Insights into the Functional Responses of Four Neotropical-Native Parasitoids to Enhance Their Role as Biocontrol Agents Against Anastrepha fraterculus Pest Populations
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing and Sources
2.2. Experimental Setup
2.3. Functional Response Analysis
2.4. Rearing Optimization
3. Results
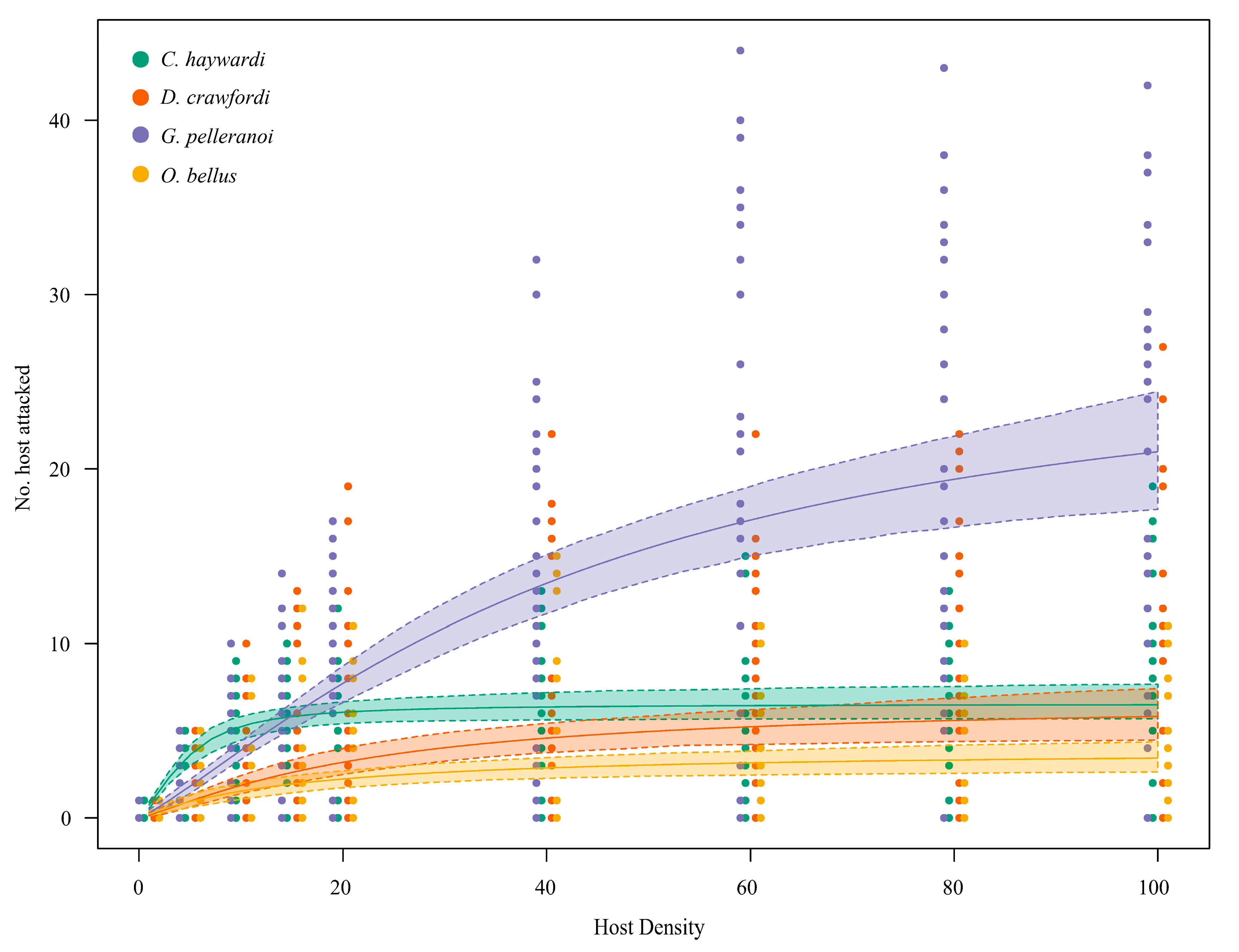
3.1. Functional Response Analysis
3.2. Rearing Optimization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hernández-Ortiz, V.; Hernández-López, M.; Steck, G.J. Morfología y Taxonomía de Tephritidae: Especies de Importancia Económica y Cuarentenaria en América. In Moscas de la Fruta: Fundamentos y Procedimientos para su Manejo; Montoya, P., Toledo, J., Hernández, E., Eds.; S y G editores: Mexico City, Mexico, 2020; pp. 71–116. [Google Scholar]
- Guillén, D.; Sánchez, R. Expansion of the National Fruit Fly Control Programme in Argentina. In Area-Wide Control of Insect Pests: From Research to Field Implementation; Springer: Dordrecht, The Netherlands, 2007; pp. 653–660. ISBN 9781402060595. [Google Scholar]
- Aruani, R.; Ceresa, A.; Granados, J.C.; Taret, G.; Peruzzotti, P.; Ortiz, G. Advances in the National Fruit Fly Control and Eradication Program in Argentina. In Fruit Fly Pests: A World Assessment of Their Biology and Management; McPheron, B.A., Steck, G.J., Eds.; St. Lucie Press: DelRay Beach, FL, USA, 1996; pp. 521–530. [Google Scholar]
- Suárez, L.d.C.; Núñez-Campero, S.R.; Murúa, F.; Garcia, F.R.M.; Ovruski, S.M. Effectiveness of Diachasmimorpha longicaudata in Killing Ceratitis capitata Larvae Infesting Commercial Fruits in Dryland Agroecosystems of Western Argentina. Agronomy 2024, 14, 2418. [Google Scholar] [CrossRef]
- Vera, M.T.; Cáceres, C.; Wornoayporn, V.; Islam, A.; Robinson, A.S.; De la Vega, M.H.; Hendrichs, J.; Cayol, J.P. Mating Incompatibility Among Populations of the South American Fruit Fly Anastrepha fraterculus (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 2006, 99, 387–397. [Google Scholar] [CrossRef]
- Cladera, J.L.; Vilardi, J.C.; Juri, M.; Paulin, L.E.; Giardini, M.C.; Gómez Cendra, P.V.; Segura, D.F.; Lanzavecchia, S.B. Genetics and Biology of Anastrepha fraterculus: Research Supporting the Use of the Sterile Insect Technique (SIT) to Control This Pest in Argentina. BMC Genet. 2014, 15, S12. [Google Scholar] [CrossRef] [PubMed]
- Garcia, F.R.M.; Ovruski, S.M.; Suárez, L.; Cancino, J.; Liburd, O.E. Biological Control of Tephritid Fruit Flies in the Americas and Hawaii: A Review of the Use of Parasitoids and Predators. Insects 2020, 11, 662. [Google Scholar] [CrossRef]
- Montoya, P.; López, P.; Cruz, J.; López, F.; Cadena, C.; Cancino, J.; Liedo, P. Effect of Diachasmimorpha longicaudata Releases on the Native Parasitoid Guild Attacking Anastrepha spp. Larvae in Disturbed Zones of Chiapas, Mexico. BioControl 2017, 62, 581–593. [Google Scholar] [CrossRef]
- Dias, N.P.; Montoya, P.; Nava, D.E. A 30-Year Systematic Review Reveals Success in Tephritid Fruit Fly Biological Control Research. Entomol. Exp. Appl. 2022, 170, 370–384. [Google Scholar] [CrossRef]
- Cancino, J.; Colmenares, M.; Pérez-López, S.; Brindis-Santos, I.; Hilerio, A.; Suárez, L.; Ovruski, S.M. Influence of Anastrepha Host Fruit Size over Parasitism by Diachasmimorpha longicaudata in Open-Field Augmentative Releases. Biocontrol Sci. Technol. 2024, 34, 812–827. [Google Scholar] [CrossRef]
- Sivinski, J.; Vulinec, K.; Menezes, E.; Aluja, M. The Bionomics of Coptera haywardi (Ogloblin) (Hymenoptera: Diapriidae) and other Pupal Parasitoids of Tephritid Fruit Flies (Diptera). Biol. Control 1998, 202, 193–202. [Google Scholar] [CrossRef]
- Aluja, M. Fruit Fly (Diptera: Tephritidae) Research in Latin America: Myths, Realities and Dreams. Anais da Sociedade Entomológica do Brasil 1999, 28, 565–594. [Google Scholar] [CrossRef]
- Aluja, M.; Rull, J. Managing Pestiferous Fruit Flies (Diptera: Tephritidae) through Environmental Manipulation. In Biorational Tree Fruit Pest Management; CABI: Wallingford, UK, 2009; pp. 171–213. ISBN 9781845934842. [Google Scholar]
- Aluja, M. Future Trends in Fruit Fly Management. In Fruit Fly Pests; CRC Press: Boca Raton, FL, USA, 1996; pp. 309–320. [Google Scholar]
- Aluja, M.; Sivinski, J.; Van Driesche, R.; Anzures-Dadda, A.; Guillén, L. Pest Management through Tropical Tree Conservation. Biodivers. Conserv. 2014, 23, 831–853. [Google Scholar] [CrossRef]
- Sivinski, J.M. The Past and Potential of Biological Control of Fruit Flies. In Fruit Fly Pests; CRC Press: Boca Raton, FL, USA, 1996; pp. 369–375. [Google Scholar]
- Poncio, S.; Montoya, P.; Cancino, J.; Nava, D.E. Best Host Age of Anastrepha obliqua (Diptera: Tephritidae) for Multiplication of Four Native Parasitoids from the Americas. J. Insect Sci. 2018, 18, 36. [Google Scholar] [CrossRef]
- Cancino, J.; Ayala, A.; Ríos, L.; López, P.; Suárez, L.; Ovruski, S.M.; Hendrichs, J. Increasing Radiation Doses in Anastrepha obliqua (Diptera: Tephritidae) Larvae Improve Parasitoid Mass-Rearing Attributes. Bull. Entomol. Res. 2022, 112, 807–817. [Google Scholar] [CrossRef]
- Montoya, P.; Cancino, J.; Zenil, M.; Santiago, G.; Gutierrez, J.M. The Augmentative Biological Control Component in the Mexican National Campaign Against Anastrepha spp. Fruit Flies. In Area-Wide Control of Insect Pests: From Research to Field Implementation; Springer: Dordrecht, The Netherlands, 2007; pp. 661–670. ISBN 9781402060595. [Google Scholar]
- Núñez-Campero, S.R.; González, C.; Rull, J.; Ovruski, S.M. Maximum Entropy (MaxEnt) as Extreme Distribution Indicator of Two Neotropical Fruit Fly Parasitoids in Irrigated Drylands of Argentina. Bull. Entomol. Res. 2022, 112, 636–645. [Google Scholar] [CrossRef]
- Knipling, E.F. The Basic Principles of Insect Population Suppression and Management; U.S. Department of Agriculture, Ed.; Agriculture Handbook; US Department of Agriculture: Washington, DC, USA, 1979; Volume 512, 623p.
- Knipling, E.F. Principles of Insect Parasitism Analyzed from New Perspectives: Practical Implications for Regulating Insect Populations by Biological Means; Agriculture Handbook; U.S. Department of Agriculture: Washington, DC, USA, 1992; Volume 693, 337p.
- Knipling, E.F. Sterile Insect and Parasite Augmentation Techniques: Unexploited Solutions for Many Insect Pest Problems. Fla. Entomol. 1998, 81, 134–160. [Google Scholar]
- Sivinski, J. Augmentative Biological Control: Research and Methods to Help Make It Work. CABI Rev. 2013, 1–11. [Google Scholar] [CrossRef]
- Wyckhuys, K.A.G.; Orankanok, W.; Ketelaar, J.W.; Rauf, A.; Goergen, G.; Neuenschwander, P. Biological Control: Cornerstone of Area-Wide-Integrated Pest Management for the Cassava Mealybug in Tropical Asia. In Area-Wide Integrated Pest Management: Development and Field Application; Hendrichs, J., Pereira, R., Vreysen, M.J.B., Eds.; CRC Press: Boca Ratón, FL, USA, 2021; pp. 17–32. ISBN 978-0-367-76986-4. [Google Scholar]
- van Lenteren, J.C.; Nicoli, G.; Heinz, K.M.; Parella, M.P. Quality Control of Mass-Produced Beneficial Insects. In Biocontrol in Protected Culture; Ball Publishing: Batavia, The Netherland, 2004; pp. 503–526. [Google Scholar]
- Núñez-Campero, S.R.; Aluja, M.; Rull, J.; Ovruski, S.M. Comparative Demography of Three Neotropical Larval-Prepupal Parasitoid Species Associated with Anastrepha fraterculus (Diptera: Tephritidae). Biol. Control 2014, 69, 8–17. [Google Scholar] [CrossRef]
- Godfray, H.C.J. Parasitoids. In Encyclopedia of Biodiversity; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1–13. [Google Scholar]
- Holling, C.S. The Components of Predation as Revealed by a Study of Small-Mammal Predation of the European Pine Sawfly. Can. Entomol. 1959, 91, 293–320. [Google Scholar] [CrossRef]
- Holling, C.S. The Functional Response of Invertebrate Predators to Prey Density. Mem. Entomol. Soc. Can. 1966, 98, 5–86. [Google Scholar] [CrossRef]
- Oaten, A.; Murdoch, W.W. Functional Response and Stability in Predator-Prey Systems. Am. Nat. 1975, 109, 289–298. [Google Scholar] [CrossRef]
- Papanikolaou, N.E.; Kypraios, T.; Moffat, H.; Fantinou, A.; Perdikis, D.P.; Drovandi, C. Predators’ Functional Response: Statistical Inference, Experimental Design, and Biological Interpretation of the Handling Time. Front. Ecol. Evol. 2021, 9, 740848. [Google Scholar] [CrossRef]
- DeLong, J.P.; Uiterwaal, S.F. Predator Functional Responses and the Biocontrol of Aphids and Mites. BioControl 2022, 67, 161–172. [Google Scholar] [CrossRef]
- Williams, R.J.; Martinez, N.D. Stabilization of Chaotic and Non-Permanent Food-Web Dynamics. Eur. Phys. J. B 2004, 38, 297–303. [Google Scholar] [CrossRef]
- Sinclair, A.R.E.; Pech, R.P.; Dickman, C.R.; Hik, D.; Mahon, P.; Newsome, A.E. Predicting Effects of Predation on Conservation of Endangered Prey. Conserv. Biol. 1998, 12, 564–575. [Google Scholar] [CrossRef]
- Twardochleb, L.A.; Novak, M.; Moore, J.W. Using the Functional Response of a Consumer to Predict Biotic Resistance to Invasive Prey. Ecol. Appl. 2012, 22, 1162–1171. [Google Scholar] [CrossRef]
- Griffen, B.D. Considerations when Applying the Consumer Functional Response Measured Under Artificial Conditions. Front. Ecol. Evol. 2021, 9, 713147. [Google Scholar] [CrossRef]
- Pritchard, D.W.; Paterson, R.A.; Bovy, H.C.; Barrios-O’Neill, D. Frair: An R Package for Fitting and Comparing Consumer Functional Responses. Methods Ecol. Evol. 2017, 8, 1528–1534. [Google Scholar] [CrossRef]
- Aluja, M.; Sivinski, J.; Ovruski, S.M.; Guillén, L.; López, M.; Cancino, J.; Torres-Anaya, A.; Gallegos-Chan, G.; Ruiz, L.C. Colonization and Domestication of Seven Species of Native New World Hymenopterous Larval-Prepupal and Pupal Fruit Fly (Diptera: Tephritidae) Parasitoids. Biocontrol Sci. Technol. 2009, 19, 49–79. [Google Scholar] [CrossRef]
- Núñez-Campero, S.R.; Ovruski, S.M.; Aluja, M. Survival Analysis and Demographic Parameters of the Pupal Parasitoid Coptera haywardi (Hymenoptera: Diapriidae), Reared on Anastrepha fraterculus (Diptera: Tephritidae). Biol. Control 2012, 61, 40–46. [Google Scholar] [CrossRef]
- Gonçalves, R.S.; Nava, D.E.; Pereira, H.C.; Lisbôa, H.; Grützmacher, A.D.; Valgas, R.A. Biology and Fertility Life Table of Aganaspis pelleranoi (Hymenoptera: Figitidae) in Larvae of Anastrepha fraterculus and Ceratitis capitata (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 2013, 106, 791–798. [Google Scholar] [CrossRef]
- Poncio, S.; Montoya, P.; Cancino, J.; Nava, D.E. Determining the Functional Response and Mutual Interference of Utetes anastrephae (Hymenoptera: Braconidae) on Anastrepha obliqua (Diptera: Tephritidae) Larvae for Mass Rearing Purposes. Ann. Entomol. Soc. Am. 2016, 109, 518–525. [Google Scholar] [CrossRef]
- Clemente, G.; Toledo, J.; Pérez-Lachaud, G.; Valle-Mora, J.F.; Liedo, P.; Montoya, P. Functional Response and Mutual Interference in the Parasitoid Coptera haywardi (Oglobin) (Hymenoptera: Diapriidae) Attacking Anastrepha ludens (Loew) (Diptera: Tephritidae) Pupae. Bull. Entomol. Res. 2024, 114, 22–29. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 25 August 2025).
- Bolker, B.M. Ecological Models and Data in R; Princeton University Press: Princeton, NJ, USA, 2008; ISBN 9781400840908. [Google Scholar]
- Real, L.A. The Kinetics of Functional Response. Am. Nat. 1977, 111, 289–300. [Google Scholar] [CrossRef]
- Rogers, D. Random Search and Insect Population Models. J. Anim. Ecol. 1972, 41, 369–383. [Google Scholar] [CrossRef]
- Royama, T. A Comparative Study of Models for Predation and Parasitism. Popul. Ecol. 1971, 15, 1–121. [Google Scholar] [CrossRef]
- Barrios-O’Neill, D.; Kelly, R.; Dick, J.T.A.; Ricciardi, A.; Macisaac, H.J.; Emmerson, M.C. On the Context-Dependent Scaling of Consumer Feeding Rates. Ecol. Lett. 2016, 19, 668–678. [Google Scholar] [CrossRef]
- Pervez, A. Omkar Functional Responses of Coccinellid Predators: An Illustration of a Logistic Approach. J. Insect Sci. 2005, 5, 5. [Google Scholar] [CrossRef]
- Montoya, P.; Liedo, P.; Benrey, B.; Barrera, J.F.; Cancino, J.; Aluja, M. Functional Response and Superparasitism by Diachasmimorpha longicaudata (Hymenoptera: Braconidae), a Parasitoid of Fruit Flies (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 2000, 93, 47–54. [Google Scholar] [CrossRef]
- Sule, H.; Muhamad, R.; Omar, D.; Kah, A.; Hee, W. Parasitism Rate, Host Stage Preference and Functional Response of Tamarixia radiata on Diaphorina citri. Int. J. Agric. Biol. 2014, 16, 783–788. [Google Scholar]
- Holling, C.S. The Functional Response of Predators to Prey Density and Its Role in Mimicry and Population Regulation. Mem. Entomol. Soc. Can. 1965, 97, 5–60. [Google Scholar] [CrossRef]
- Hassell, M.; Lawton, J.; Beddington, J. Sigmoid Functional Responses by Invertebrate Predators and Parasitoids. J. Anim. Ecol. 1977, 46, 249–262. [Google Scholar] [CrossRef]
- Tazerouni, Z.; Talebi, A.A.; Fathipour, Y.; Soufbaf, M.; Reddy, G.V.P. Modeling Interactions and Dynamics of Aphidius matricariae and Praon volucre (Hymenoptera: Braconidae) on Two Major Aphid Species in a Greenhouse. Biol. Control 2019, 132, 110–115. [Google Scholar] [CrossRef]
- Rim, H.; Park, H.h.; Seo, M. The Effects of Host Plant Cultivars on the Functional Response of Binodoxys communis and Biological Control against Aphis gossypii. J. Asia Pac. Entomol. 2023, 26, 102078. [Google Scholar] [CrossRef]
- Bruzzone, O.A.; Aguirre, M.B.; Hill, J.G.; Virla, E.G.; Logarzo, G. Revisiting the Influence of Learning in Predator Functional Response, How It Can Lead to Shapes Different from Type III. Ecol. Evol. 2022, 12, e8593. [Google Scholar] [CrossRef]
- Marco, S.B.; Kent, M.D. Host-handling Behaviours in Parasitoids of the Black Scale: A Case for Ant-mediated Evolution. J. Anim. Ecol. 2001, 70, 237–247. [Google Scholar] [CrossRef]
- Landi, P.; McCoy, M.W.; Vonesh, J.R. Predicting Invasive Consumer Impact via the Comparative Functional Response Approach: Linking Application to Ecological Theory. Biol. Invasions 2022, 24, 3565–3579. [Google Scholar] [CrossRef]
- Van Nieuwenhove, G.A.; Bezdjian, L.P.; Schliserman, P.; Aluja, M.; Ovruski, S.M. Combined Effect of Larval and Pupal Parasitoid Use for Anastrepha fraterculus (Diptera: Tephritidae) Control. Biol. Control 2016, 95, 94–102. [Google Scholar] [CrossRef]
- Montoya, P.; Gálvez, C.; Díaz-Fleischer, F. Host availability affects the interaction between pupal parasitoid Coptera haywardi (Hym.: Diapridae) and larval-pupal parasitoid Diachasmimorpha longicaudata (Hym.: Braconidae). Bull. Entomol. Res. 2019, 109, 15–23. [Google Scholar] [CrossRef]
- Fernández-Arhex, V.; Corley, J. La Respuesta Funcional: Una Revisión y Guía Experimental. Ecol. Austral 2004, 14, 83–93. [Google Scholar]
- Montoya, P.; Pérez-Lachaud, G.; Liedo, P. Superparasitism in the Fruit Fly Parasitoid Diachasmimorpha longicaudata (Hymenoptera: Braconidae) and the Implications for Mass Rearing and Augmentative Release. Insects 2012, 3, 900–911. [Google Scholar] [CrossRef]
- Aragón, S.; Rodríguez, D.; Cantor, F. Criterios de Liberación de Encarsia formosa Gahan (Hymenoptera: Aphelinidae) Para el Control de Trialeurodes vaporariorum (Westwood) (Hemiptera: Aleyrodidae) en Tomate. Agron. Colomb. 2008, 26, 277–284. [Google Scholar]

| Parasitoid Species | FR | FR Fitted | AIC | dAIC | q | p < 0.05 |
|---|---|---|---|---|---|---|
| C. haywardi | Type II | flexpnr | 1096.814 * | 0.000 | 0.386 | 0.063 |
| rogersII | 1098.789 | 1.975 | ||||
| D. crawfordi | Type II | flexpnr | 3216.193 * | 0.000 | 0.210 | 0.095 |
| rogersII | 3217.234 | 1.041 | ||||
| G. pelleranoi | Type III | flexpnr | 3231.265 * | 0.000 | 0.242 | 0.001 |
| hassIIInr | 3237.161 | 5.896 | ||||
| rogersII | 3239.820 | 8.555 | ||||
| O. bellus | Type II | rogersII | 1347.659 * | 0.000 | 0.069 | 0.727 |
| flexpnr | 1349.531 | 1.872 |
| Parasitoid Species | Parameters | Value [CI] | SE | z-Value | p-Value |
|---|---|---|---|---|---|
| C. haywardi | b | 1.555 [0.914–3.27] | 0.430 | 3.622 | 2.20 × 10−4 * |
| q | 0.386 [0.296–0.686] | 0.208 | 1.860 | 0.063 | |
| h | 0.153 [0.114–0.172] | 0.007 | 22.835 | 2.20 × 10−16 * | |
| D. crawfordi | b | 0.178 [0.048–0.391] | 0.050 | 3.554 | 3.76 × 10−4 * |
| q | 0.210 [0.236–0.821] | 0.126 | 1.671 | 0.095 | |
| h | 0.150 [0.060–0.949] | 0.012 | 12.099 | 2.20 × 10−16 * | |
| G. pelleranoi | b | 0.328 [0.139–0.638] | 0.064 | 5.113 | 3.17 × 10−7 * |
| q | 0.242 [0.067–0.586] | 0.077 | 3.135 | 1.72 × 10−3 * | |
| h | 0.036 [0.017–0.049] | 0.002 | 13.066 | 2.20 × 10−16 * | |
| O. bellus | a | 0.274 [0.142–0.511] | 0.044 | 6.247 | 4.19 × 10−10 * |
| h | 0.255 [0.169–0.349] | 0.021 | 12.028 | 2.20 × 10−16 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Núñez-Campero, S.R.; Suárez, L.d.C.; Mello Garcia, F.R.; Cancino, J.; Montoya, P.; Ovruski, S.M. Insights into the Functional Responses of Four Neotropical-Native Parasitoids to Enhance Their Role as Biocontrol Agents Against Anastrepha fraterculus Pest Populations. Insects 2025, 16, 919. https://doi.org/10.3390/insects16090919
Núñez-Campero SR, Suárez LdC, Mello Garcia FR, Cancino J, Montoya P, Ovruski SM. Insights into the Functional Responses of Four Neotropical-Native Parasitoids to Enhance Their Role as Biocontrol Agents Against Anastrepha fraterculus Pest Populations. Insects. 2025; 16(9):919. https://doi.org/10.3390/insects16090919
Chicago/Turabian StyleNúñez-Campero, Segundo Ricardo, Lorena del Carmen Suárez, Flávio Roberto Mello Garcia, Jorge Cancino, Pablo Montoya, and Sergio Marcelo Ovruski. 2025. "Insights into the Functional Responses of Four Neotropical-Native Parasitoids to Enhance Their Role as Biocontrol Agents Against Anastrepha fraterculus Pest Populations" Insects 16, no. 9: 919. https://doi.org/10.3390/insects16090919
APA StyleNúñez-Campero, S. R., Suárez, L. d. C., Mello Garcia, F. R., Cancino, J., Montoya, P., & Ovruski, S. M. (2025). Insights into the Functional Responses of Four Neotropical-Native Parasitoids to Enhance Their Role as Biocontrol Agents Against Anastrepha fraterculus Pest Populations. Insects, 16(9), 919. https://doi.org/10.3390/insects16090919









