The Effects of Cold Acclimation on Cold Tolerance and Growth and Reproduction of Plodia interpunctella
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
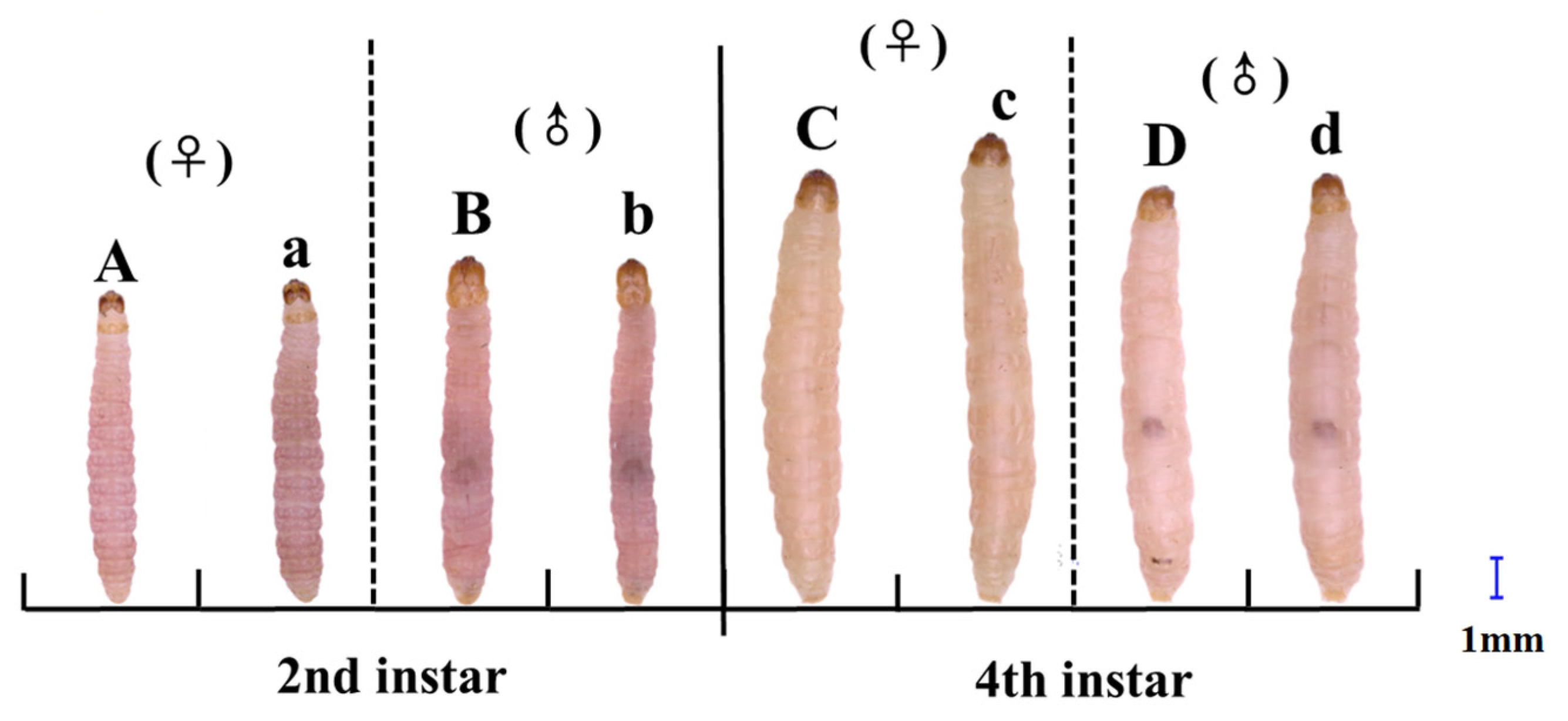
2.2. Determination of Larval Body Coloration After Cold Acclimation
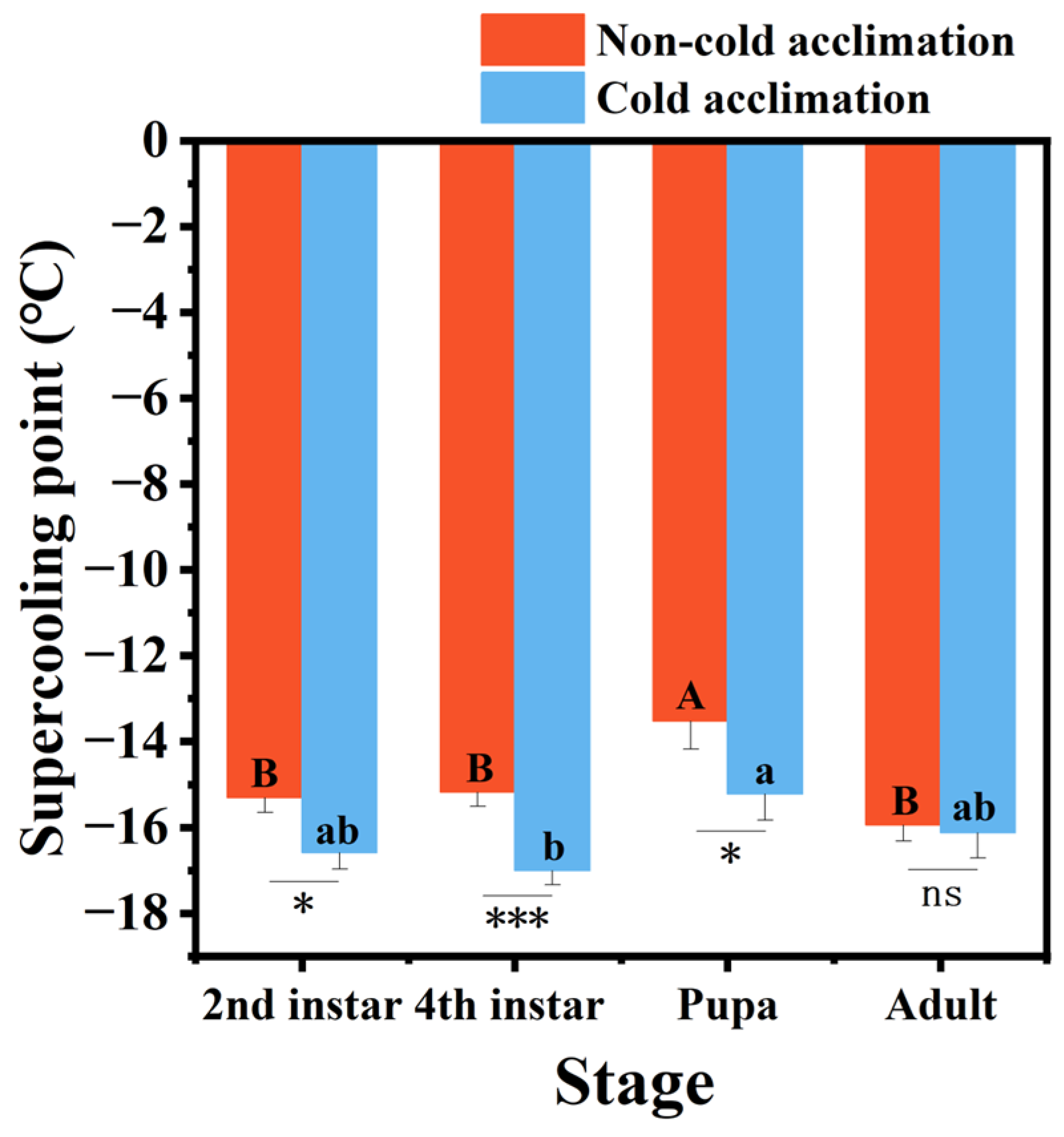
2.3. Measurement of Supercooling Point of Cold Acclimated P. interpunctella
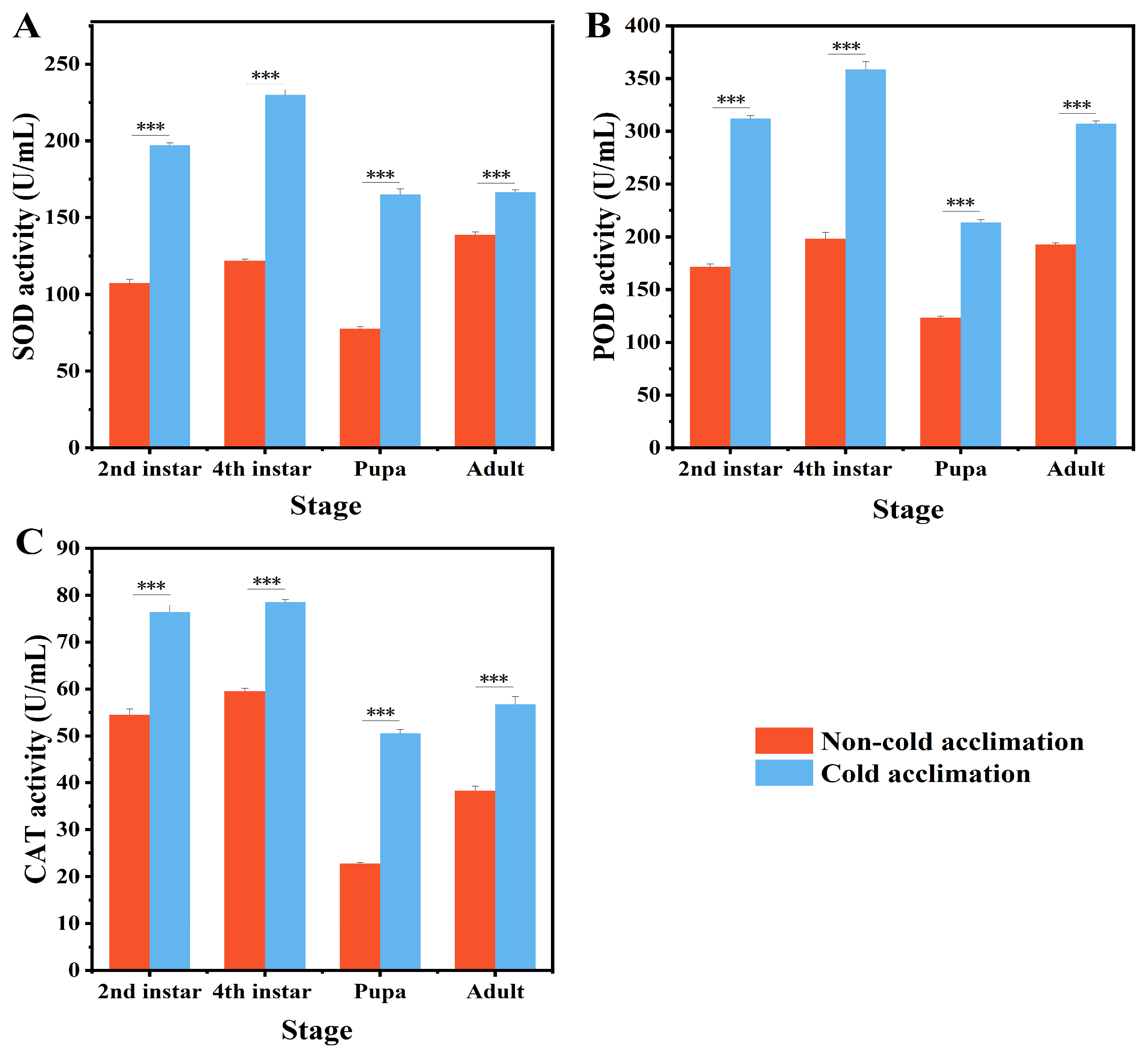
2.4. Determination of Antioxidant Enzymes Activity of Cold Acclimated P. interpunctella
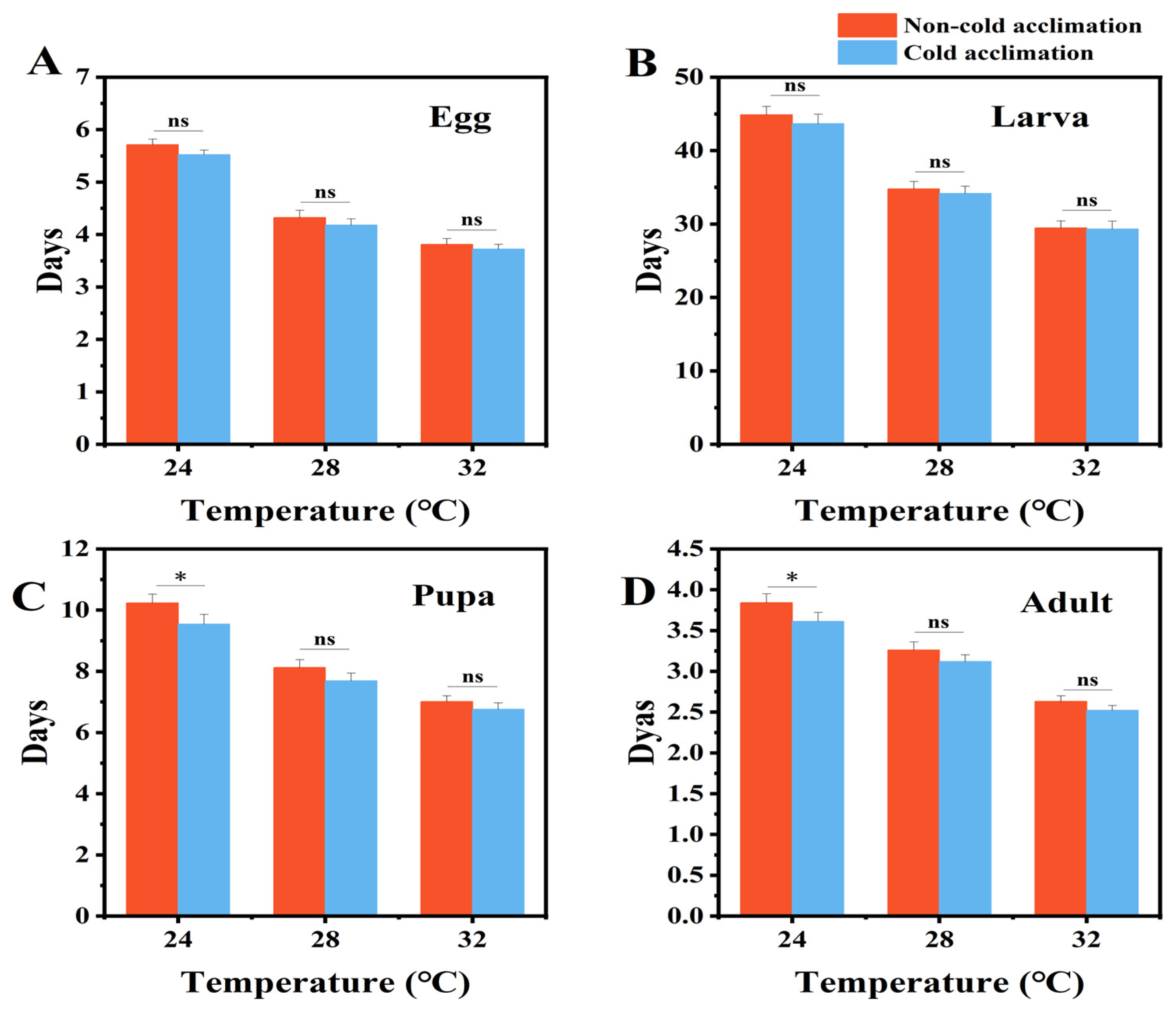
2.5. Development, Lifespan and Fecundity of Acclimated P. interpunctella Depending on Temperature
2.6. Data Analysis
3. Results
3.1. Effects of Cold Acclimation on Body Coloration
3.2. Effects of Cold Acclimation on Supercooling Point
3.3. Effects of Cold Acclimation on Antioxidant Enzyme Activities
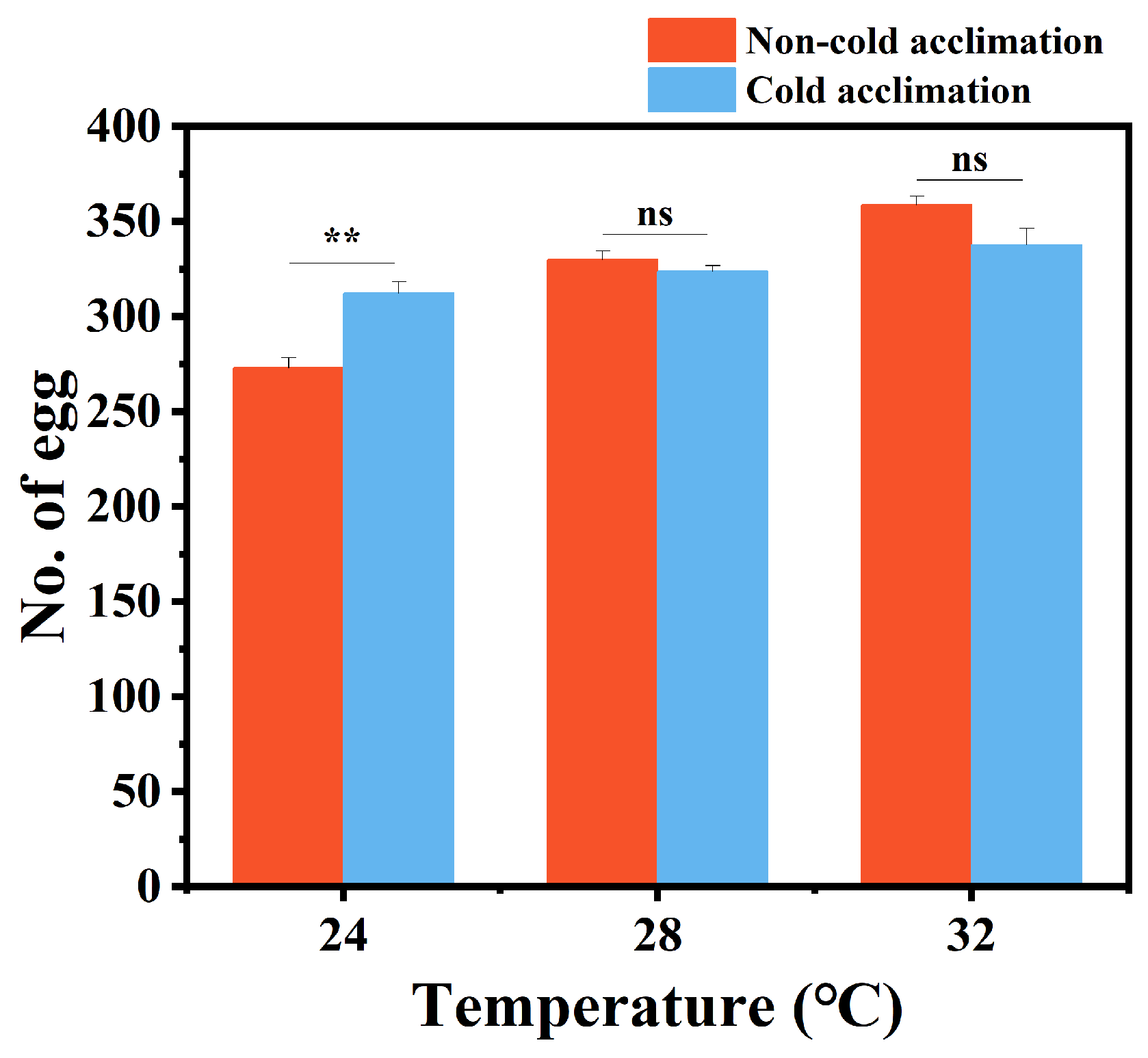
3.4. Effects of Cold Acclimation on Development, Lifespan and Reproduction
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guru, P.N.; Mridula, D.; Dukare, A.S.; Ghodki, B.M.; Paschapur, A.U.; Samal, I.; Nikhil, R.M.; Padala, V.K.; Rajashekhar, M.; Subbanna, A.R.N.S. A comprehensive review on advances in storage pest management: Current scenario and future prospects. Front. Sustain. Food Syst. 2022, 6, 993341. [Google Scholar] [CrossRef]
- Kumar, D.; Kalita, P. Reducing postharvest losses during storage of grain crops to strengthen food security in developing countries. Foods 2017, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Siddiqui, Y.; Siddique, K.H.M.; Bobo, J.A.; Ali, A. Minimizing postharvest food losses: A vital strategy to alleviate food insecurity and malnutrition in developing nations: A review. Discov. Food 2024, 4, 145. [Google Scholar] [CrossRef]
- Utono, I.M. Assessment of grain loss due to insect pest during storage for small-scale farmers of Kebbi. IOSR J. Agric. Vet. Sci. 2013, 5, 38–50. [Google Scholar] [CrossRef]
- FAO. Global Food Losses and Food Waste: Extent, Causes and Prevention; FAO: Rome, Italy, 2011; pp. 4–10. [Google Scholar]
- Luo, Y.; Huang, D.; Li, D.Y.; Wu, L.P. On-farm storage, storage losses, and the effects of loss reduction in China. Resour. Conserv. Recycl. 2020, 162, 105062. [Google Scholar] [CrossRef]
- Gamage, A.; Gangahagedara, R.; Gamage, J.; Jayasinghe, N.S.; Kodikara, N.; Suraweera, P.; Merah, O. Role of organic farming for achieving sustainability in agriculture. Farm. Syst. 2023, 1, 100005. [Google Scholar] [CrossRef]
- Hasan, M.M.; Athanassiou, C.G.; Hossain, M.A. Estimating long-term spatial distribution of Plodia interpunctella in various food facilities in Rajshahi Municipality, Bangladesh, through pheromone-baited traps. Sci. Rep. 2022, 12, 15986. [Google Scholar] [CrossRef]
- Liu, J.Y.; Yu, Z.E.; He, X.Z.; Zhou, G.X.; Guo, M.B.; Deng, J.Y. Attraction of the Indian meal moth Plodia interpunctella (Lepidoptera: Pyralidae) to commercially available vegetable oils: Implications in integrated pest management. Agriculture 2024, 14, 1526. [Google Scholar] [CrossRef]
- Ndomo-Moualeu, A.F.; Ulrichs, C.; Radek, R.; Adler, C. Structure and distribution of antennal sensilla in the Indian meal moth, Plodia interpunctella (Hübner, 1813) (Lepidoptera: Pyralidae). J. Stored Prod. Res. 2014, 59, 66–75. [Google Scholar] [CrossRef]
- Mohandass, S.; Arthur, F.H.; Zhu, K.Y.; Throne, J.E. Biology and management of Plodia interpunctella (Lepidoptera: Pyralidae) in stored products. J. Stored Prod. Res. 2007, 43, 302–311. [Google Scholar] [CrossRef]
- Agrafioti, P.; Kaloudis, E.; Kateris, D.; Athanassiou, C.G. Assessment of phosphine resistance in major stored-product insects in greece using two diagnostic protocols. Insects 2024, 15, 802. [Google Scholar] [CrossRef] [PubMed]
- Fields, P.G.; White, N.D.G. Alternatives to methyl bromide treatments for stored-product and quarantine insects. Annu. Rev. Entomol. 2002, 47, 331–359. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.C.; Ge, L.Q.; Liu, F.; Song, Q.S.; Stanley, D. Pesticide-induced planthopper population resurgence in rice cropping systems. Annu. Rev. Entomol. 2020, 65, 409–429. [Google Scholar] [CrossRef]
- Romero, A.; Potter, M.F.; Potter, D.A.; Haynes, K.F. Insecticide resistance in the bed bug: A factor in the pest’s sudden resurgence? J. Med. Entomol. 2007, 44, 175–178. [Google Scholar] [PubMed]
- Li, Y.B. Effects of long-term low oxygen storage treatment on survival of rice weevil (Sitophilus oryzae) and confused flour beetle (Tribolium confusum). J. Econ. Entomol. 2022, 115, 1712–1718. [Google Scholar] [CrossRef]
- Ziegler, V.; Paraginski, R.T.; Ferreira, C.D. Grain storage systems and effects of moisture, temperature and time on grain quality—A review. J. Stored Prod. Res. 2021, 91, 101770. [Google Scholar] [CrossRef]
- Ahmadi, F.; Moharramipour, S.; Mikani, A. The effect of temperature and photoperiod on diapause induction in pupae of Scrobipalpa ocellatella (Lepidoptera: Gelechiidae). Environ. Entomol. 2018, 47, 1314–1322. [Google Scholar] [CrossRef]
- Stejskal, V.; Vendl, T.; Li, Z.H.; Aulicky, R. Minimal thermal requirements for development and activity of stored product and food industry pests (Acari, Coleoptera, Lepidoptera, Psocoptera, Diptera and Blattodea): A review. Insects 2019, 10, 149. [Google Scholar] [CrossRef]
- Denlinger, D.L. Insect diapause: From a rich history to an exciting future. J. Exp. Biol. 2023, 226, 245329. [Google Scholar] [CrossRef]
- Bale, J.S.; Masters, G.J.; Hodkinson, I.D.; Awmack, C.; Bezemer, T.M.; Brown, V.K.; Butterfield, J.; Buse, A.; Coulson, J.C.; Farrar, J.; et al. Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Glob. Change Biol. 2002, 8, 1–16. [Google Scholar] [CrossRef]
- Gu, S.H. Temporal changes in PTTH/Egf signaling and ERK target gene expressions during chilling in Bombyx mori eggs. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2025, 307, 111884. [Google Scholar] [CrossRef]
- Denlinger, D.L. Regulation of diapause. Annu. Rev. Entomol. 2002, 47, 93–122. [Google Scholar] [CrossRef]
- Boardman, L.; Sørensen, J.G.; Johnson, S.A.; Terblanche, J.S. Interactions between controlled atmospheres and low temperature tolerance: A review of biochemical mechanisms. Front. Physiol. 2011, 2, 92. [Google Scholar] [CrossRef]
- Scaccini, D.; Vanishvili, L.; Tirello, P.; Walton, V.M.; Duso, C.; Pozzebon, A. Lethal and sub-lethal effects of low-temperature exposures on Halyomorpha halys (Hemiptera: Pentatomidae) adults before and after overwintering. Sci. Rep. 2020, 10, 15231. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Y.; Gu, X.P.; Peng, X.Q.; Tao, M.; Peng, L.; Chen, G.H.; Zhang, X.M. Effect of short-term low temperature on the growth, development, and reproduction of Bactrocera tau (Diptera: Tephritidae) and Bactrocera cucurbitae. J. Econ. Entomol. 2020, 113, 2141–2149. [Google Scholar] [CrossRef]
- Teets, N.M.; Denlinger, D.L. Physiological mechanisms of seasonal and rapid cold-hardening in insects. Physiol. Entomol. 2013, 38, 105–116. [Google Scholar] [CrossRef]
- Rajamohan, A.; Sinclair, B.J. Short-term hardening effects on survival of acute and chronic cold exposure by Drosophila melanogaster larvae. J. Insect Physiol. 2008, 54, 708–718. [Google Scholar] [CrossRef]
- Findsen, A.; Lembcke, J.A.; Calderon, S.; Overgaard, J. Rapid cold hardening improves recovery of ion homeostasis and chill coma recovery time in the migratory locust, Locusta migratoria. J. Exp. Biol. 2013, 216, 1630–1637. [Google Scholar]
- Teets, N.M.; Elnitsky, M.A.; Benoit, J.B.; Lee, R.E.; Denlinger, D.L. Rapid cold-hardening in larvae of the Antarctic midge Belgica antarctica: Cellular cold-sensing and a role for calcium. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R1938–R1946. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.Y.; Dai, L.L.; Ning, H.; Kang, X.T.; Sun, Y.Y.; Chen, H. Effects of cold stress on metabolic regulation in the overwintering larvae of the Chinese white pine beetle, Dendroctonus armandi. J. Insect Physiol. 2025, 168, 836–850. [Google Scholar] [CrossRef]
- Lopez-Martinez, G.; Elnitsky, M.A.; Benoit, J.B.; Lee, R.E.; Denlinger, D.L. High resistance to oxidative damage in the Antarctic midge Belgica antarctica, and developmentally linked expression of genes encoding superoxide dismutase, catalase and heat shock proteins. Insect Biochem. Mol. Biol. 2008, 38, 796–804. [Google Scholar] [CrossRef]
- Jia, F.X.; Dou, W.; Hu, F.; Wang, J.J. Effects of thermal stress on lipid peroxidation and antioxidant enzyme activities of oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae). Florida Entomol. 2011, 94, 956–963. [Google Scholar] [CrossRef]
- Yuan, J.W.; Zheng, Y.T.; Zhang, Y.W.; Bai, J.; Qin, J.; Du, Y.Z. Differential regulation of antioxidant enzymes in Frankliniella occidentalis (Thysanoptera: Thripidae) exposed to thermal stress. PeerJ 2021, 9, e12089. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; He, S.Q.; Liu, X.W.; Huang, Z.J.; Chen, F.J.; Gui, F.R. Effect of elevated CO2 on the interaction between invasive thrips, Frankliniella occidentalis, and its host kidney bean, Phaseolus vulgaris. Pest Manag. Sci. 2018, 74, 2717–2724. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.H.; Huang, H.; Wang, J.J. Antioxidant responses of citrus red mite, Panonychus citri (McGregor) (Acari: Tetranychidae), exposed to thermal stress. J. Insect Physiol. 2010, 56, 1871–1876. [Google Scholar] [CrossRef]
- Farahani, S.; Bandani, A.R.; Alizadeh, H.; Goldansaz, S.H.; Whyard, S. Differential expression of heat shock proteins and antioxidant enzymes in response to temperature, starvation, and parasitism in the carob moth larvae, Ectomyelois ceratoniae (Lepidoptera: Pyralidae). PLoS ONE 2020, 15, e0228104. [Google Scholar] [CrossRef]
- Storey, K.B.; Storey, J.M. Insect cold hardiness: Metabolic, gene, and protein adaptation. Can. J. Zool. 2012, 90, 456–475. [Google Scholar] [CrossRef]
- Li, X.J.; Han, Z.Q.; Lin, Q.; Wu, Z.D.; Chen, L.; Zhang, Q. Smart cooling-aeration guided by aeration window model for paddy stored in concrete silos in a depot of Guangzhou, China. Comput. Electron. Agric. 2020, 173, 105452. [Google Scholar] [CrossRef]
- Morales-Quiros, A.; Campabadal, C.; Maier, D.E.; Lazzari, S.M.N.; Lazzari, F.A.; Phillips, T.W. Chilled aeration to control pests and maintain grain quality during summer storage of wheat in the North Central Region of Kansas. Appl. Eng. Agric. 2019, 35, 657–668. [Google Scholar] [CrossRef]
- Nath, B.B. Editorial: Physiological adaptations of insects exposed to different stress conditions, Volume II. Front. Physiol. 2025, 16, 1582575. [Google Scholar] [CrossRef]
- Teets, N.M.; Marshall, K.E.; Reynolds, J.A. Molecular mechanisms of winter survival. Annu. Rev. Entomol. 2023, 68, 319–339. [Google Scholar] [CrossRef] [PubMed]
- Adler, C. Low temperature to control Plodia interpunctella and Stegobium paniceum. Julius-Kühn-Archiv 2010, 425, 167–178. [Google Scholar]
- Sibilia, C.D.; Brosko, K.A.; Hickling, C.H.; Thompson, L.M.; Grayson, K.L.; Olson, J.R. Thermal physiology and developmental plasticity of pigmentation in the Harlequin Bug (Hemiptera: Pentatomidae). J. Insect Sci. 2018, 18, 4. [Google Scholar] [CrossRef]
- Aguilon, D.J.; Velasco, L.R. Effects of larval rearing temperature and host plant condition on the development, survival, and coloration of African armyworm, Spodoptera exempta Walker (Lepidoptera: Noctuidae). J. Environ. Sci. Manag. 2015, 18, 54–60. [Google Scholar] [CrossRef]
- Kutch, I.C.; Sevgili, H.; Wittman, T.; Fedorka, K.M. Thermoregulatory strategy may shape immune investment in Drosophila melanogaster. J. Exp. Biol. 2014, 217, 3664–3669. [Google Scholar] [CrossRef] [PubMed]
- Fedorka, K.M.; Lee, V.; Winterhalter, W.E. Thermal environment shapes cuticle melanism and melanin-based immunity in the ground cricket Allonemobius socius. Evol. Ecol. 2013, 27, 521–531. [Google Scholar] [CrossRef]
- Khabir, M.; Izadi, H.; Mahdian, K. The supercooling point depression is the leading cold tolerance strategy for the variegated ladybug, Hippodamia variegata (Goezel). Front. Physiol. 2023, 14, 1323701. [Google Scholar] [CrossRef]
- Ditrich, T. Supercooling point is an individually fixed metric of cold tolerance in Pyrrhocoris apterus. J. Therm. Biol. 2018, 74, 208–213. [Google Scholar] [CrossRef]
- Colinet, H.; Renault, D.; Hance, T.; Vernon, P. The impact of fluctuating thermal regimes on the survival of a cold-exposed parasitic wasp, Aphidius colemani. Physiol. Entomol. 2006, 31, 234–240. [Google Scholar] [CrossRef]
- Kučera, L.; Moos, M.; Štětina, T.; Korbelová, J.; Vodrážka, P.; Des Marteaux, L.; Grgac, R.; Hůla, P.; Rozsypal, J.; Faltus, M.; et al. A mixture of innate cryoprotectants is key for freeze tolerance and cryopreservation of a drosophilid fly larva. J. Exp. Biol. 2022, 225, jeb243934. [Google Scholar] [CrossRef]
- Jia, Q.Q.; Li, S. Mmp-induced fat body cell dissociation promotes pupal development and moderately averts pupal diapause by activating lipid metabolism. Proc. Natl. Acad. Sci. USA 2023, 120, e2215214120. [Google Scholar] [CrossRef]
- Weaving, H.; Terblanche, J.S.; Pottier, P.; English, S. Meta analysis reveals weak but pervasive plasticity in insect thermal limits. Nat. Commun. 2022, 13, 5292. [Google Scholar] [CrossRef]
- Carrillo, M.A.; Cannon, C.A. Supercooling point variability in the Indian meal moth, Plodia interpunctella (Hübner) (Lepidoptera: Pyralidae). J. Stored Prod. Res. 2005, 41, 556–564. [Google Scholar] [CrossRef]
- Hemmati, C.; Moharramipour, S.; Talebi, A.A. Effects of cold acclimation, cooling rate and heat stress on cold tolerance of the potato tuber moth Phthorimaea operculella (Lepidoptera: Gelechiidae). Eur. J. Entomol. 2014, 111, 487–494. [Google Scholar] [CrossRef]
- Mohammadzadeh, M.; Izadi, H. Cold acclimation of Trogoderma granarium Everts is tightly linked to regulation of enzyme activity, energy content, and ion concentration. Front. Physiol. 2018, 9, 1427. [Google Scholar] [CrossRef]
- Wen, X.; Wang, S.; Duman, J.G.; Arifin, J.F.; Juwita, V.; Goddard, W.A.; Rios, A.; Liu, F.; Kim, S.; Abrol, R.; et al. Antifreeze proteins govern the precipitation of trehalose in a freezing-avoiding insect at low temperature. Proc. Natl. Acad. Sci. USA 2016, 113, 6683–6688. [Google Scholar] [CrossRef] [PubMed]
- Zachariassen, K.E.; Kristiansen, E. Ice nucleation and antinucleation in nature. Cryobiology 2000, 41, 257–279. [Google Scholar] [CrossRef]
- Lubawy, J.; Chowański, S.; Adamski, Z.; Słocińska, M. Mitochondria as a target and central hub of energy division during cold stress in insects. Front. Zool. 2022, 19, 1. [Google Scholar] [CrossRef]
- Lalouette, L.; Williams, C.M.; Hervant, F.; Sinclair, B.J.; Renault, D. Metabolic rate and oxidative stress in insects exposed to low temperature thermal fluctuations. Comp Biochem. Physiol. A Mol. Integr. Physiol. 2011, 158, 229–234. [Google Scholar] [CrossRef] [PubMed]
- El-Saadi, M.I.; MacMillan, H.A.; Ferguson, L.V. Cold-induced immune activation in chill-susceptible insects. Curr. Opin. Insect Sci. 2023, 55, 100969. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Enriquez, T.; Colinet, H. Cold acclimation triggers major transcriptional changes in Drosophila suzukii. BMC Genom. 2019, 20, 413. [Google Scholar] [CrossRef]
- Khodayari, S.; Derocles, S.A.P.; Renault, D. Effects of duration and type of cold acclimation on the subsequent cold tolerance of a tenebrionid beetle. J. Therm. Biol. 2025, 129, 104100. [Google Scholar] [CrossRef]
- Shi, F.; Xing, Y.; Niu, Y.; Cheng, L.; Xu, Y.; Li, X.; Ren, L.; Zong, S.; Tao, J. Unveiling winter survival strategies: Physiological and metabolic responses to cold stress of Monochamus saltuarius larvae during overwintering. Pest Manag. Sci. 2024, 80, 5656–5671. [Google Scholar] [CrossRef]
- MacMillan, H.A.; Knee, J.M.; Dennis, A.B.; Udaka, H.; Marshall, K.E.; Merritt, T.J.S.; Sinclair, B.J. Cold acclimation wholly reorganizes the Drosophila melanogaster transcriptome and metabolome. Sci. Rep. 2016, 6, 28999. [Google Scholar] [CrossRef]
- Naeemullah, M.; Tanaka, K.; Tsumuki, H.; Takeda, M. Relationship of cold tolerance to developmental determination in the Indian meal moth, Plodia interpunctella (Lepidoptera: Phycitidae). Appl. Entomol. Zool. 1999, 34, 267–276. [Google Scholar] [CrossRef]
- Ayar, A.; Uysal, H.; Altun, D. The effects of cold shock on the longevity in Oregon R wild and vestigial mutant of Drosophila melanogaster (Diptera: Drosophilidae). Ekoloji 2010, 19, 38–44. [Google Scholar] [CrossRef]
- Winkler, A.; Jung, J.; Kleinhenz, B.; Racca, P. A review on temperature and humidity effects on Drosophila suzukii population dynamics. Agric. For. Entomol. 2020, 22, 179–192. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Z.; Zhang, H.; Lu, S.; Chen, M. The Effects of Cold Acclimation on Cold Tolerance and Growth and Reproduction of Plodia interpunctella. Insects 2025, 16, 927. https://doi.org/10.3390/insects16090927
Shi Z, Zhang H, Lu S, Chen M. The Effects of Cold Acclimation on Cold Tolerance and Growth and Reproduction of Plodia interpunctella. Insects. 2025; 16(9):927. https://doi.org/10.3390/insects16090927
Chicago/Turabian StyleShi, Zhuoke, Huiyuan Zhang, Shaohua Lu, and Mingshun Chen. 2025. "The Effects of Cold Acclimation on Cold Tolerance and Growth and Reproduction of Plodia interpunctella" Insects 16, no. 9: 927. https://doi.org/10.3390/insects16090927
APA StyleShi, Z., Zhang, H., Lu, S., & Chen, M. (2025). The Effects of Cold Acclimation on Cold Tolerance and Growth and Reproduction of Plodia interpunctella. Insects, 16(9), 927. https://doi.org/10.3390/insects16090927






