Warmer Temperature Accelerates the Aging-Dependent Decrease in Female Ovary Size, Delays Male Accessory Gland Development, and Accelerates Aging-Dependent Changes in Reproductive Gene Expression in Anopheles gambiae Mosquitoes
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
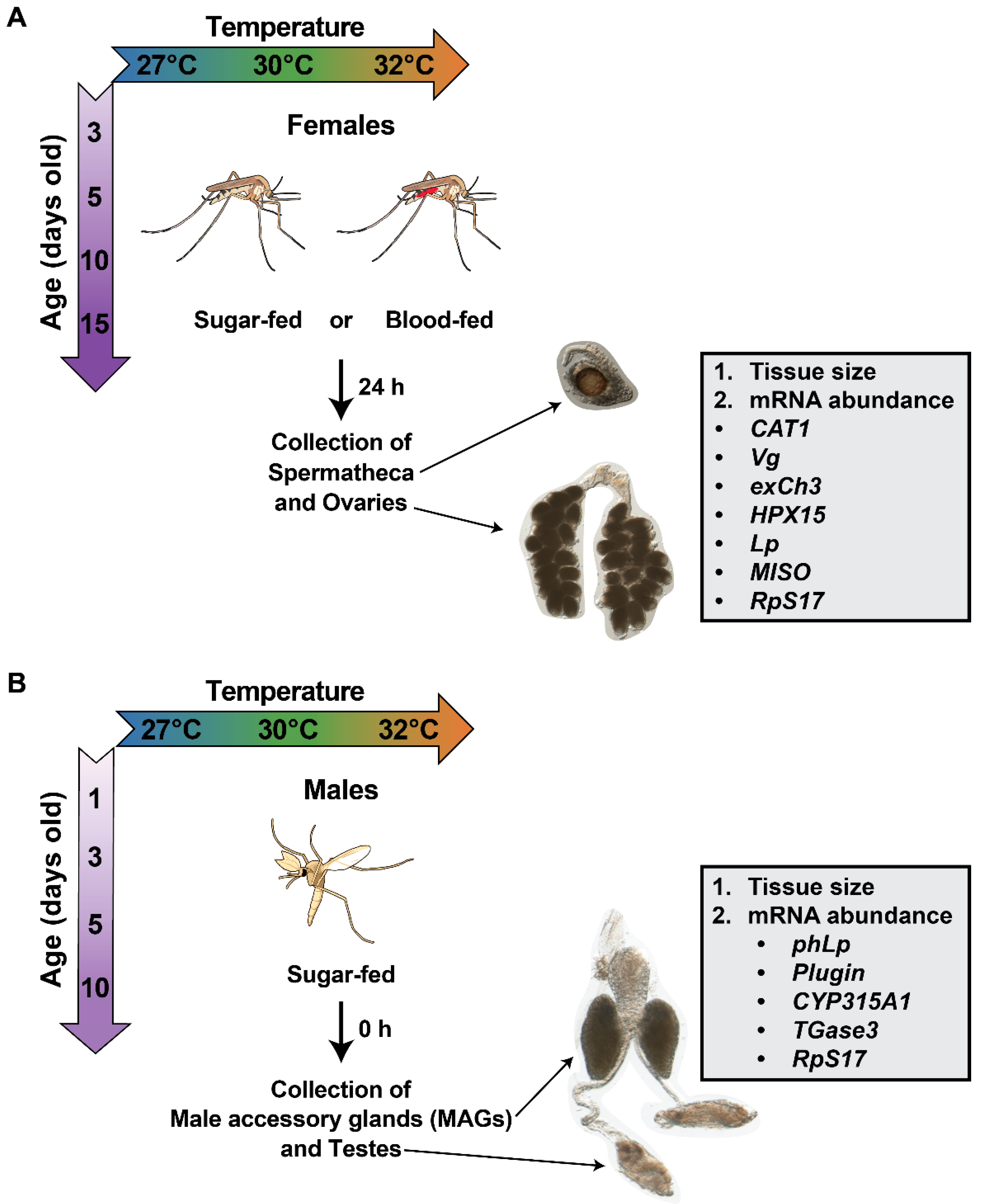
2.1. Mosquito Rearing, Feeding, and Dissection
2.2. Measuring Reproductive Tissue Size
2.3. Measuring Reproductive Tissue mRNA Abundance by Quantitative Real-Time PCR
3. Results
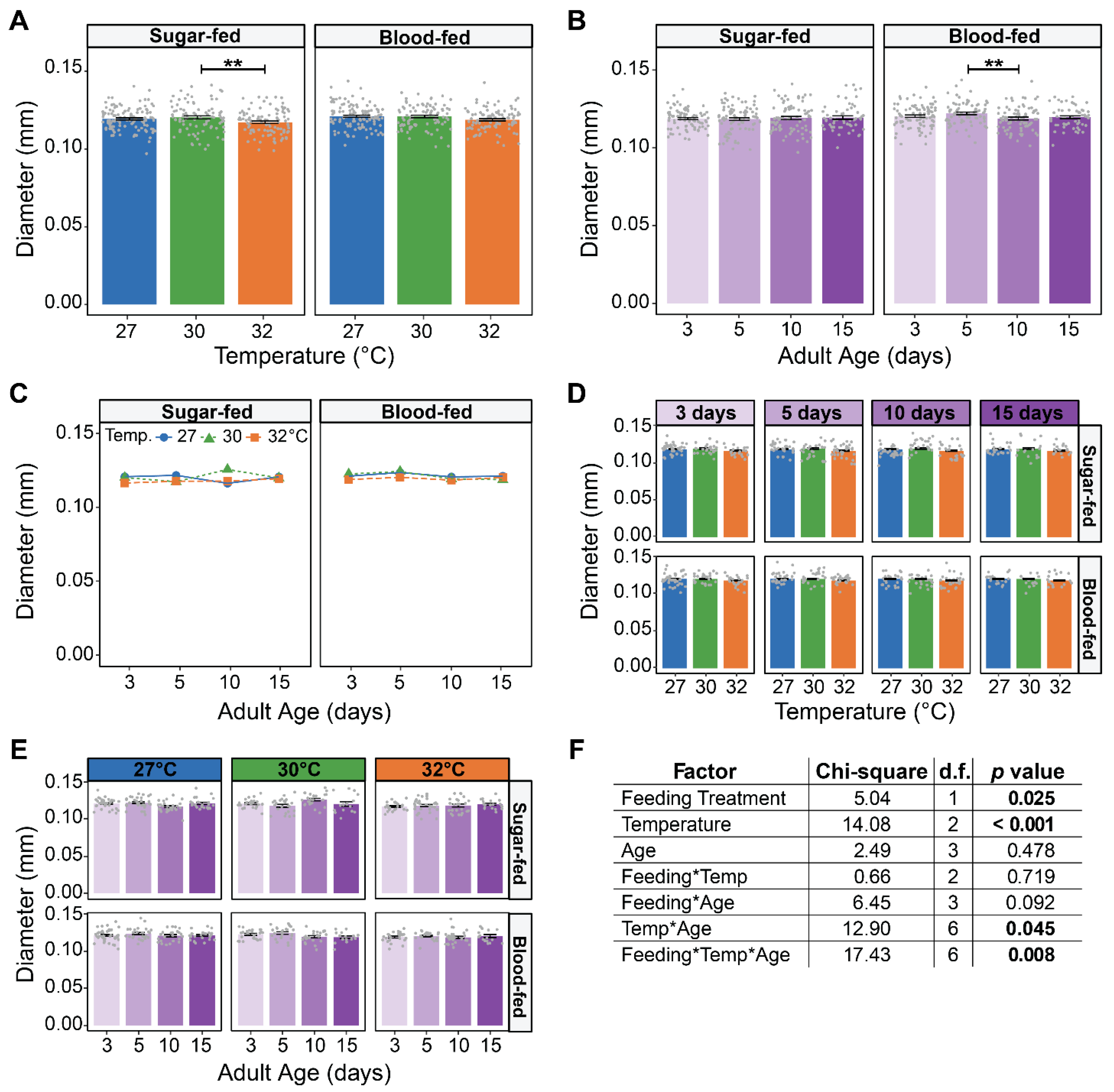
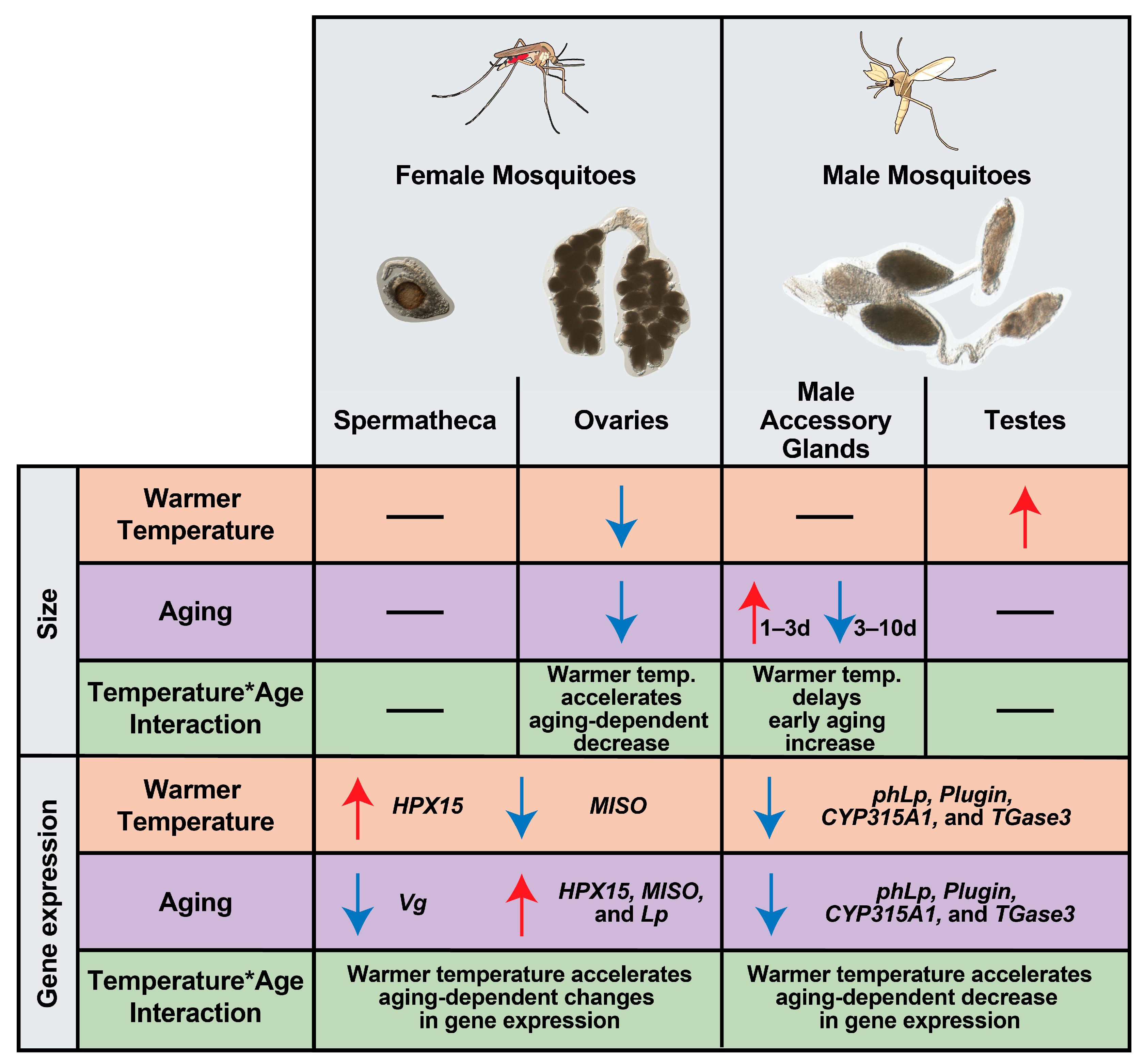
3.1. Neither Warmer Temperature, Aging, nor Feeding Affect the Size of a Female’s Spermatheca
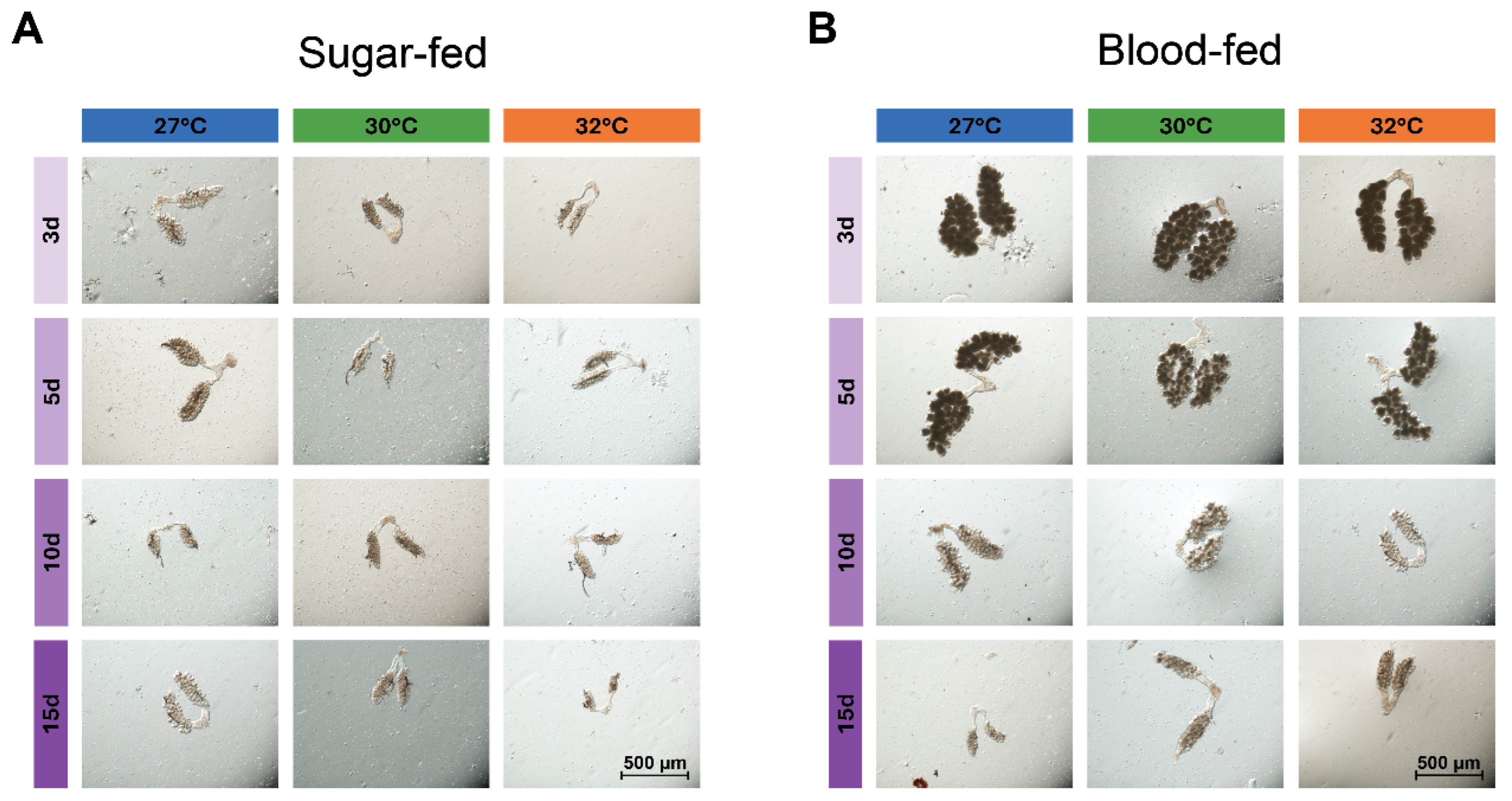
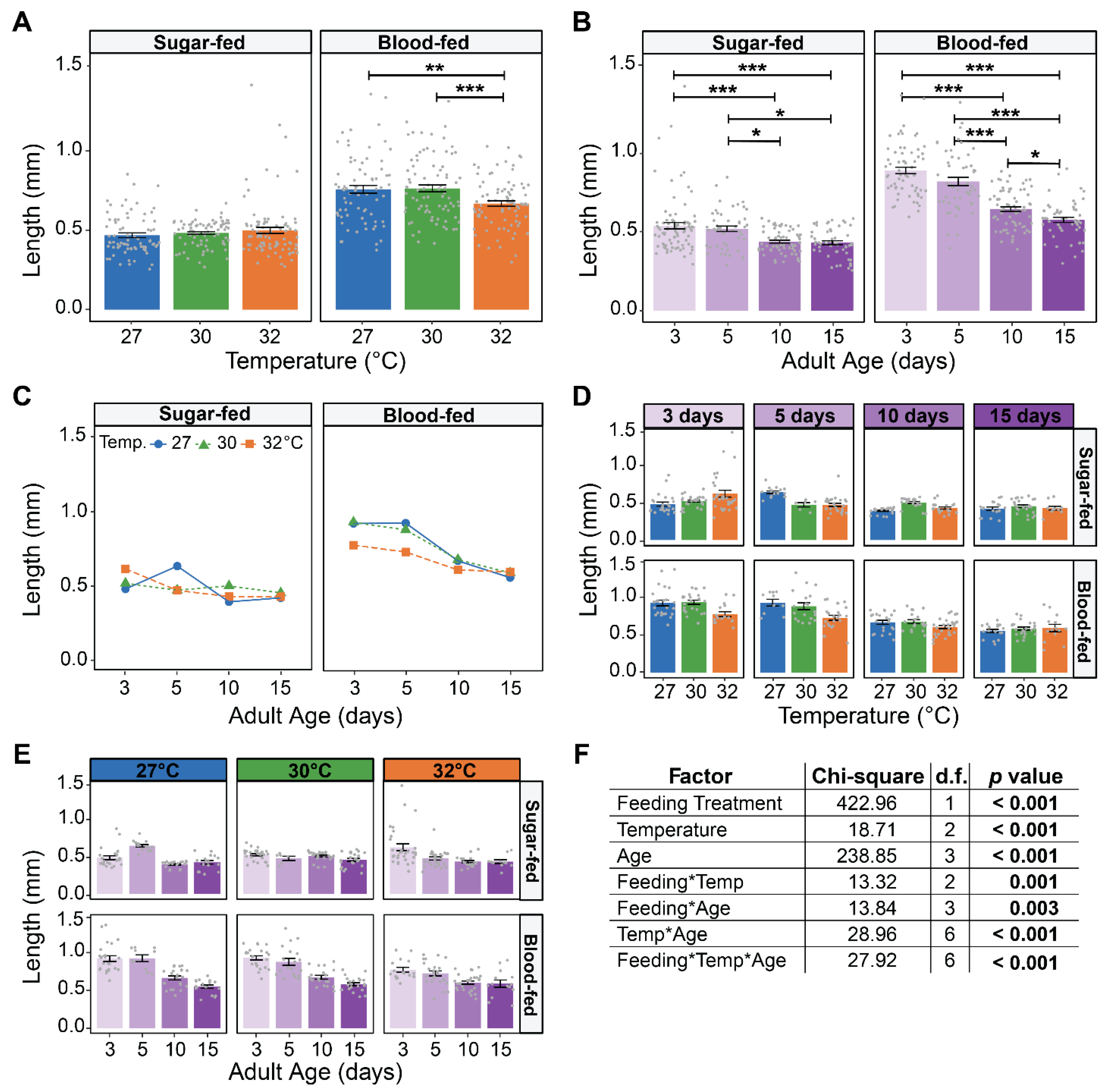
3.2. Warmer Temperature Accelerates the Aging-Dependent Decrease in the Size of the Ovaries of Blood-Fed Females
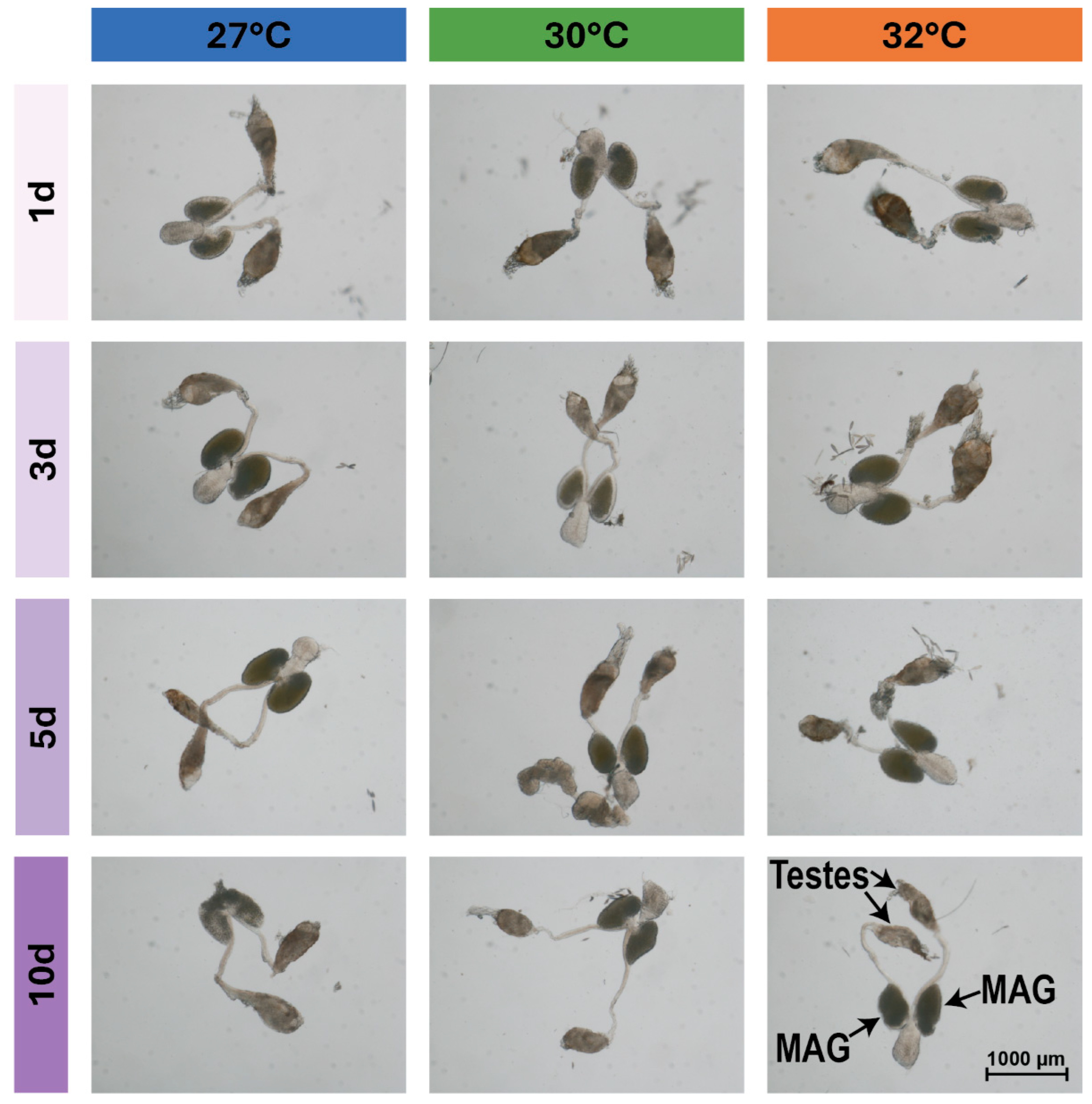
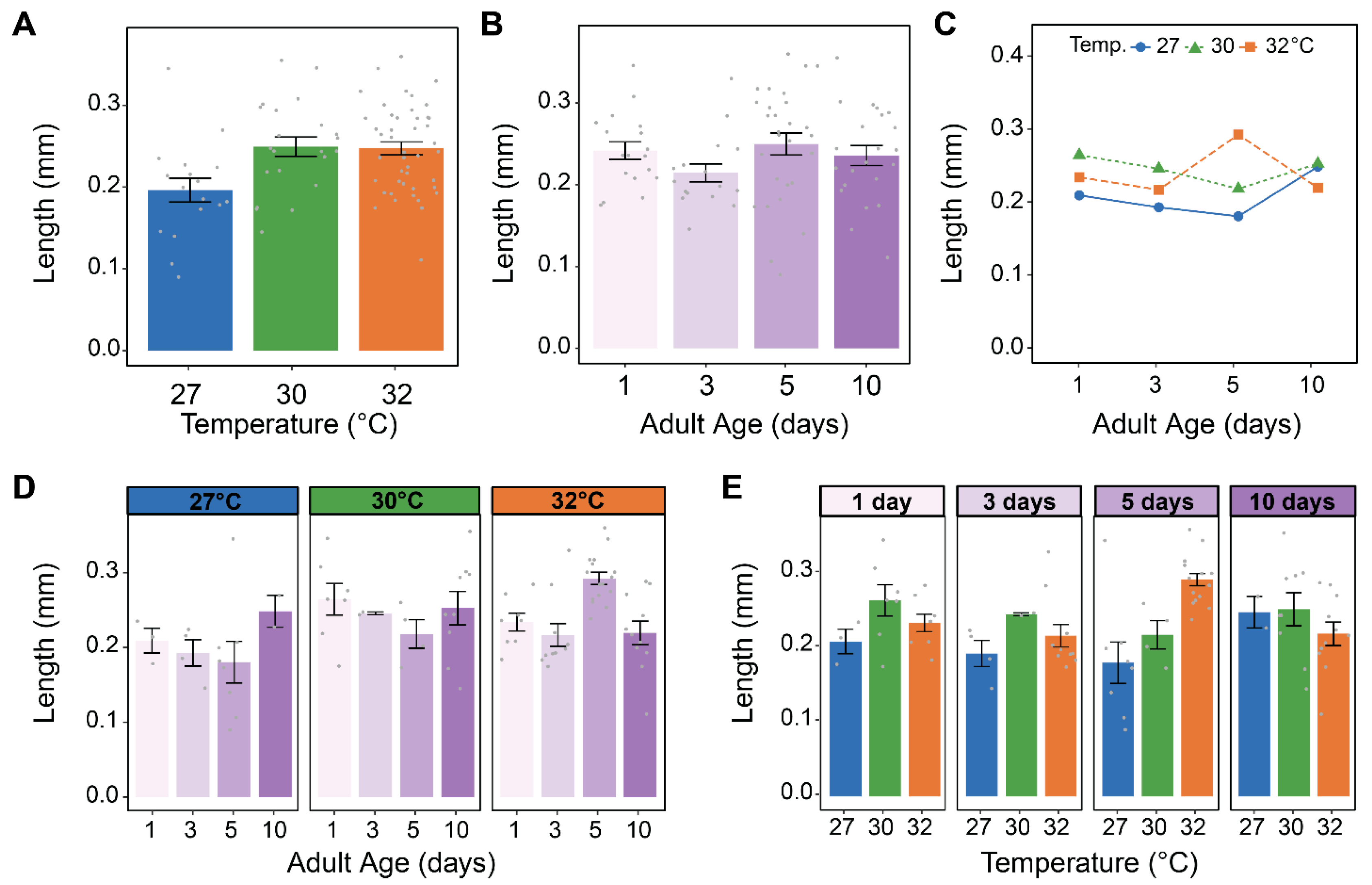
3.3. Warmer Temperature Lessens and Delays the Increase in the Size of Male Accessory Glands That Occurs Early in Life
3.4. Warmer Temperature Increases the Size of the Testes
3.5. In Female Reproductive Tissues, Warmer Temperature Accelerates an Aging-Dependent Decrease in Vg Expression but an Increase in MISO and HPX15 Expression
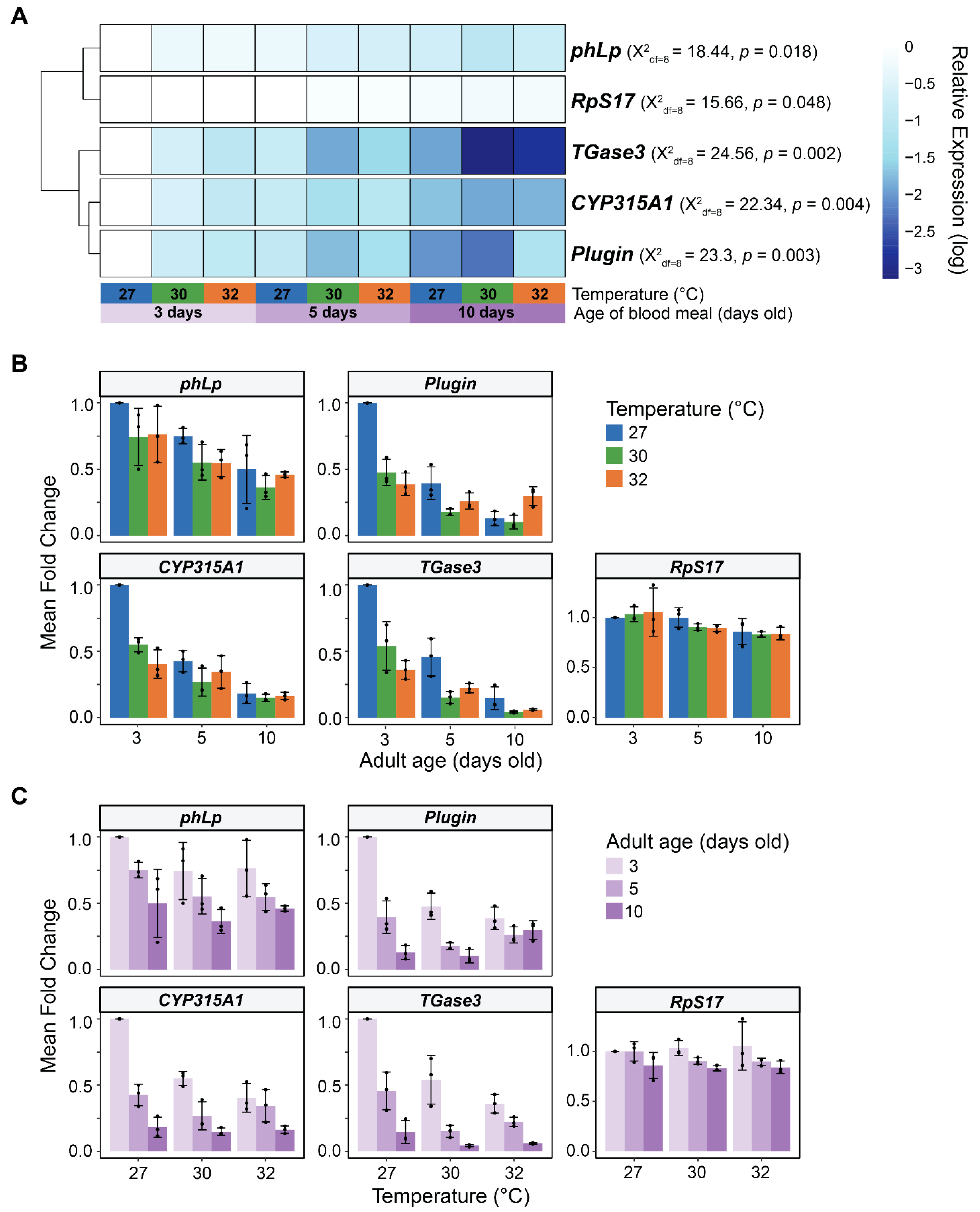
3.6. In Male Reproductive Tissues, Warmer Temperature Accelerates the Aging-Dependent Decrease in the Expression of Plugin, TGase3, phLP, and CYP315A1
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MAGs | Male Accessory Glands |
| Temp | Temperature |
| d.f. | Degrees of freedom |
| 20E | 20-hydroxyecdysone |
| JH | Juvenile hormone |
References
- Attardo, G.M.; Hansen, I.A.; Raikhel, A.S. Nutritional regulation of vitellogenesis in mosquitoes: Implications for anautogeny. Insect Biochem. Mol. Biol. 2005, 35, 661–675. [Google Scholar] [CrossRef]
- Raikhel, A.S. The cell biology of mosquito vitellogenesis. Memórias Inst. Oswaldo Cruz 1987, 82 (Suppl. S3), 93–101. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Yang, L.; He, Q.; Zhou, S. Regulatory mechanisms of vitellogenesis in insects. Front. Cell Dev. Biol. 2021, 8, 593613. [Google Scholar] [CrossRef]
- Clements, A.N.; Boocock, M.R. Ovarian development in mosquitoes: Stages of growth and arrest, and follicular resorption. Physiol. Entomol. 1984, 9, 1–8. [Google Scholar] [CrossRef]
- Roy, S.; Smykal, V.; Johnson, L.; Saha, T.T.; Zou, Z.; Raikhel, A.S. Regulation of reproductive processes in female mosquitoes. In Advances in Insect Physiology; Raikhel, A.S., Ed.; Academic Press: Cambridge, MA, USA, 2016; Volume 51, pp. 115–144. [Google Scholar]
- Clifton, M.E.; Noriega, F.G. The fate of follicles after a blood meal is dependent on previtellogenic nutrition and juvenile hormone in Aedes aegypti. J. Insect Physiol. 2012, 58, 1007–1019. [Google Scholar] [CrossRef]
- Hernández-Martínez, S.; Cardoso-Jaime, V.; Nouzova, M.; Michalkova, V.; Ramirez, C.E.; Fernandez-Lima, F.; Noriega, F.G. Juvenile hormone controls ovarian development in female Anopheles albimanus mosquitoes. Sci. Rep. 2019, 9, 2127. [Google Scholar] [CrossRef]
- Ekoka, E.; Maharaj, S.; Nardini, L.; Dahan-Moss, Y.; Koekemoer, L.L. 20-Hydroxyecdysone (20E) signaling as a promising target for the chemical control of malaria vectors. Parasites Vectors 2021, 14, 86. [Google Scholar] [CrossRef]
- Hansen, I.A.; Attardo, G.M.; Rodriguez, S.D.; Drake, L.L. Four-way regulation of mosquito yolk protein precursor genes by juvenile hormone-, ecdysone-, nutrient-, and insulin-like peptide signaling pathways. Front. Physiol. 2014, 5, 103. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.B.; Wheelock, G.D.; Hagedorn, H.H.; Baker, F.C.; Tsai, L.W.; Schooley, D.A. Juvenile hormone and juvenile hormone esterase in adult females of the mosquito Aedes aegypti. J. Insect Physiol. 1986, 32, 867–877. [Google Scholar] [CrossRef]
- Shapiro, J.P.; Hagedorn, H.H. Juvenile hormone and the development of ovarian responsiveness to a brain hormone in the mosquito, Aedes aegypti. Gen. Comp. Endocrinol. 1982, 46, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Saha, T.T.; Roy, S.; Shin, S.W.; Backman, T.W.H.; Girke, T.; White, K.P.; Raikhel, A.S. Juvenile hormone and its receptor, methoprene-tolerant, control the dynamics of mosquito gene expression. Proc. Natl. Acad. Sci. USA 2013, 110, E2173–E2181. [Google Scholar] [CrossRef] [PubMed]
- Klowden, M.J. Endocrine aspects of mosquito reproduction. Arch. Insect Biochem. Physiol. 1997, 35, 491–512. [Google Scholar] [CrossRef]
- Raikhel, A.; Brown, M.; Belles, X. Hormonal control of reproductive processes. In Reproductive Biology of Invertebrates, Vol. 12, Part B: Progress in Vitellogenesis; Raikhel, A.S., Sappington, T.W., Eds.; CRC Press: Boca Raton, FL, USA, 2005; Volume 3, pp. 433–491. [Google Scholar]
- Santos, C.G.; Humann, F.C.; Hartfelder, K. Juvenile hormone signaling in insect oogenesis. Curr. Opin. Insect Sci. 2019, 31, 43–48. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, S.S.; Rodovalho, C.M.; Gaviraghi, A.; Mota, M.B.S.; Jablonka, W.; Rocha-Santos, C.; Nunes, R.D.; Sá-Guimarães, T.D.E.; Oliveira, D.S.; Melo, A.C.A.; et al. Aedes aegypti post-emergence transcriptome: Unveiling the molecular basis for the hematophagic and gonotrophic capacitation. PLoS Neglected Trop. Dis. 2021, 15, e0008915. [Google Scholar] [CrossRef]
- Zhu, J.; Noriega, F.G. The role of juvenile hormone in mosquito development and reproduction. In Advances in Insect Physiology; Raikhel, A.S., Ed.; Academic Press: Cambridge, MA, USA, 2016; Volume 51, pp. 93–113. [Google Scholar]
- Hernández-Martínez, S.; Mayoral, J.G.; Li, Y.; Noriega, F.G. Role of juvenile hormone and allatotropin on nutrient allocation, ovarian development and survivorship in mosquitoes. J. Insect Physiol. 2007, 53, 230–234. [Google Scholar] [CrossRef]
- Caroci, A.S.; Li, Y.; Noriega, F.G. Reduced juvenile hormone synthesis in mosquitoes with low teneral reserves reduces ovarian previtellogenic development in Aedes aegypti. J. Exp. Biol. 2004, 207, 2685–2690. [Google Scholar] [CrossRef]
- Pondeville, E.; Maria, A.; Jacques, J.-C.; Bourgouin, C.; Dauphin-Villemant, C. Anopheles gambiae males produce and transfer the vitellogenic steroid hormone 20-hydroxyecdysone to females during mating. Proc. Natl. Acad. Sci. USA 2008, 105, 19631–19636. [Google Scholar] [CrossRef]
- Baldini, F.; Gabrieli, P.; Rogers, D.W.; Catteruccia, F. Function and composition of male accessory gland secretions in Anopheles gambiae: A comparison with other insect vectors of infectious diseases. Pathog. Glob. Health 2012, 106, 82–93. [Google Scholar] [CrossRef]
- Baldini, F.; Gabrieli, P.; South, A.; Valim, C.; Mancini, F.; Catteruccia, F. The interaction between a sexually transferred steroid hormone and a female protein regulates oogenesis in the malaria mosquito Anopheles gambiae. PLoS Biol. 2013, 11, e1001695. [Google Scholar] [CrossRef]
- Gabrieli, P.; Kakani, E.G.; Mitchell, S.N.; Mameli, E.; Want, E.J.; Mariezcurrena Anton, A.; Serrao, A.; Baldini, F.; Catteruccia, F. Sexual transfer of the steroid hormone 20E induces the postmating switch in Anopheles gambiae. Proc. Natl. Acad. Sci. USA 2014, 111, 16353–16358. [Google Scholar] [CrossRef]
- Pondeville, E.; Puchot, N.; Lang, M.; Cherrier, F.; Schaffner, F.; Dauphin-Villemant, C.; Bischoff, E.; Bourgouin, C. Evolution of sexually-transferred steroids and mating-induced phenotypes in Anopheles mosquitoes. Sci. Rep. 2019, 9, 4669. [Google Scholar] [CrossRef] [PubMed]
- Degner, E.C.; Ahmed-Braimah, Y.H.; Borziak, K.; Wolfner, M.F.; Harrington, L.C.; Dorus, S. Proteins, transcripts, and genetic architecture of seminal fluid and sperm in the mosquito Aedes aegypti. Mol. Cell. Proteom. 2019, 18, S6–S22. [Google Scholar] [CrossRef]
- Peng, D.; Kakani, E.G.; Mameli, E.; Vidoudez, C.; Mitchell, S.N.; Merrihew, G.E.; Maccoss, M.J.; Adams, K.; Rinvee, T.A.; Shaw, W.R.; et al. A male steroid controls female sexual behaviour in the malaria mosquito. Nature 2022, 608, 93–97. [Google Scholar] [CrossRef]
- Raikhel, A.S.; Dhadialla, T.S. Accumulation of yolk proteins in insect oocytes. Annu. Rev. Entomol. 1992, 37, 217–251. [Google Scholar] [CrossRef]
- Nicholson, A.J. The development of the ovary and ovarian egg of a mosquito, Anopheles maculipennis, Meig. J. Cell Sci. 1921, S2-65, 395–448. [Google Scholar] [CrossRef]
- Airs, P.M.; Nazarchyk, M.J.; Tucker, B.J.; Bartholomay, L.C. Characterizing oogenesis and programmed cell death in the eastern tree hole mosquito Aedes (Protomacleaya) triseriatus. Front. Insect Sci. 2023, 2, 1073308. [Google Scholar] [CrossRef] [PubMed]
- Gillies, M.T. Observations on nulliparous and parous rates in some common East African mosquitoes. Ann. Trop. Med. Parasitol. 1963, 57, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.E.; Estévez-Lao, T.Y.; McCabe, T.C.; Hillyer, J.F. Warmer temperature accelerates reproductive senescence in mosquitoes. Front. Physiol. 2025, 16, 1610310. [Google Scholar] [CrossRef] [PubMed]
- Neven, L.G. Physiological responses of insects to heat. Postharvest Biol. Technol. 2000, 21, 103–111. [Google Scholar] [CrossRef]
- Angilletta, M.J., Jr.; Huey, R.B.; Frazier, M.R. Thermodynamic effects on organismal performance: Is hotter better? Physiol. Biochem. Zool. 2010, 83, 197–206. [Google Scholar] [CrossRef]
- Martin, L.E.; Ruiz, M.; Hillyer, J.F. Senescence of humoral antimicrobial immunity occurs in infected mosquitoes when the temperature is higher. J. Exp. Biol. 2024, 227, jeb248149. [Google Scholar] [CrossRef]
- Barr, J.S.; Estevez-Lao, T.Y.; Khalif, M.; Saksena, S.; Yarlagadda, S.; Farah, O.; Shivere, Y.; Hillyer, J.F. Temperature and age, individually and interactively, shape the size, weight, and body composition of adult female mosquitoes. J. Insect Physiol. 2023, 148, 104525. [Google Scholar] [CrossRef]
- Barr, J.S.; Martin, L.E.; Tate, A.T.; Hillyer, J.F. Warmer environmental temperature accelerates aging in mosquitoes, decreasing longevity and worsening infection outcomes. Immun. Ageing 2024, 21, 61. [Google Scholar] [CrossRef] [PubMed]
- Agyekum, T.P.; Arko-Mensah, J.; Botwe, P.K.; Hogarh, J.N.; Issah, I.; Dwomoh, D.; Billah, M.K.; Dadzie, S.K.; Robins, T.G.; Fobil, J.N. Effects of elevated temperatures on the growth and development of adult Anopheles gambiae (s.l.) (Diptera: Culicidae) mosquitoes. J. Med. Entomol. 2022, 59, 1413–1420. [Google Scholar] [CrossRef]
- Martin, L.E.; Hillyer, J.F. Higher temperature accelerates the aging-dependent weakening of the melanization immune response in mosquitoes. PLoS Pathog. 2024, 20, e1011935. [Google Scholar] [CrossRef]
- Murdock, C.C.; Paaijmans, K.P.; Bell, A.S.; King, J.G.; Hillyer, J.F.; Read, A.F.; Thomas, M.B. Complex effects of temperature on mosquito immune function. Proc. R. Soc. Lond. B Biol. Sci. 2012, 279, 3357–3366. [Google Scholar] [CrossRef] [PubMed]
- Mordecai, E.A.; Caldwell, J.M.; Grossman, M.K.; Lippi, C.A.; Johnson, L.R.; Neira, M.; Rohr, J.R.; Ryan, S.J.; Savage, V.; Shocket, M.S.; et al. Thermal biology of mosquito-borne disease. Ecol. Lett. 2019, 22, 1690–1708. [Google Scholar] [CrossRef] [PubMed]
- Agyekum, T.P.; Botwe, P.K.; Arko-Mensah, J.; Issah, I.; Acquah, A.A.; Hogarh, J.N.; Dwomoh, D.; Robins, T.G.; Fobil, J.N. A systematic review of the effects of temperature on Anopheles mosquito development and survival: Implications for malaria control in a future warmer climate. Int. J. Environ. Res. Public Health 2021, 18, 7255. [Google Scholar] [CrossRef]
- Huxley, P.J.; Murray, K.A.; Pawar, S.; Cator, L.J. The effect of resource limitation on the temperature dependence of mosquito population fitness. Proc. R. Soc. Lond. B Biol. Sci. 2021, 288, 20203217. [Google Scholar] [CrossRef]
- Ciota, A.T.; Matacchiero, A.C.; Kilpatrick, A.M.; Kramer, L.D. The effect of temperature on life history traits of Culex mosquitoes. J. Med. Entomol. 2014, 51, 55–62. [Google Scholar] [CrossRef]
- Ezeakacha, N.F.; Yee, D.A. The role of temperature in affecting carry-over effects and larval competition in the globally invasive mosquito Aedes albopictus. Parasites Vectors 2019, 12, 1–11. [Google Scholar] [CrossRef]
- Costa, E.A.P.D.A.; Santos, E.M.D.M.; Correia, J.C.; Albuquerque, C.M.R.D. Impact of small variations in temperature and humidity on the reproductive activity and survival of Aedes aegypti (Diptera, Culicidae). Rev. Bras. Entomol. 2010, 54, 488–493. [Google Scholar] [CrossRef]
- Gandara, A.C.P.; Drummond-Barbosa, D. Chronic exposure to warm temperature causes low sperm abundance and quality in Drosophila melanogaster. Sci. Rep. 2023, 13, 12331. [Google Scholar] [CrossRef] [PubMed]
- Sales, K.; Thomas, P.; Gage, M.J.G.; Vasudeva, R. Experimental heatwaves reduce the effectiveness of ejaculates at occupying female reproductive tracts in a model insect. R. Soc. Open Sci. 2024, 11, 231949. [Google Scholar] [CrossRef]
- Sales, K.; Vasudeva, R.; Dickinson, M.E.; Godwin, J.L.; Lumley, A.J.; Michalczyk, Ł.; Hebberecht, L.; Thomas, P.; Franco, A.; Gage, M.J.G. Experimental heatwaves compromise sperm function and cause transgenerational damage in a model insect. Nat. Commun. 2018, 9, 4771. [Google Scholar] [CrossRef]
- Ryan, S.J.; Ben-Horin, T.; Johnson, L.R. Malaria control and senescence: The importance of accounting for the pace and shape of aging in wild mosquitoes. Ecosphere 2015, 6, art170. [Google Scholar] [CrossRef]
- Müller, L.; Fülöp, T.; Pawelec, G. Immunosenescence in vertebrates and invertebrates. Immun. Ageing 2013, 10, 12. [Google Scholar] [CrossRef]
- Zajitschek, F.; Zajitschek, S.; Bonduriansky, R. Senescence in wild insects: Key questions and challenges. Funct. Ecol. 2020, 34, 26–37. [Google Scholar] [CrossRef]
- Styer, L.M.; Carey, J.R.; Wang, J.L.; Scott, T.W. Mosquitoes do senesce: Departure from the paradigm of constant mortality. Am. J. Trop. Med. Hyg. 2007, 76, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Somé, B.M.; Guissou, E.; Da, D.F.; Richard, Q.; Choisy, M.; Yameogo, K.B.; Hien, D.F.; Yerbanga, R.S.; Ouedraogo, G.A.; Dabiré, K.R.; et al. Mosquito ageing modulates the development, virulence and transmission potential of pathogens. Proc. R. Soc. B Biol. Sci. 2024, 291, 20232097. [Google Scholar] [CrossRef]
- Hillyer, J.F.; Schmidt, S.L.; Fuchs, J.F.; Boyle, J.P.; Christensen, B.M. Age-associated mortality in immune challenged mosquitoes (Aedes aegypti) correlates with a decrease in haemocyte numbers. Cell. Microbiol. 2005, 7, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Barr, J.S.; Saksena, S.R.; Hillyer, J.F. Cellular immune senescence in mosquitoes accelerates when the temperature is warmer. Dev. Comp. Immunol. 2025, 168, 105396. [Google Scholar] [CrossRef] [PubMed]
- McCann, S.; Day, J.F.; Allan, S.; Lord, C.C. Age modifies the effect of body size on fecundity in Culex quinquefasciatus Say (Diptera: Culicidae). J. Vector Ecol. 2009, 34, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.T.; Silveira, I.D.d.; Tátila-Ferreira, A.; David, M.R.; Chouin-Carneiro, T.; Van den Wouwer, L.; Maes, L.; Maciel-de-Freitas, R. The impact of the age of first blood meal and Zika virus infection on Aedes aegypti egg production and longevity. PLoS ONE 2018, 13, e0200766. [Google Scholar] [CrossRef]
- Ramírez-Sánchez, L.F.; Hernández, B.J.; Guzmán, P.A.; Alfonso-Parra, C.; Avila, F.W. The effects of female age on blood-feeding, insemination, sperm storage, and fertility in the dengue vector mosquito Aedes aegypti (Diptera: Culicidae). J. Insect Physiol. 2023, 150, 104570. [Google Scholar] [CrossRef]
- Sawadogo, S.P.; Diabaté, A.; Toé, H.K.; Sanon, A.; Lefevre, T.; Baldet, T.; Gilles, J.; Simard, F.; Gibson, G.; Sinkins, S.; et al. Effects of age and size on Anopheles gambiae s.s. male mosquito mating success. J. Med. Entomol. 2013, 50, 285–293. [Google Scholar] [CrossRef]
- Ponlawat, A.; Harrington, L.C. Age and body size influence male sperm capacity of the Dengue vector Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2007, 44, 422–426. [Google Scholar] [CrossRef]
- Verhoek, B.A.; Takken, W. Age effects on the insemination rate of Anopheles gambiae s.l. in the laboratory. Entomol. Exp. Appl. 1994, 72, 167–172. [Google Scholar] [CrossRef]
- Meuti, M.E.; Short, S.M. Physiological and environmental factors affecting the composition of the ejaculate in mosquitoes and other insects. Insects 2019, 10, 74. [Google Scholar] [CrossRef]
- Herrera-Cruz, M.; Abraham, S.; Nuñez-Beverido, N.; Flores-Estévez, N.; Reyes-Hernández, M.; Alvarado, M.; Pérez-Staples, D. Male age and strain affect ejaculate quality in the Mexican fruit fly. Insect Sci. 2018, 25, 703–711. [Google Scholar] [CrossRef]
- Mitchell, S.N.; Catteruccia, F. Anopheline reproductive biology: Impacts on vectorial capacity and potential avenues for malaria control. Cold Spring Harb. Perspect. Med. 2017, 7, a025593. [Google Scholar] [CrossRef]
- Couper, L.I.; Farner, J.E.; Caldwell, J.M.; Childs, M.L.; Harris, M.J.; Kirk, D.G.; Nova, N.; Shocket, M.; Skinner, E.B.; Uricchio, L.H.; et al. How will mosquitoes adapt to climate warming? eLife 2021, 10, e69630. [Google Scholar] [CrossRef] [PubMed]
- Pörtner, H.O.; Roberts, D.C.; Tignor, M.; Poloczanska, E.S.; Mintenbeck, K.; Alegría, A.; Craig, M.; Langsdorf, S.; Löschke, S.; Möller, V.; et al. IPCC, 2022: Climate Change 2022: Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022. [Google Scholar]
- Takken, W.; Charlwood, D.; Lindsay, S.W. The behaviour of adult Anopheles gambiae, sub-Saharan Africa’s principal malaria vector, and its relevance to malaria control: A review. Malar. J. 2024, 23, 161. [Google Scholar] [CrossRef] [PubMed]
- Oliva, C.F.; Benedict, M.Q.; Lempérière, G.; Gilles, J. Laboratory selection for an accelerated mosquito sexual development rate. Malar. J. 2011, 10, 135. [Google Scholar] [CrossRef]
- Dahan, Y.L.; Koekemoer, L.L. Analysis of the genitalia rotation in the male Anopheles funestus (Diptera: Culicidae). Acta Trop. 2014, 132, S20–S25. [Google Scholar] [CrossRef]
- Provost, M.W.; Lum, P.T.M.; Branch, N. Rotation of male terminalia in Aedes taeniorhynchus (Diptera: Culicidae) as affected by temperature. Ann. Entomol. Soc. Am. 1961, 54, 896–900. [Google Scholar] [CrossRef]
- Howell, P.I.; Knols, B.G. Male mating biology. Malar. J. 2009, 8, S8. [Google Scholar] [CrossRef]
- McGillycuddy, M.; Popovic, G.; Bolker, B.M.; Warton, D.I. Parsimoniously fitting large multivariate random effects in glmmTMB. J. Stat. Softw. 2025, 112, 1–19. [Google Scholar] [CrossRef]
- Brooks, M.E.; Kristensen, K.; Benthem, K.J.V.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Mächler, M.; Bolker, B.M. glmmTMB balances speed and flexibility among packages forzero-inflated generalized linear mixed modeling. R J. 2017, 9, 378. [Google Scholar] [CrossRef]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models, v0.4.7; R, 2024. [CrossRef]
- Cribari-Neto, F.; Zeileis, A. Beta regression in R. J. Stat. Softw. 2010, 34, 1–24. [Google Scholar] [CrossRef]
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means, 1.8.3; R, 2022. [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kolde, R. Pheatmap: Pretty Heatmaps, v1.0.12; R, 2019. [CrossRef]
- Pascini, T.V.; Martins, G.F. The insect spermatheca: An overview. Zoology 2017, 121, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Shaw, W.R.; Teodori, E.; Mitchell, S.N.; Baldini, F.; Gabrieli, P.; Rogers, D.W.; Catteruccia, F. Mating activates the heme peroxidase HPX15 in the sperm storage organ to ensure fertility in Anopheles gambiae. Proc. Natl. Acad. Sci. USA 2014, 111, 5854–5859. [Google Scholar] [CrossRef] [PubMed]
- Pascini, T.V.; Ramalho-Ortigão, M.; Ribeiro, J.M.; Jacobs-Lorena, M.; Martins, G.F. Transcriptional profiling and physiological roles of Aedes aegypti spermathecal-related genes. BMC Genom. 2020, 21, 143. [Google Scholar] [CrossRef]
- Hagedorn, H.H.; Fallon, A.M. Ovarian control of vitellogenin synthesis by the fat body in Aedes aegypti. Nature 1973, 244, 103–105. [Google Scholar] [CrossRef]
- Moura, A.S.; Costa-da-Silva, A.L.; Peixoto, P.S.; Maciel, C.; Cardoso, A.F. Vitellogenin genes are transcribed in Culex quinquefasciatus ovary. Memórias Inst. Oswaldo Cruz 2023, 118, e220143. [Google Scholar] [CrossRef] [PubMed]
- Itoe, M.A.; Shaw, W.R.; Stryapunina, I.; Vidoudez, C.; Peng, D.; Du, E.W.; Rinvee, T.A.; Singh, N.; Yan, Y.; Hulai, O.; et al. Maternal lipid mobilization is essential for embryonic development in the malaria vector Anopheles gambiae. PLoS Biol. 2024, 22, e3002960. [Google Scholar] [CrossRef]
- Gondim, K.C.; Majerowicz, D. Lipophorin: The lipid shuttle. In Lipophorin: The Lipid Shuttle; Advances in Experimental Medicine and Biology; Springer International Publishing: Berlin, Germany, 2024. [Google Scholar] [CrossRef]
- Pinch, M.; Mitra, S.; Rodriguez, S.D.; Li, Y.; Kandel, Y.; Dungan, B.; Holguin, F.O.; Attardo, G.M.; Hansen, I.A. Fat and happy: Profiling mosquito fat body lipid storage and composition post-blood meal. Front. Insect Sci. 2021, 1, 693168. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, W.; Zhao, X.; Yu, Z.; Guo, H.; Yang, Y.; Moussian, B.; Zhu, K.Y.; Zhang, J. Lipophorin receptor is required for the accumulations of cuticular hydrocarbons and ovarian neutral lipids in Locusta migratoria. Int. J. Biol. Macromol. 2023, 236, 123746. [Google Scholar] [CrossRef]
- Wang, X.; Hou, Y.; Saha, T.T.; Pei, G.; Raikhel, A.S.; Zou, Z. Hormone and receptor interplay in the regulation of mosquito lipid metabolism. Proc. Natl. Acad. Sci. USA 2017, 114, E2709–E2718. [Google Scholar] [CrossRef]
- DeJong, R.J.; Miller, L.M.; Molina-Cruz, A.; Gupta, L.; Kumar, S.; Barillas-Mury, C. Reactive oxygen species detoxification by catalase is a major determinant of fecundity in the mosquito Anopheles gambiae. Proc. Natl. Acad. Sci. USA 2007, 104, 2121–2126. [Google Scholar] [CrossRef]
- Diaz-Albiter, H.; Mitford, R.; Genta, F.A.; Sant’Anna, M.R.; Dillon, R.J. Reactive oxygen species scavenging by catalase is important for female Lutzomyia longipalpis fecundity and mortality. PLoS ONE 2011, 6, e17486. [Google Scholar] [CrossRef] [PubMed]
- Sim, C.; Denlinger, D.L. Catalase and superoxide dismutase-2 enhance survival and protect ovaries during overwintering diapause in the mosquito Culex pipiens. J. Insect Physiol. 2011, 57, 628–634. [Google Scholar] [CrossRef]
- Dao, A.; Kassogue, Y.; Adamou, A.; Diallo, M.; Yaro, A.S.; Traore, S.F.; Lehmann, T. Reproduction-longevity trade-off in Anopheles gambiae (Diptera: Culicidae). J. Med. Entomol. 2010, 47, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Le, B.V.; Nguyen, J.B.; Logarajah, S.; Wang, B.; Marcus, J.; Williams, H.P.; Catteruccia, F.; Baxter, R.H.G. Characterization of Anopheles gambiae transglutaminase 3 (AgTG3) and Iits native substrate plugin. J. Biol. Chem. 2013, 288, 4844–4853. [Google Scholar] [CrossRef] [PubMed]
- Rogers, D.W.; Baldini, F.; Battaglia, F.; Panico, M.; Dell, A.; Morris, H.R.; Catteruccia, F. Transglutaminase-mediated semen coagulation controls sperm storage in the malaria mosquito. PLoS Biol. 2009, 7, e1000272. [Google Scholar] [CrossRef]
- Lyu, X.Y.; Wang, X.L.; Geng, D.Q.; Jiang, H.; Zou, Z. Juvenile hormone acts on male accessory gland function via regulating l-asparaginase expression and triacylglycerol mobilization in Aedes aegypti. Insect Sci. 2023, 30, 81–94. [Google Scholar] [CrossRef]
- Stanley, D.; Kim, Y. Prostaglandins and other eicosanoids in insects: Biosynthesis and biological actions. Front. Physiol. 2019, 9, 1927. [Google Scholar] [CrossRef]
- Pondeville, E.; David, J.-P.; Guittard, E.; Maria, A.; Jacques, J.-C.; Ranson, H.; Bourgouin, C.; Dauphin-Villemant, C. Microarray and RNAi analysis of P450s in Anopheles gambiae male and female steroidogenic tissues: CYP307A1 is required for ecdysteroid synthesis. PLoS ONE 2013, 8, e79861. [Google Scholar] [CrossRef]
- Hu, J.; Medison, R.G.; Zhang, S.; Ma, P.; Shi, C. Impacts of non-lethal high-temperature stress on the development and reproductive organs of Bradysia odoriphaga. Insects 2022, 13, 74. [Google Scholar] [CrossRef]
- Gandara, A.C.P.; Drummond-Barbosa, D. Warm and cold temperatures have distinct germline stem cell lineage effects during Drosophila oogenesis. Development 2022, 149, dev200149. [Google Scholar] [CrossRef] [PubMed]
- Blanckenhorn, W.U.; Henseler, C. Temperature-dependent ovariole and testis maturation in the yellow dung fly. Entomol. Exp. Appl. 2005, 116, 159–165. [Google Scholar] [CrossRef]
- Pan, L.; Chen, S.; Weng, C.; Call, G.; Zhu, D.; Tang, H.; Zhang, N.; Xie, T. Stem cell aging is controlled both intrinsically and extrinsically in the Drosophila ovary. Cell Stem Cell 2007, 1, 458–469. [Google Scholar] [CrossRef]
- Michalkova, V.; Benoit, J.B.; Attardo, G.M.; Medlock, J.; Aksoy, S. Amelioration of reproduction-associated oxidative stress in a viviparous insect is critical to prevent reproductive senescence. PLoS ONE 2014, 9, e87554. [Google Scholar] [CrossRef]
- González-Tokman, D.; Villada-Bedoya, S.; Hernández, A.; Montoya, B. Antioxidants, oxidative stress and reactive oxygen species in insects exposed to heat. Curr. Res. Insect Sci. 2025, 7, 100114. [Google Scholar] [CrossRef]
- González-Tokman, D.; Córdoba-Aguilar, A.; Dáttilo, W.; Lira-Noriega, A.; Sánchez-Guillén, R.A.; Villalobos, F. Insect responses to heat: Physiological mechanisms, evolution and ecological implications in a warming world. Biol. Rev. Camb. Philos. Soc. 2020, 95, 802–821. [Google Scholar] [CrossRef]
- Briegel, H. Fecundity, metabolism, and body size in Anopheles (Diptera: Culicidae), vectors of malaria. J. Med. Entomol. 1990, 27, 839–850. [Google Scholar] [CrossRef]
- Briegel, H. Metabolic relationship between female body size, reserves, and fecundity of Aedes aegypti. J. Insect Physiol. 1990, 36, 165–172. [Google Scholar] [CrossRef]
- Stryapunina, I.; Itoe, M.A.; Trinh, Q.; Vidoudez, C.; Du, E.; Mendoza, L.; Hulai, O.; Kauffman, J.; Carew, J.; Shaw, W.R.; et al. Precise coordination between nutrient transporters ensures fertility in the malaria mosquito Anopheles gambiae. PLoS Genet. 2024, 20, e1011145. [Google Scholar] [CrossRef]
- Raikhel, A.S.; Kokoza, V.A.; Zhu, J.; Martin, D.; Wang, S.-F.; Li, C.; Sun, G.; Ahmed, A.; Dittmer, N.; Attardo, G. Molecular biology of mosquito vitellogenesis: From basic studies to genetic engineering of antipathogen immunity. Insect Biochem. Mol. Biol. 2002, 32, 1275–1286. [Google Scholar] [CrossRef]
- Helinski, M.E.H.; Harrington, L.C. Male mating history and body size influence female fecundity and longevity of the Dengue vector Aedes aegypti. J. Med. Entomol. 2011, 48, 202–211. [Google Scholar] [CrossRef]
- Wigby, S.; Perry, J.C.; Kim, Y.H.; Sirot, L.K. Developmental environment mediates male seminal protein investment in Drosophila melanogaster. Funct. Ecol. 2016, 30, 410–419. [Google Scholar] [CrossRef]
- Hansen, I.A.; Attardo, G.M.; Park, J.H.; Peng, Q.; Raikhel, A.S. Target of rapamycin-mediated amino acid signaling in mosquito anautogeny. Proc. Natl. Acad. Sci. USA 2004, 101, 10626–10631. [Google Scholar] [CrossRef]
- Driver, C.J.I.; Lamb, M.J. Metabolic changes in ageing Drosophila melanogaster. Exp. Gerontol. 1980, 15, 167–175. [Google Scholar] [CrossRef]
- Costanzo, K.; Occhino, D. Effects of temperature on blood feeding and activity levels in the tiger mosquito, Aedes albopictus. Insects 2023, 14, 752. [Google Scholar] [CrossRef]
- Agyekum, T.P.; Arko-Mensah, J.; Botwe, P.K.; Hogarh, J.N.; Issah, I.; Dwomoh, D.; Billah, M.K.; Dadzie, S.K.; Robins, T.G.; Fobil, J.N. Effects of elevated temperatures on the development of immature stages of Anopheles gambiae (s.l.) mosquitoes. Trop. Med. Int. Health 2022, 27, 338–346. [Google Scholar] [CrossRef]
- Ukubuiwe, A.C.; Olayemi, I.K.; Arimoro, F.O.; Omalu, I.C.J.; Baba, B.M.; Ukubuiwe, C.C.; Odeyemi, M.O.; Adeniyi, K.A. Influence of rearing-water temperature on life stages’ vector attributes, distribution and utilisation of metabolic reserves in Culex quinquefasciatus (Diptera: Culicidae): Implications for disease transmission and vector control. J. Basic Appl. Zool. 2018, 79, 32. [Google Scholar] [CrossRef]
- Klepsatel, P.; Gáliková, M.; Xu, Y.; Kühnlein, R.P. Thermal stress depletes energy reserves in Drosophila. Sci. Rep. 2016, 6, 33667. [Google Scholar] [CrossRef] [PubMed]
- Dittmer, J.; Alafndi, A.; Gabrieli, P. Fat body–specific vitellogenin expression regulates host-seeking behaviour in the mosquito Aedes albopictus. PLoS Biol. 2019, 17, e3000238. [Google Scholar] [CrossRef] [PubMed]
- Briegel, H.; Hörler, E. Multiple blood meals as a reproductive strategy in Anopheles (Diptera: Culicidae). J. Med. Entomol. 1993, 30, 975–985. [Google Scholar] [CrossRef]
- Takken, W.; Klowden, M.J.; Chambers, G.M. Articles: Effect of body size on host seeking and blood meal utilization in Anopheles gambiae sensu stricto (Diptera: Culicidae): The disadvantage of being small. J. Med. Entomol. 1998, 35, 639–645. [Google Scholar] [CrossRef]
- Fernandes, L.; Briegel, H. Reproductive physiology of Anopheles gambiae and Anopheles atroparvus. J. Vector Ecol. 2005, 30, 11–26. [Google Scholar]
- Clements, A.N.; Potter, S.A. The fine structure of the spermathecae and their ducts in the mosquito Aedes aegypti. J. Insect Physiol. 1967, 13, 1825–1836. [Google Scholar] [CrossRef]
- Pascini, T.V.; Ramalho-Ortigäo, J.M.; Martins, G.F. The fine structure of the spermatheca in Anopheles aquasalis (Diptera: Culicidae). Ann. Entomol. Soc. Am. 2013, 106, 857–867. [Google Scholar] [CrossRef]
- Pereira, J.; Rios, T.; Amorim, J.; Faria-Reis, A.; de Almeida, E.; Neves, M.; Santos-Araújo, S.; Selim, L.; Bertuci, F.; Silva, M.B.; et al. Functional characterization of vitellogenin unveils novel roles in RHBP uptake and lifespan regulation in the insect vector Rhodnius prolixus. Insect Biochem. Mol. Biol. 2025, 180, 104301. [Google Scholar] [CrossRef]
- Chen, D.; Han, H.-L.; Li, W.-J.; Wang, J.-J.; Wei, D. Expression and role of vitellogenin genes in ovarian development of Zeugodacus cucurbitae. Insects 2022, 13, 452. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.D.; Weiner, A.J.; Goralski, T.J.; Mahowald, A.P. The follicle cells are a major site of vitellogenin synthesis in Drosophila melanogaster. Dev. Biol. 1982, 89, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Rogers, D.W.; Whitten, M.M.; Thailayil, J.; Soichot, J.; Levashina, E.A.; Catteruccia, F. Molecular and cellular components of the mating machinery in Anopheles gambiae females. Proc. Natl. Acad. Sci. USA 2008, 105, 19390–19395. [Google Scholar] [CrossRef] [PubMed]
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef]
- Shaw, W.R.; Attardo, G.M.; Aksoy, S.; Catteruccia, F. A comparative analysis of reproductive biology of insect vectors of human disease. Curr. Opin. Insect Sci. 2015, 10, 142–148. [Google Scholar] [CrossRef]
- Borovsky, D.; Carlson, D.A.; Hancock, R.G.; Rembold, H.; van Handel, E. De novo biosynthesis of juvenile hormone III and I by the accessory glands of the male mosquito. Insect Biochem. Mol. Biol. 1994, 24, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Clifton, M.E.; Correa, S.; Rivera-Perez, C.; Nouzova, M.; Noriega, F.G. Male Aedes aegypti mosquitoes use JH III transferred during copulation to influence previtellogenic ovary physiology and affect the reproductive output of female mosquitoes. J. Insect Physiol. 2014, 64, 40–47. [Google Scholar] [CrossRef]
- Benoit, J.B.; Denlinger, D.L. Meeting the challenges of on-host and off-host water balance in blood-feeding arthropods. J. Insect Physiol. 2010, 56, 1366–1376. [Google Scholar] [CrossRef]
- Canyon, D.V.; Hii, J.L.; Müller, R. Adaptation of Aedes aegypti (Diptera: Culicidae) oviposition behavior in response to humidity and diet. J. Insect Physiol. 1999, 45, 959–964. [Google Scholar] [CrossRef]
- Canyon, D.V.; Muller, R.; Hii, J.L. Aedes aegypti disregard humidity-related conditions with adequate nutrition. Trop. Biomed. 2013, 30, 1–8. [Google Scholar]
- Lega, J.; Brown, H.E.; Barrera, R. Aedes aegypti (Diptera: Culicidae) abundance model improved with relative humidity and precipitation-driven egg hatching. J. Med. Entomol. 2017, 54, 1375–1384. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.J.; Pascual, M.; Wimberly, M.C.; Johnson, L.R.; Murdock, C.C. Humidity—The overlooked variable in the thermal biology of mosquito-borne disease. Ecol. Lett. 2023, 26, 1029–1049. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, L.E.; Estévez-Lao, T.Y.; Grant, M.I.; Shastri, N.Y.; Hillyer, J.F. Warmer Temperature Accelerates the Aging-Dependent Decrease in Female Ovary Size, Delays Male Accessory Gland Development, and Accelerates Aging-Dependent Changes in Reproductive Gene Expression in Anopheles gambiae Mosquitoes. Insects 2025, 16, 921. https://doi.org/10.3390/insects16090921
Martin LE, Estévez-Lao TY, Grant MI, Shastri NY, Hillyer JF. Warmer Temperature Accelerates the Aging-Dependent Decrease in Female Ovary Size, Delays Male Accessory Gland Development, and Accelerates Aging-Dependent Changes in Reproductive Gene Expression in Anopheles gambiae Mosquitoes. Insects. 2025; 16(9):921. https://doi.org/10.3390/insects16090921
Chicago/Turabian StyleMartin, Lindsay E., Tania Y. Estévez-Lao, Megan I. Grant, Norbu Y. Shastri, and Julián F. Hillyer. 2025. "Warmer Temperature Accelerates the Aging-Dependent Decrease in Female Ovary Size, Delays Male Accessory Gland Development, and Accelerates Aging-Dependent Changes in Reproductive Gene Expression in Anopheles gambiae Mosquitoes" Insects 16, no. 9: 921. https://doi.org/10.3390/insects16090921
APA StyleMartin, L. E., Estévez-Lao, T. Y., Grant, M. I., Shastri, N. Y., & Hillyer, J. F. (2025). Warmer Temperature Accelerates the Aging-Dependent Decrease in Female Ovary Size, Delays Male Accessory Gland Development, and Accelerates Aging-Dependent Changes in Reproductive Gene Expression in Anopheles gambiae Mosquitoes. Insects, 16(9), 921. https://doi.org/10.3390/insects16090921






