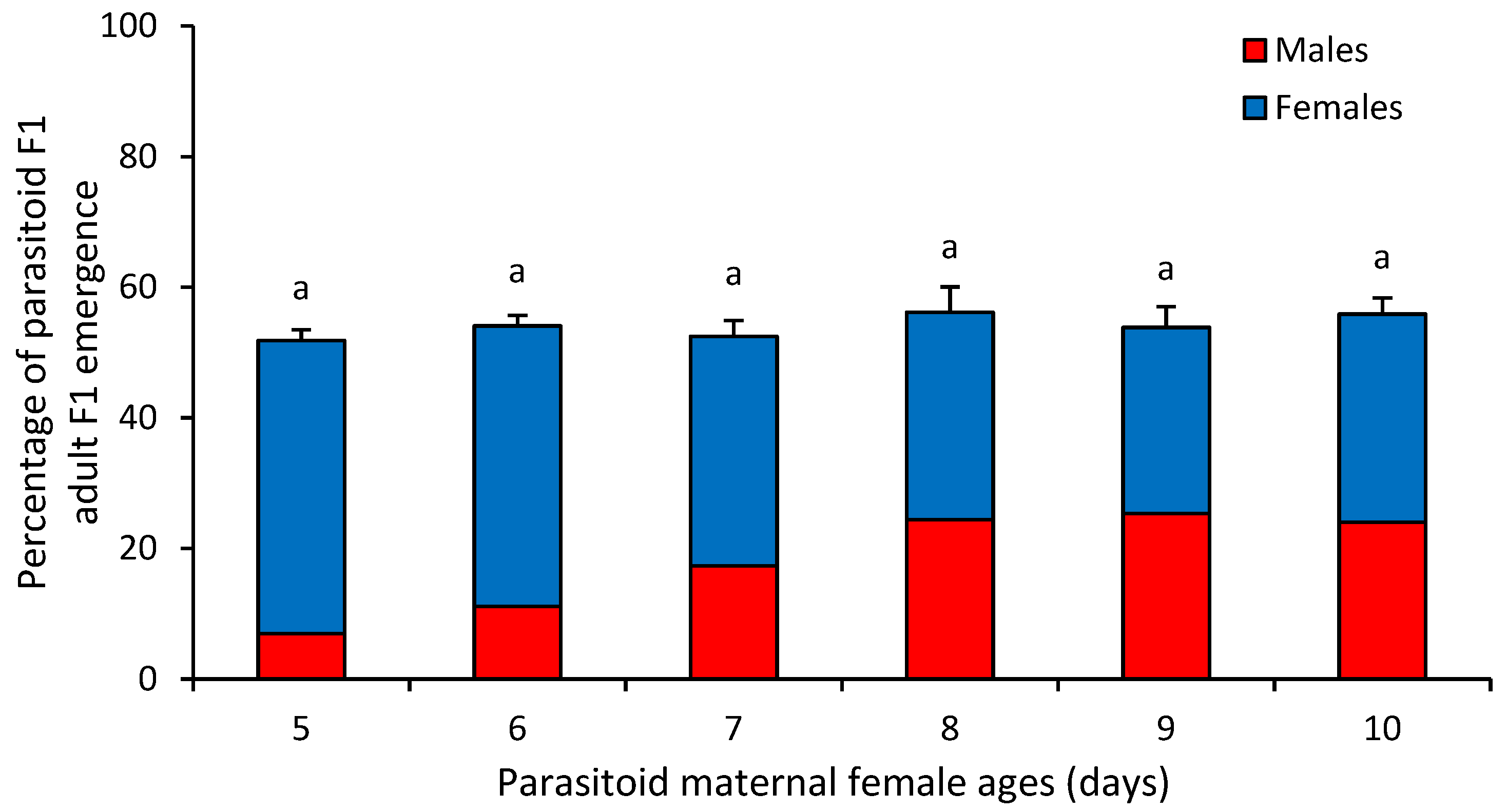
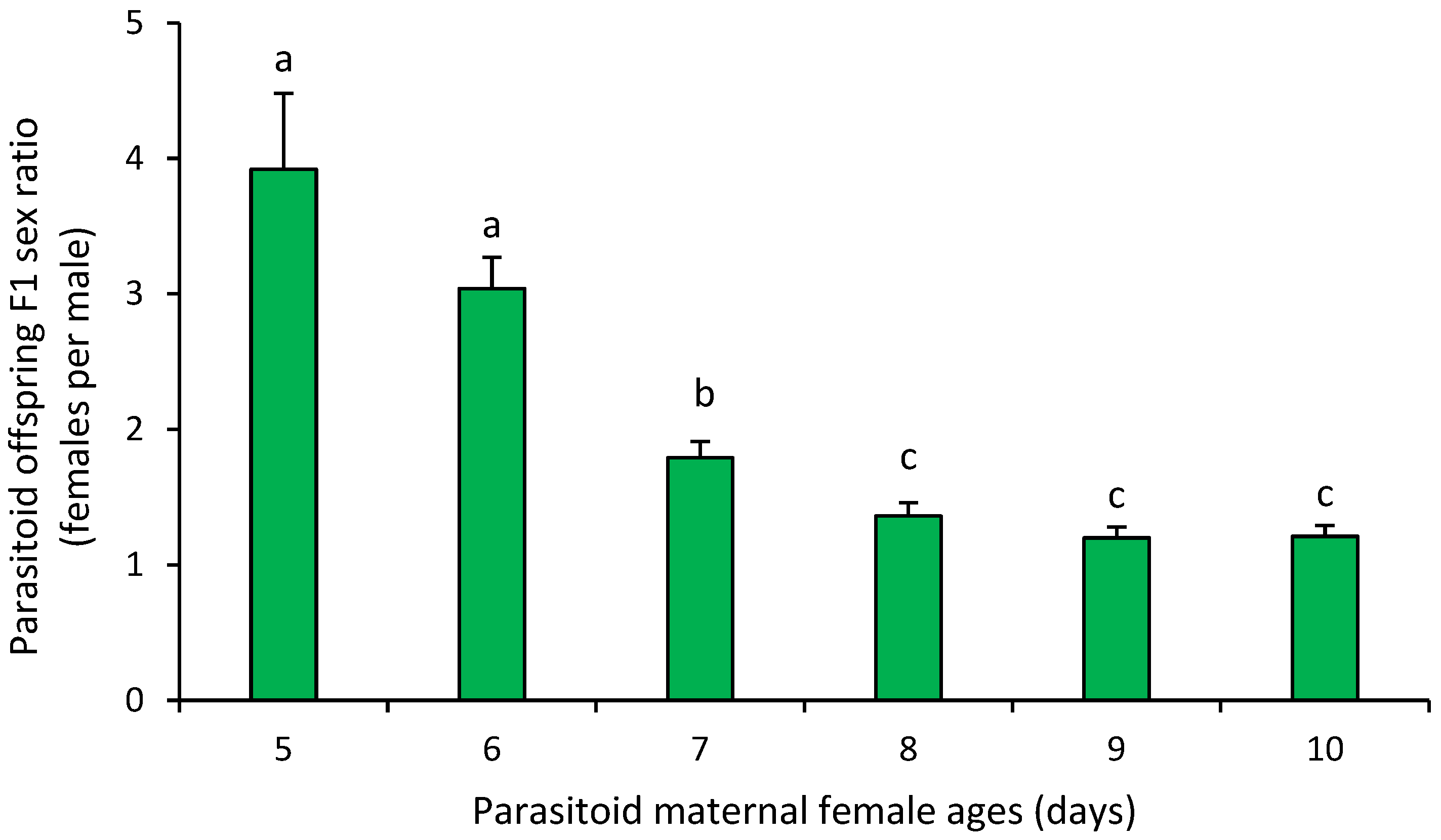
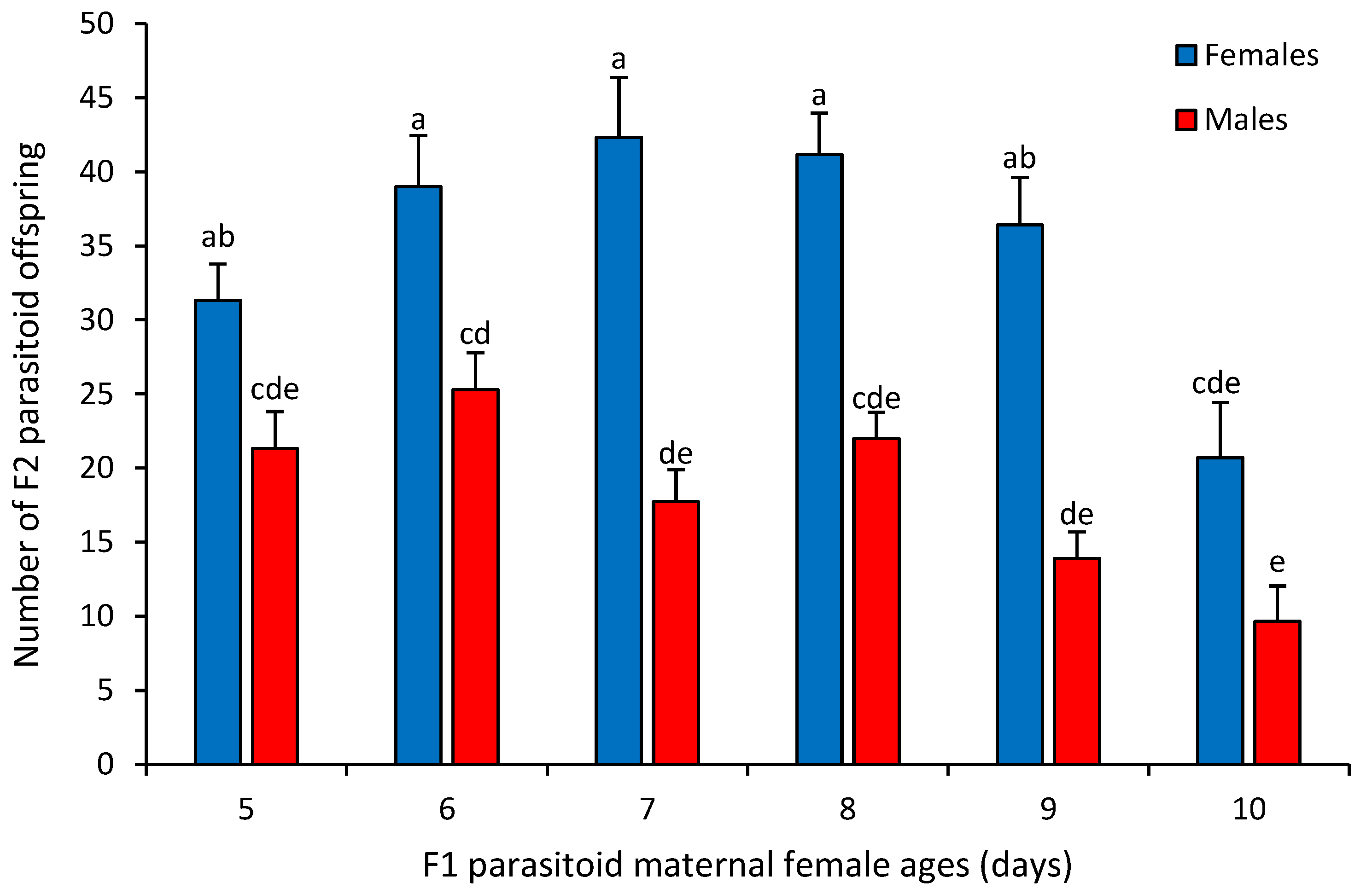
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heimpel, G.E.; Rosehnheim, J.A.; Mangel, M. Egg limitation, host quality, and dynamic behavior by a parasitoid in the field. Ecology 1996, 77, 2410–2420. [Google Scholar] [CrossRef]
- Lebreton, S.; Chevrier, C.; Darrouzet, E. Sex allocation strategies in response to conspecifics’ offspring sex ratio in solitary parasitoids. Behav. Ecol. 2010, 21, 107–112. [Google Scholar] [CrossRef]
- Ueno, T. Age-dependent constraints of sex allocation in a parasitoid wasp. Psyche 2014, 1, 363174. [Google Scholar] [CrossRef]
- Pannebakker, B.A.; Cook, N.; van den Heuvel, J.; van de Zande, L.; Shuker, D.M. Genomics of sex allocation in the parasitoid wasp Nasonia vitripennis. BMC Genom. 2020, 21, 499. [Google Scholar] [CrossRef] [PubMed]
- Ellers, J.; Sevenster, J.G.; Driessen, G. Egg load evolution in parasitoids. Am. Nat. 2000, 156, 650–665. [Google Scholar] [CrossRef]
- Iqbal, M.S.; Abdin, Z.; Arshad, M.S.; Kosar Abbas, S.; Tahir, M.; Jamil, A.; Manzoor, A. The role of parasitoid age on the fecundity and sex ratio of the parasitoid, Aenasius bambawalei (Hayat) (Hymenoptera: Encyrtidae). Pak. J. Zool. 2016, 48, 67–72. [Google Scholar]
- Zuim, V.; de Souza-Rodrigues, H.; Pratissoli, D.; Braz-Torres, J.; Ferreira-Melo-Fragoso, D.; Oliveira de Freitas-Bueno, R.C. Age and density of eggs of Helicoverpa armigera influence on Trichogramma pretiosum parasitism. Acta Sci. Biol. Sci. 2017, 39, 513–520. [Google Scholar] [CrossRef][Green Version]
- Liu, P.-C.; Wang, Z.-Y.; Qi, M.; Hu, H.-Y. Testing the local mate competition rule in a quasi-gregarious parasitoid with facultative superparasitism. Behav. Ecol. 2023, 34, 287–296. [Google Scholar] [CrossRef]
- Thiel, A.; Hoffmeister, T.S. Knowing your habitat: Linking patch-encounter rate and patch exploitation in parasitoids. Behav. Ecol. 2004, 15, 419–425. [Google Scholar] [CrossRef][Green Version]
- Wajnberg, E. Time allocation strategies in insect parasitoids: From ultimate predictions to proximate behavioral mechanisms. Behav. Ecol. Sociobiol. 2006, 60, 589–611. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Wang, Y.F.; Yin, S.Y.; Liu, P.C.; Hu, H.Y. Oviposition experience promotes active reproductive behaviour in a synovigenic parasitoid. J. Hymenopt. Res. 2023, 95, 1–12. [Google Scholar] [CrossRef]
- Ksentini, I.; Jardak, T.; Zeghal, N. Parasitoid age and host quality side effects on the parasitization behavior of Trichogramma oleae and Trichogramma cacoeciae (Hymenoptera: Trichogrammatidae). J. Entomol. Zool. Stud. 2018, 6, 2004–2009. [Google Scholar]
- Iqbal, A.; Yang-Yang, H.; Yong-Ming, C.; Ali, A.; Monticelli, L.S.; Desneux, N.; Lian-Sheng, Z. Impact of Trichogramma parasitoid age on the outcome of multiparasitism in the factitious host eggs of Chinese oak silkworm, Antheraea pernyi. J. Pest Sci. 2020, 93, 1347–1357. [Google Scholar] [CrossRef]
- Queiroz, A.P.; Oliveira-Costa, C.; Favetti, B.M.; Vieira-Silva, G.; Bueno, A.F. Effects of parasitoid and host age on the parasitism of Trichogramma pretiosum on eggs of Anticarsia gemmatalis. Rev. Bras. Entomol. 2020, 64, e2019105. [Google Scholar] [CrossRef]
- Mawela, K.V.; Kfiri, R.; Krüger, K. Age-related reproductive biology of Trichogrammatoidea lutea on eggs of the African bollworm Helicoverpa armigera. Bull. Insectol. 2021, 74, 123–128. [Google Scholar]
- Queiroz, A.P.; Favetti, B.M.; Hayashida, R.; Grande, M.L.M.; Neiva, M.M.; Panizzi, A.R.; Bueno, A.F. Effect of the ages of parasitoid and host eggs on Telenomus podisi (Hymenoptera: Platygasteridae) parasitism. Neotrop. Entomol. 2019, 48, 974–982. [Google Scholar] [CrossRef]
- Montoya, P.; Liedo, P.; Benrey, B.; Cancino, J.; Barrera, J.F.; Sivinski, J.; Aluja, M. Biological control of Anastrepha spp. (Diptera: Tephritidae) in mango orchards through augmentative releases of Diachasmimorpha longicaudata (Ashmead) (Hymenoptera: Braconidae). Biol. Control 2000, 18, 216–224. [Google Scholar] [CrossRef]
- Meirelles, R.N.; Rodrigues Redaelli, L.; Bernardes Ourique, C. Comparative biology of Diachasmimorpha longicaudata (Hymenoptera: Braconidae) reared on Anastrepha fraterculus and Ceratitis capitata (Diptera: Tephritidae). Fla. Entomol. 2013, 96, 412–418. [Google Scholar] [CrossRef]
- Cancino, J.; Ruiz, L.; López, L.; Moreno, F.M. Cría masiva de parasitoides. In Moscas de la Fruta. Fundamentos y Procedimientos para su Control, 2nd ed.; Montoya, P., Toledo, J., Hernández, E., Eds.; S y G Editores: Mexico City, Mexico, 2020; pp. 463–481. [Google Scholar]
- Sivinski, J.; Calkins, C.O.; Baranowski, R.; Harris, D.; Brambila, J.; Diaz, J.; Burns, R.E.; Holler, T.; Dodson, G. Suppression of a Caribbean fruit fly (Anastrepha suspensa (Loew)) (Diptera: Tephritidae) population through augmentative releases of the parasitoid Diachasmimorpha longicaudata (Ashmead) (Hymenoptera: Braconidae). Biol. Control 1996, 16, 177–185. [Google Scholar] [CrossRef]
- Montoya, P.; Cancino, J.; Zenil, M.; Santiago, G.; Gutierrez, J.M. The augmentative biological control component in the Mexican national campaign against Anastrepha spp. fruit flies. In Area-Wide Control of Insect Pests: From Research to Field Implementation; Vreysen, M.J.B., Robinson, A.S., Hendrichs, J., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 661–670. [Google Scholar]
- Vargas, R.I.; Leblanc, L.; Harris, E.J.; Manoukis, N.C. Regional suppression of Bactrocera fruit flies (Diptera: Tephritidae) in the Pacific through biological control and prospects for future introductions into other areas of the world. Insects 2012, 3, 727–742. [Google Scholar] [CrossRef]
- de Pedro, L.; Tormos, J.; Harbi, A.; Ferrara, F.; Sabater-Muñoz, B.; Asís, J.D.; Beitia, F. Combined use of the larvo-pupal parasitoids Diachasmimorpha longicaudata and Aganaspis daci for biological control of the medfly. Ann. Appl. Biol. 2019, 174, 40–50. [Google Scholar] [CrossRef]
- Dias, N.P.; Montoya, P.; Nava, D.E. A 30-year systematic review reveals success in tephritid fruit fly biological control research. Entomol. Exp. Appl. 2022, 170, 370–384. [Google Scholar] [CrossRef]
- Garcia, F.R.M.; Ovruski, S.M.; Suárez, L.; Cancino, J.; Liburd, O.E. Biological control of tephritid fruit flies in the Americas and Hawaii: A review of the use of parasitoids and predators. Insects 2020, 11, 662. [Google Scholar] [CrossRef]
- Montoya, P.; Liedo, P.; Benrey, B.; Cancino, J.; Barrera, J.F.; Sivinski, J.; Aluja, M. Functional response and superparasitism by Diachasmimorpha longicaudata (Hymenoptera: Braconidae), a parasitoid of fruit flies (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 2000, 93, 47–54. [Google Scholar] [CrossRef]
- Montoya, P.; Cancino, J.; Pérez-Lachaud, G.; Liedo, P. Host size, superparasitism and sex ratio in mass-reared Diachasmimorpha longicaudata, a fruit fly parasitoid. BioControl 2011, 56, 11–17. [Google Scholar] [CrossRef]
- López, P.; Rosales, D.; Flores, S.; Montoya, P. Mutual interference in the mass-reared fruit fly parasitoid, Diachasmimorpha longicaudata (Hymenoptera: Braconidae). BioControl 2021, 66, 649–658. [Google Scholar] [CrossRef]
- González, P.I.; Montoya, P.; Pérez-Lachaud, G.; Cancino, J.; Liedo, P. Superparasitism in mass-reared Diachasmimorpha longicaudata (Ashmead) (Hymenoptera: Braconidae), a parasitoid of fruit flies (Diptera: Tephritidae). Biol. Control 2007, 40, 320–326. [Google Scholar] [CrossRef]
- SAS Institute Inc. JMP®, version 11.1.1; SAS Institute Inc.: Cary, NC, USA, 2013.
- Sirot, E.; Bernstein, C. Food searching and superparasitism in solitary parasitoids. Acta Oecol. 1997, 18, 63–72. [Google Scholar] [CrossRef]
- Montoya, P.; López, P.; Cruz, J.; López, F.; Cadena, C.; Cancino, J.; Liedo, P. Effect of Diachasmimorpha longicaudata releases on the native parasitoid guild attacking Anastrepha spp. larvae in disturbed zones of Chiapas, Mexico. BioControl 2017, 62, 581–593. [Google Scholar] [CrossRef]
- van Alphen, J.J.M.; Visser, M.E. Superparasitism as an adaptive strategy for insect parasitoids. Ann. Rev. Entomol. 1992, 35, 59–79. [Google Scholar] [CrossRef]
- Devescovi, F.; Bachmann, G.E.; Nussenbaum, A.L.; Viscarret, M.M.; Cladera, J.L.; Segura, D.F. Effects of superparasitism on immature and adult stages of Diachasmimorpha longicaudata Ashmead (Hymenoptera: Braconidae) reared on Ceratitis capitata Wiedemann (Diptera: Tephritidae). Bull. Entomol. Res. 2017, 107, 756–767. [Google Scholar] [CrossRef]
- Suzuki, Y.; Tsuji, H.; Sasakawa, M. Sex allocation and effects of superparasitism on secondary sex ratios in the gregarious parasitoid, Trichogramma chilonis (Hymenoptera: Trichogrammatidae). Anim. Behav. 1984, 32, 478–484. [Google Scholar] [CrossRef]
- Mackauer, M.; Chow, A. Females of the parasitoid wasp, Dendrocerus carpenteri (Hymenoptera: Megaspilidae), adjust offspring sex allocation when competing for hosts. Eur. J. Entomol. 2016, 113, 542–550. [Google Scholar] [CrossRef]
- Tunca, H.; Buradino, M.; Colombel, E.A.; Tabone, E. Tendency and consequences of superparasitism for the parasitoid Ooencyrtus pityocampae (Hymenoptera: Encyrtidae) in parasitizing a new laboratory host, Philosamia ricini (Lepidoptera: Saturniidae). Eur. J. Entomol. 2016, 113, 51–59. [Google Scholar] [CrossRef]
- Darrouzet, E.; Imbert, E.; Chevier, C. Self-superparasitism consequences for offspring sex ratio in the solitary ectoparasitoid Eupelmus vuilleti. Entomol. Exp. Appl. 2003, 109, 167–171. [Google Scholar] [CrossRef]
- Gandolfi, M.; Mattiacci, L.; Dorn, S. Mechanisms of behavioral alterations of parasitoids reared in artificial systems. J. Chem. Ecol. 2003, 29, 1871–1887. [Google Scholar] [CrossRef]
- Postic, E.Y.; Outreman, Y.; Derocles, S.; Granado, C.; Le Ralec, A. Genetics of wild and mass-reared populations of a generalist aphid parasitoid and improvement of biological control. PLoS ONE 2021, 16, e0249893. [Google Scholar] [CrossRef]
- Sousa, J.M.; Spence, J.R. Effects of mating status and parasitoid density on superparasitism and offspring fitness in Tiphodytes gerriphagus (Hymenoptera: Scelionidae). Ann. Entomol. Soc. Am. 2000, 93, 548–553. [Google Scholar] [CrossRef]
- Altafini, D.L.; Rodrigues-Redaelli, L.; Mundstock-Jahnke, S. Superparasitism of Ceratitis capitata and Anastrepha fraterculus (Diptera: Tephritidae) by Diachasmimorpha longicaudata (Hymenoptera: Braconidae). Fla. Entomol. 2013, 96, 391–395. [Google Scholar] [CrossRef]
- Tormos, J.; Asís, J.; Sabater-Muñoz, B.; Baños, L.; Gayubo, S.; Beitia, F. Superparasitism in laboratory rearing of Spalangia cameroni (Hymenoptera: Pteromalidae), a parasitoid of medfly (Diptera: Tephritidae). Bull. Entomol. Res. 2012, 102, 51–61. [Google Scholar] [CrossRef]
- Montoya, P.; Pérez-Lachaud, G.; Liedo, P. Superparasitism in the fruit fly parasitoid Diachasmimorpha longicaudata (Hymenoptera: Braconidae) and the implications for mass rearing and augmentative release. Insects 2012, 3, 900–911. [Google Scholar] [CrossRef]
- Santolamazza-Carbone, S.; Cordero Rivera, A. Superparasitism and sex ratio adjustment in a wasp parasitoid: Results at variance with local mate competition? Oecologia 2003, 136, 365–373. [Google Scholar] [CrossRef]
- da Cruz, C.G.; Alvarenga, C.D.; do Carmo Oliveira, P.C.; Souza Conceição, E.R.; Cardoso dos Santos, Z.; Giustolin, T.A.; da Cruz Souza, M.D. Density of Diachasmimorpha longicaudata (Ashmead) and host Ceratitis capitata (Wied) larvae for the increase of parasitoid female production. Arq. Inst. Biol. 2018, 85, 1–6. [Google Scholar] [CrossRef][Green Version]
- Cancino, J.; López, F.; Montoya, P. Packaging conditions for the field release of the fruit fly parasitoid Diachasmimorpha longicaudata (Hymenoptera: Braconidae). Austral Entomol. 2017, 56, 261–267. [Google Scholar] [CrossRef]
- Pen, I.; Weissing, F.J. Optimal sex allocation: Steps towards a mechanistic theory. In Sex Ratios: Concepts and Research Methods; Hardy, I.C.W., Ed.; Cambridge University Press: Cambridge, UK, 2002; pp. 26–46. [Google Scholar] [CrossRef]
- King, B.H. Offspring sex ratios in parasitoid wasps. Q. Rev. Biol. 1987, 62, 367–396. [Google Scholar] [CrossRef]
- King, B.H. Sex ratio manipulation by parasitoid wasps. In Evolution and Diversity of Sex Ratio in Insects and Mites; Wrensch, D.L., Ebbert, M., Eds.; Chapman & Hall: New York, NY, USA, 1993; pp. 418–441. [Google Scholar]
- Ode, P.J.; Hardy, I.C.W. Parasitoid sex ratios and biological control. In Behavioral Ecology of Insect Parasitoids; Wajnberg, E., Bernstein, C., van Alphen, J., Eds.; Blackwell: Malden, MA, USA, 2008; pp. 253–291. [Google Scholar]
- Nadel, H.; Luck, R.F. Span of female emergence and male sperm depletion in the female-biased, quasi-gregarious parasitoid, Pachycrepoideus vindemiae (Hymenoptera: Pteromalidae). Ann. Entomol. Soc. Am. 1985, 78, 410–414. [Google Scholar] [CrossRef]
- Pandey, S.; Singh, R. Effect of parental age at coition on reproduction of Lysiphlebia mirzai (Hymenoptera: Braconidae). Entomol. Gen. 1998, 23, 187–193. [Google Scholar] [CrossRef]
- Suzuki, Y.; Hiehata, K. Mating systems and sex ratios in the egg parasitoids, Trichogramma dendrolimi and T. papilionis (Hymenoptera: Trichogrammatidae). Anim. Behav. 1985, 33, 1223–1227. [Google Scholar] [CrossRef]
- Bressac, C.; Khanh, H.D.T.; Chevrier, C. Effects of age and repeated mating on male sperm supply and paternity in a parasitoid wasp. Entomol. Exp. Appl. 2009, 130, 207–213. [Google Scholar] [CrossRef]
- El-Sabrout, A.M.; Hegazi, E.; Khafagi, W.; Bressac, C. Sperm production ss reduced after a heatwave at the pupal stage in the males of the parasitoid wasp Microplitis rufiventris Kok (Hymenoptera; Braconidae). Insects 2021, 12, 862. [Google Scholar] [CrossRef]
- Hernandez, E.; Rivera, P.; Aceituno-Medina, M.; Orozco-Davila, D. Yeast efficacy for mass rearing of Anastrepha ludens, A. obliqua and Ceratitis capitata (Diptera: Tephritidae). Acta Zool. Mex. 2016, 32, 240–252. [Google Scholar] [CrossRef][Green Version]
- Harvey, J.A.; Poelman, E.H.; Tanaka, T. Intrinsic inter- and intraspecific competition in parasitoid wasps. Annu. Rev. Entomol. 2013, 58, 333–351. [Google Scholar] [CrossRef]
- Cusumano, A.; Peri, E.; Boivin, G.; Colazza, S. Fitness costs of intrinsic competition in two egg parasitoids of a true bug. J. Insect Physiol. 2015, 81, 52–59. [Google Scholar] [CrossRef]
- Akman-Gündüz, E.; Gülel, A. Investigation of fecundity and sex ratio in the parasitoid Bracon hebetor Say (Hymenoptera: Braconidae) in relation to parasitoid age. Turk. J. Zool. 2005, 29, 291–294. Available online: https://journals.tubitak.gov.tr/zoology/vol29/iss4/1 (accessed on 27 August 2025).
- Uçkan, F.; Gülel, A. Age-related fecundity and sex ratio variation in Apanteles galleriae (Hym., Braconidae) and host effect on fecundity and sex ratio of its hyperparasitoid Dibrachys boarmiae (Hym., Pteromalidae). J. Appl. Entomol. 2002, 126, 534–537. [Google Scholar] [CrossRef]
| Parasitoid Female Age (Days) | Parameters * | ||
|---|---|---|---|
| Dead Parasitized Hosts | Scars on Puparia | Parasitoid First Instars | |
| 5 | 3.0 ± 0.5 a | 13.6 ± 1.1 a | 5.4 ± 0.6 a |
| 6 | 3.2 ± 0.5 a | 8.8 ± 0.8 b | 2.7 ± 0.3 b |
| 7 | 3.8 ± 0.5 a | 9.6 ± 1.1 ab | 2.9 ± 0.3 b |
| 8 | 1.7 ± 0.4 b | 5.8 ± 0.4 bc | 2.3 ± 0.3 bc |
| 9 | 1.7 ± 0.3 b | 4.0 ± 0.8 c | 1.1 ± 0.3 c |
| 10 | 1.5 ± 0.3 b | 3.7 ± 0.6 c | 1.0 ± 0.2 c |
| Statistical parameters | F9,20 = 18.97, p < 0.0001 | F9,20 = 21.91, p < 0.0001 | F9,20 = 18.18, p < 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cancino, J.; Ayala, A.; Flores-Sarmiento, E.; Moreno, F.d.M.; Suárez, L.d.C.; Ovruski, S.M.; Montoya, P. Egg Allocation on Anastrepha ludens Larvae by Mass-Reared Diachasmimorpha longicaudata Females. Insects 2025, 16, 926. https://doi.org/10.3390/insects16090926
Cancino J, Ayala A, Flores-Sarmiento E, Moreno FdM, Suárez LdC, Ovruski SM, Montoya P. Egg Allocation on Anastrepha ludens Larvae by Mass-Reared Diachasmimorpha longicaudata Females. Insects. 2025; 16(9):926. https://doi.org/10.3390/insects16090926
Chicago/Turabian StyleCancino, Jorge, Amanda Ayala, Erick Flores-Sarmiento, Flor de María Moreno, Lorena del Carmen Suárez, Sergio Marcelo Ovruski, and Pablo Montoya. 2025. "Egg Allocation on Anastrepha ludens Larvae by Mass-Reared Diachasmimorpha longicaudata Females" Insects 16, no. 9: 926. https://doi.org/10.3390/insects16090926
APA StyleCancino, J., Ayala, A., Flores-Sarmiento, E., Moreno, F. d. M., Suárez, L. d. C., Ovruski, S. M., & Montoya, P. (2025). Egg Allocation on Anastrepha ludens Larvae by Mass-Reared Diachasmimorpha longicaudata Females. Insects, 16(9), 926. https://doi.org/10.3390/insects16090926






