Transcriptomics and Metabolomics Combined to Analyze the Response Mechanism of Silkworm Eggs to High-Temperature Stress
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Transcriptome Sequencing and Analysis
2.2.1. RNA Extraction and Detection
2.2.2. Transcriptome Sequencing
2.2.3. Data Quality Control and Sequence Alignment
2.2.4. Gene Expression Level Quantification and Differential Analysis
2.2.5. RT-qPCR Validation
2.3. Metabolomic Analysis
2.3.1. Metabolome Sample Extraction
2.3.2. LC-MS Analysis
2.3.3. Metabolite Identification and Quantitative Analysis
2.3.4. Integrated Analysis of Transcriptome and Metabolome Integrated Transcriptome and Metabolome Analysis
3. Results and Analysis
3.1. Transcriptomic Analysis of Silkworm Embryo Response to High-Temperature Stress
3.1.1. Sequencing Data Quality Control
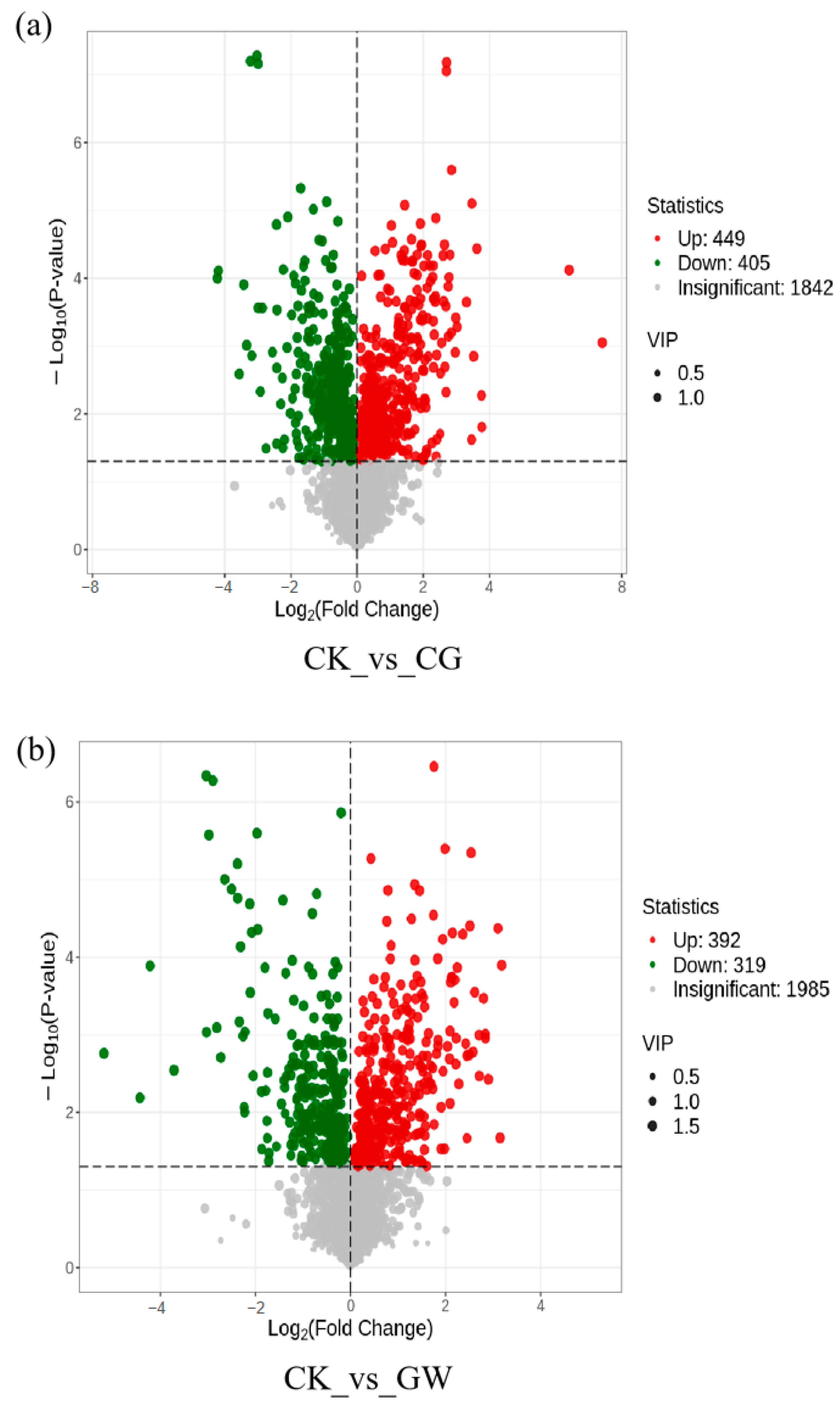
3.1.2. Statistics of DEGs
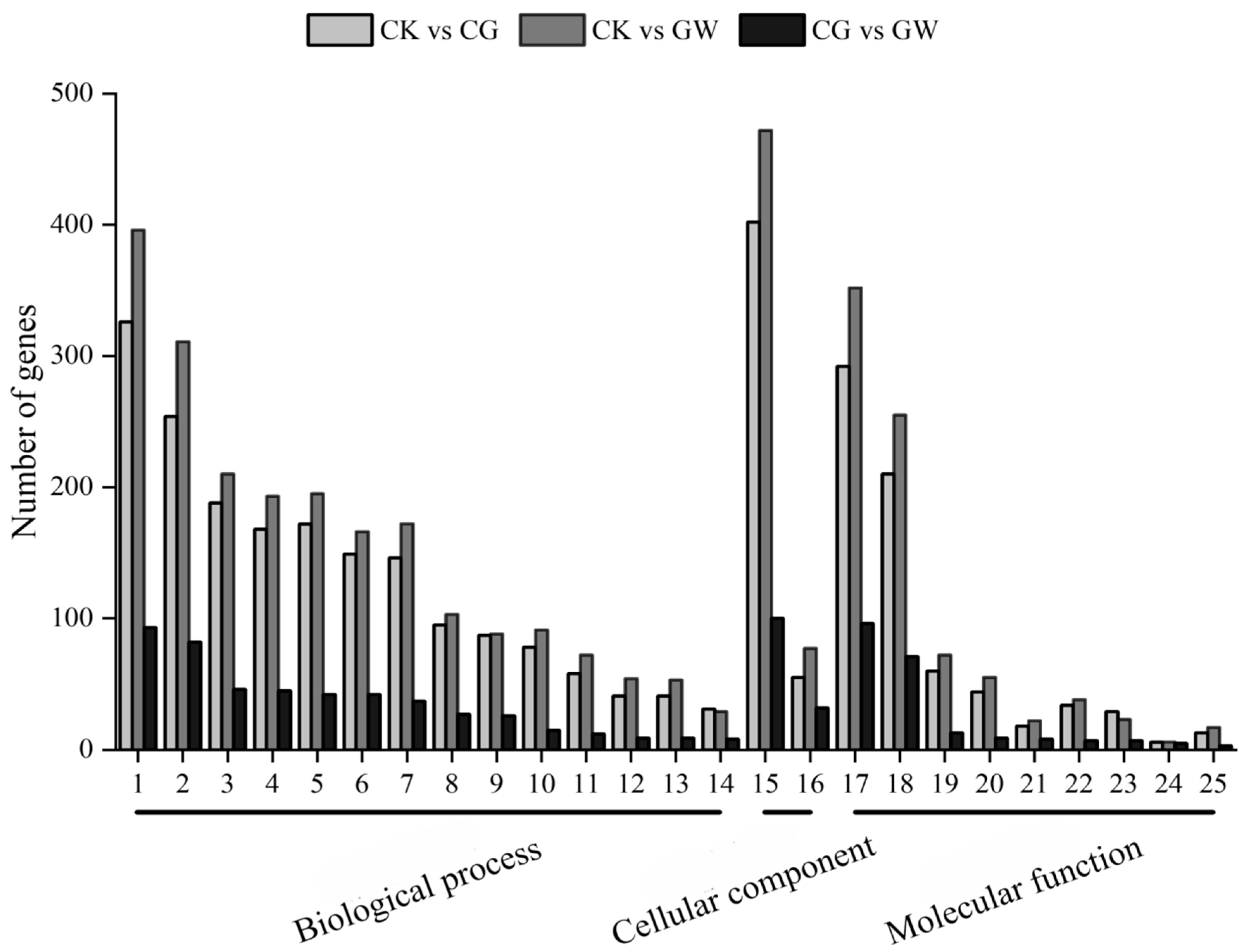
3.1.3. GO Functional Classification of DEGs
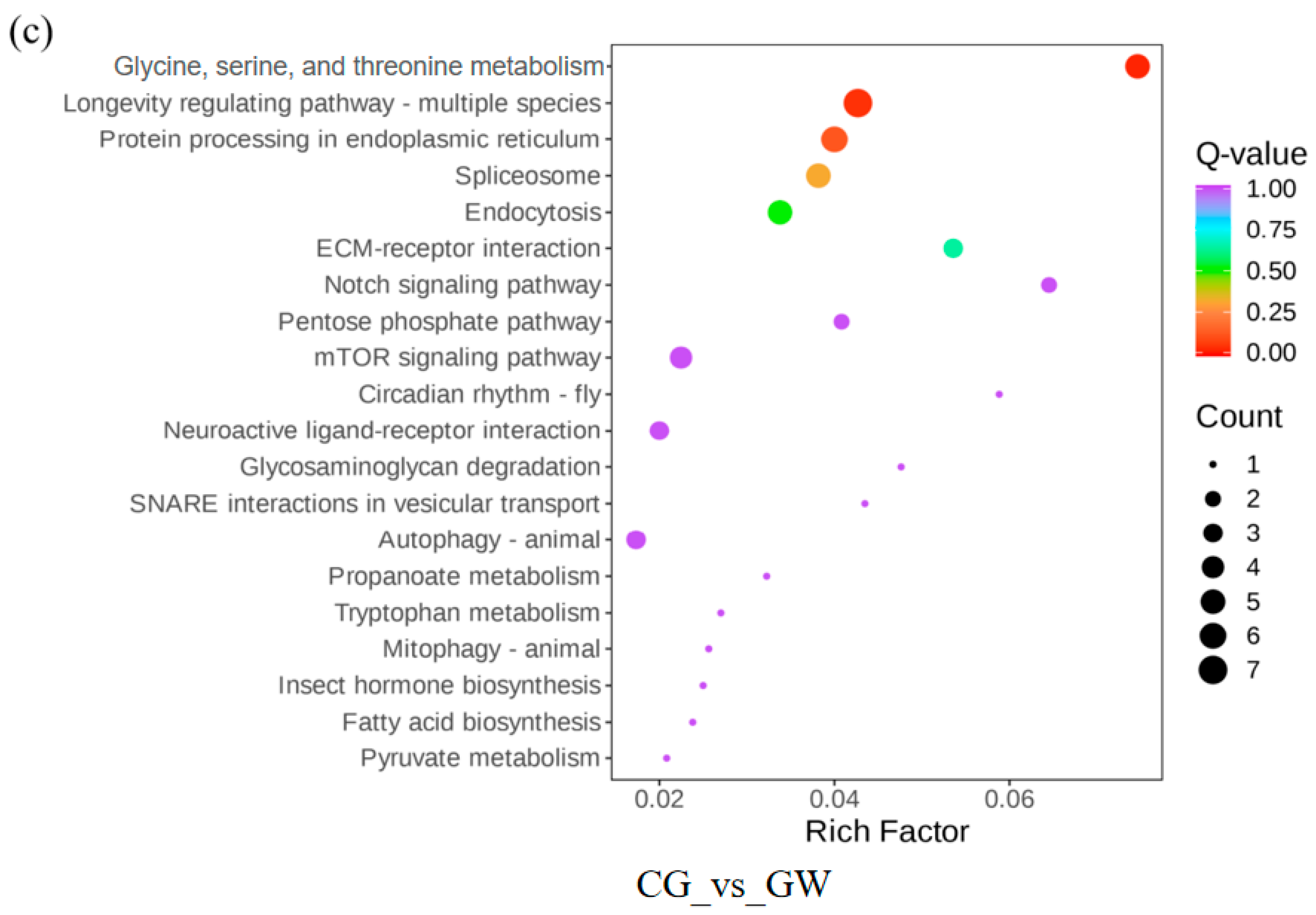
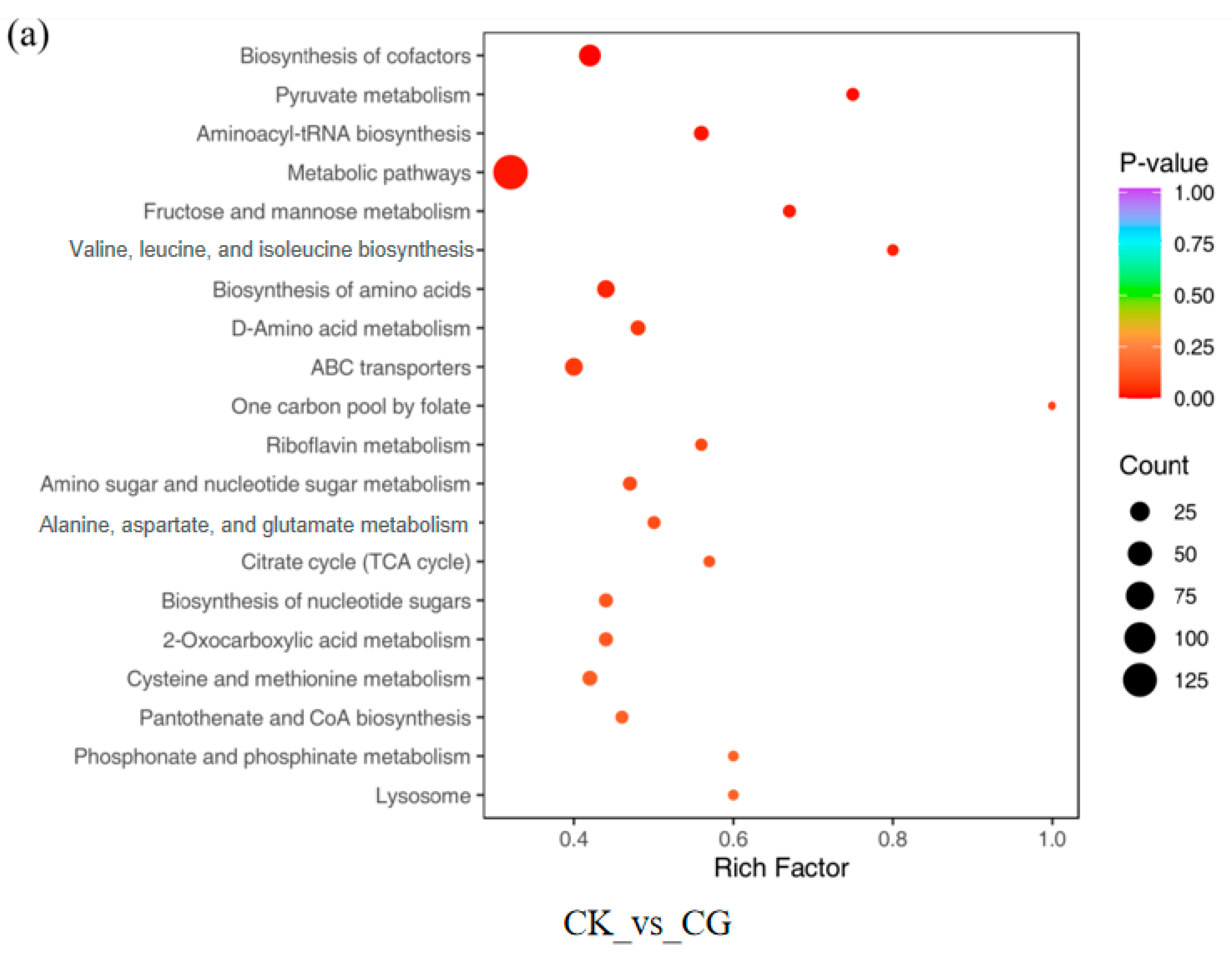
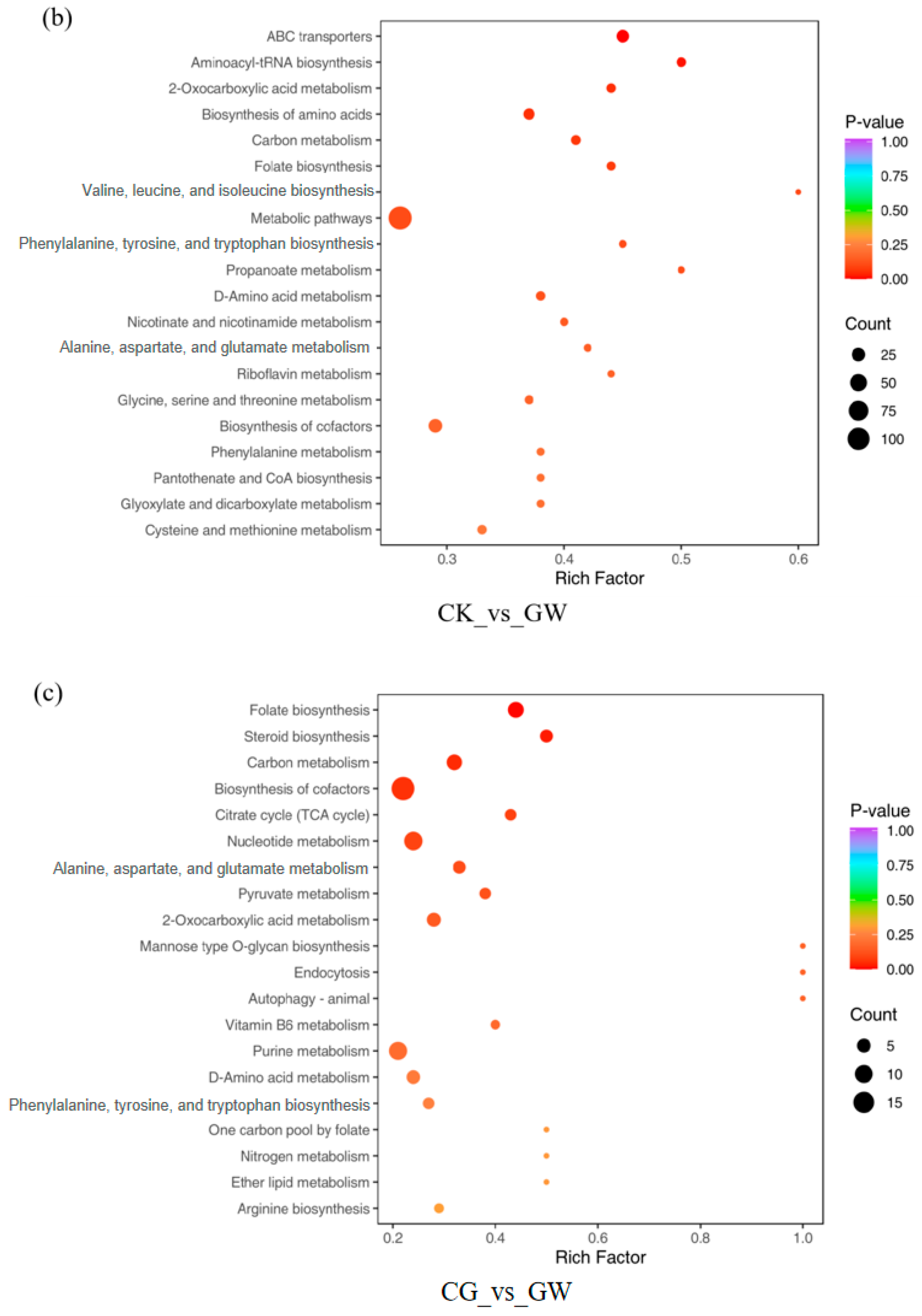
3.1.4. KEGG Pathway Enrichment Analysis of DEGs
3.1.5. Screening and Identification of DEGs
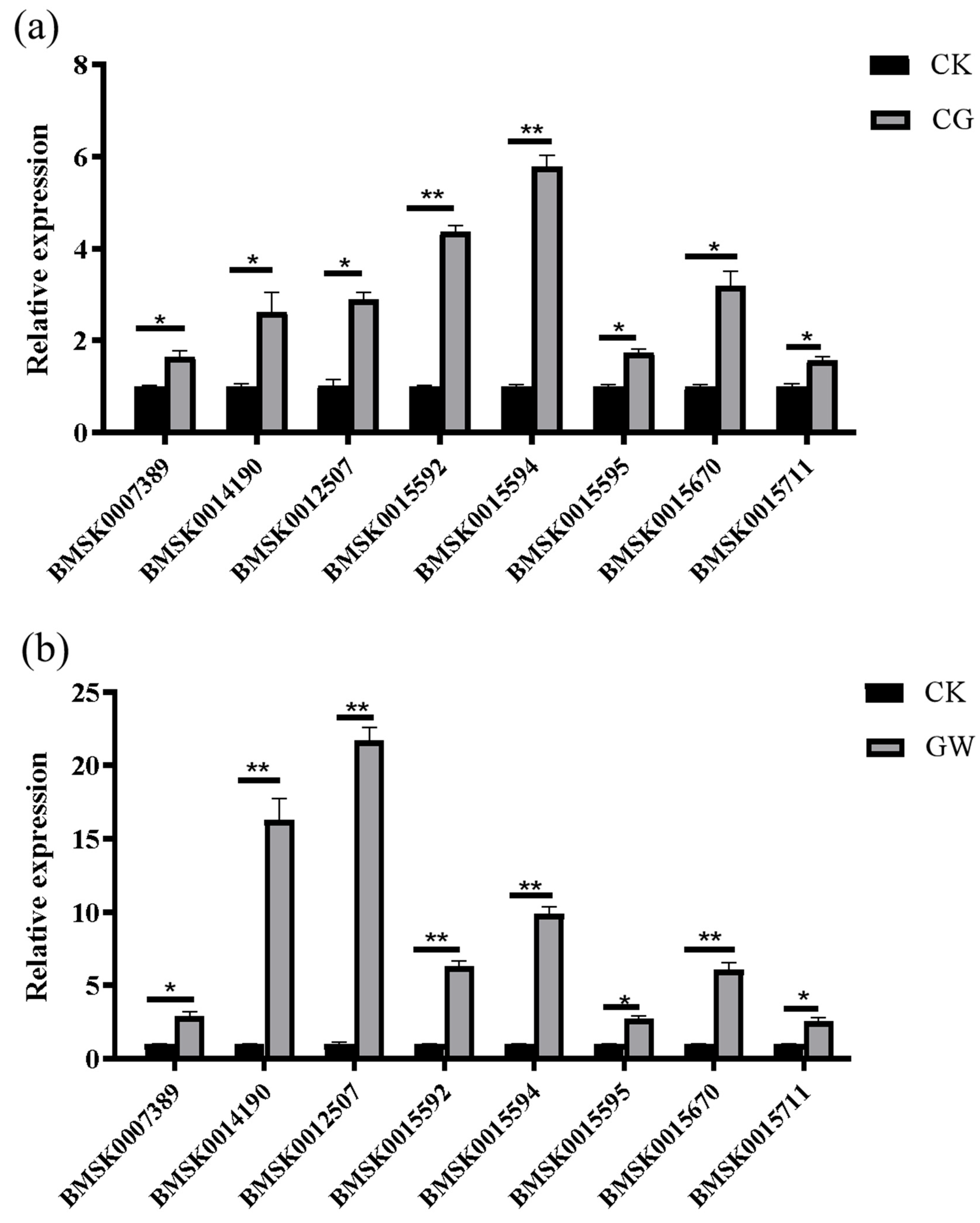
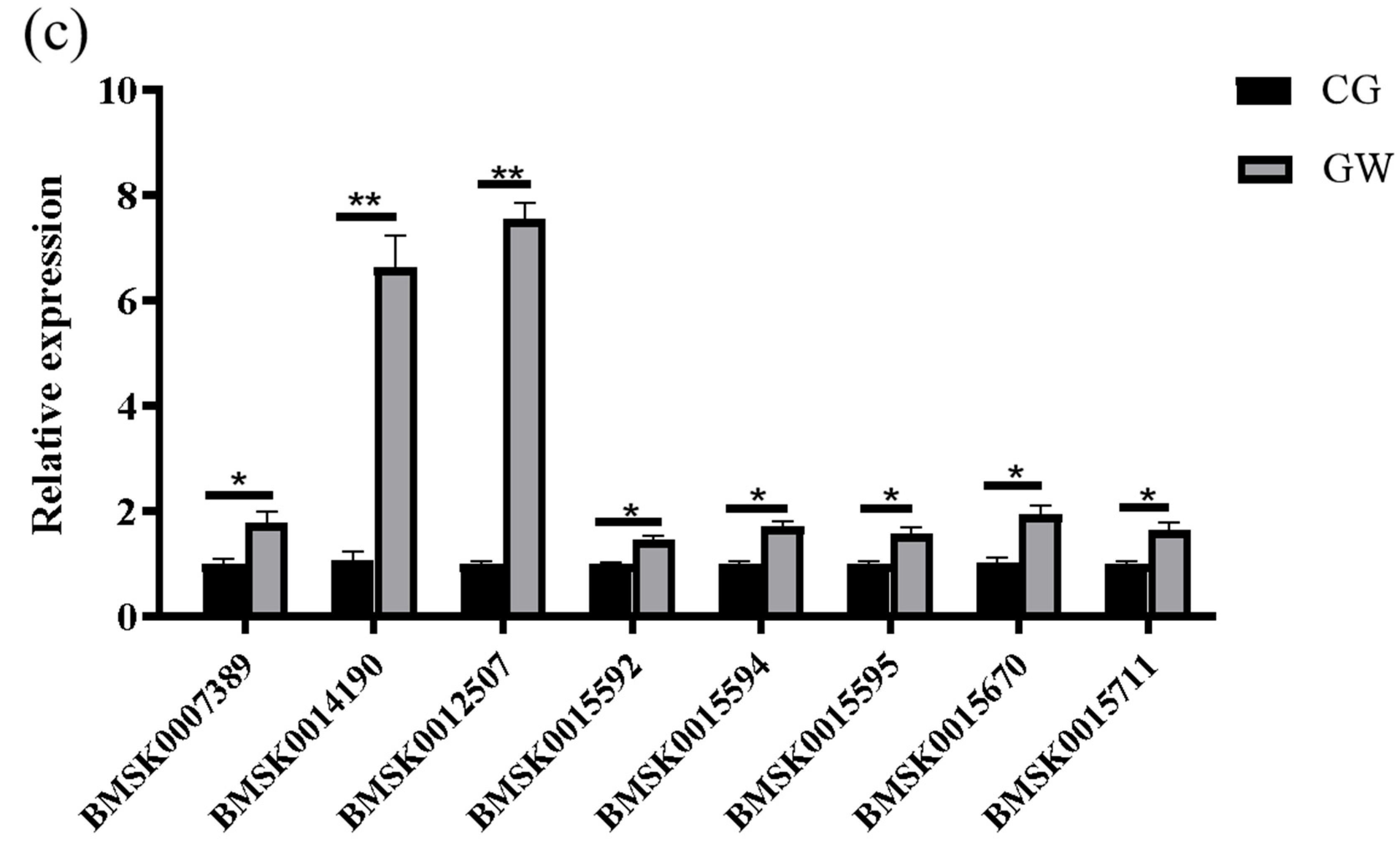
3.1.6. RT-qPCR Validation of Transcriptome Data
3.2. Metabolomic Analysis of Silkworm Embryo Response to High-Temperature Stress
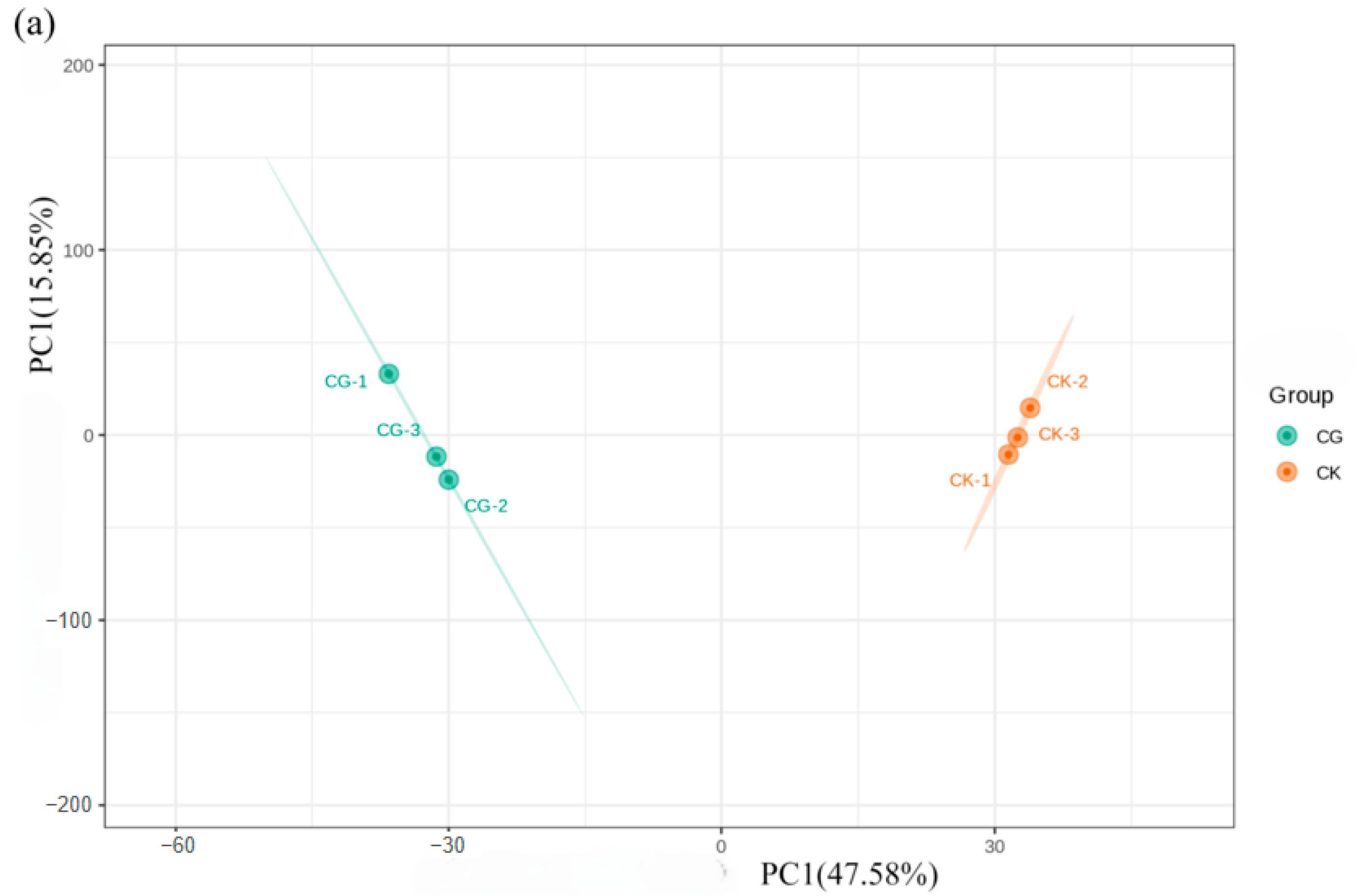
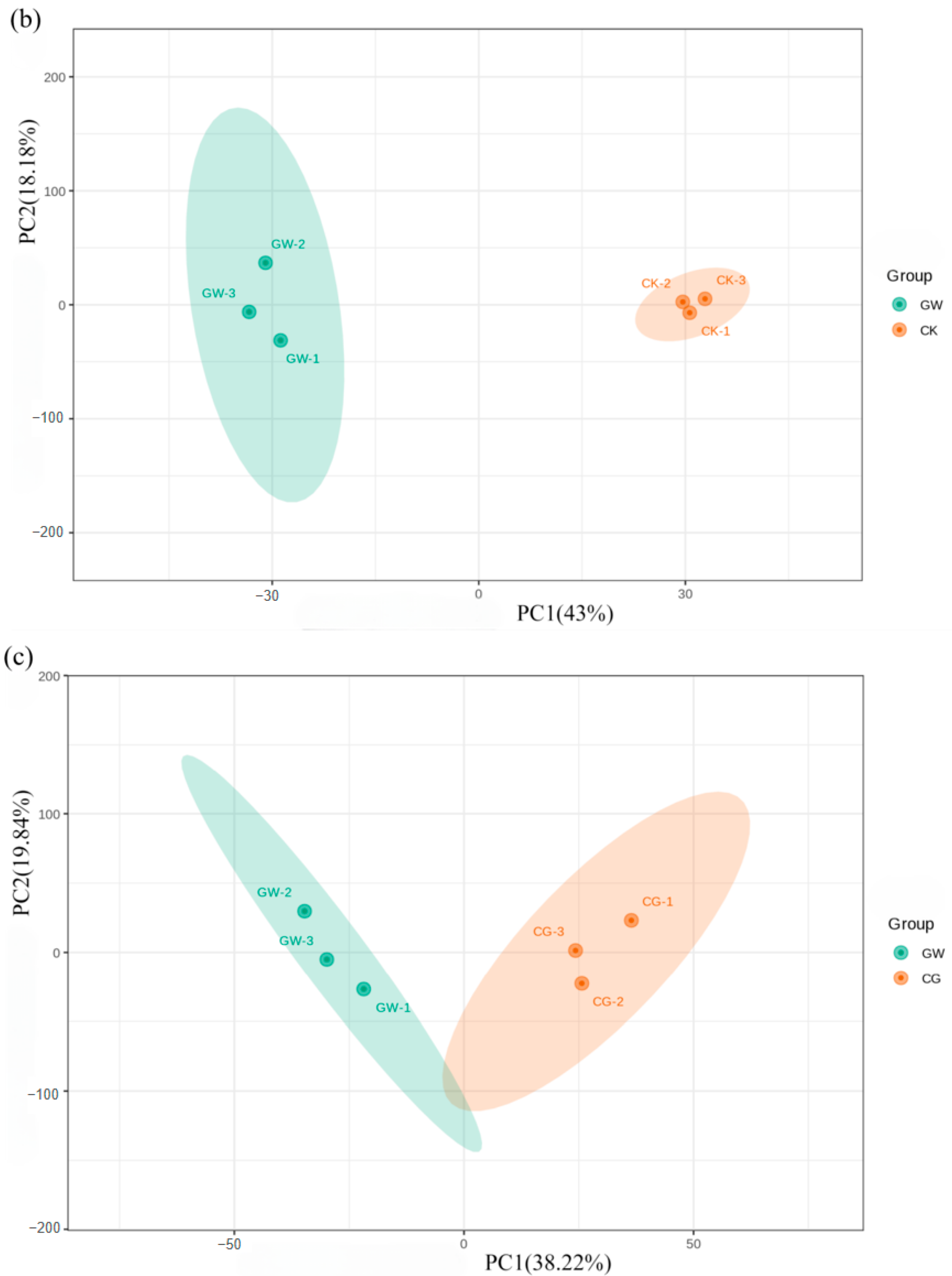
3.2.1. Metabolic Profile Analysis
3.2.2. Statistics of DMs and KEGG Pathway Enrichment Analysis
3.2.3. Screening and Identification of DMs
3.3. Integrated Transcriptome and Metabolome Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hance, T.; van Baaren, J.; Vernon, P.; Boivin, G. Impact of extreme temperatures on parasitoids in a climate change perspective. Annu. Rev. Entomol. 2007, 52, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Angilletta, M.J.; Cooper, B.S.; Schuler, M.S.; Boyles, J.G. The evolution of thermal physiology in endotherms. Front. Biosci. 2010, 2, 861–881. [Google Scholar] [CrossRef]
- Abdel-Hady, A.A.A.; Ramadan, M.M.; Lü, J.; Hashem, A.S. High-temperature shock consequences on the red flour beetle (Tribolium castaneum) and the rice weevil (Sitophilus oryzae). J. Therm. Biol. 2021, 100, 103062. [Google Scholar] [CrossRef]
- Wang, J.; Mu, Y.L.; Yang, C.; Huang, X.D.; Wang, Y.C.; Yu, H.P.; Chen, X.S. Effects of high and low temperature stress on development and cold tolerance of Spodoptera frugiperda (Smith) (Lepidoptera:Noctuidae). J. Mt. Agr. Biol. 2020, 41, 18–25. [Google Scholar]
- Zhao, F.; Bu, Y.L.; Han, H.; Cheng, Z.; Wang, D.; He, C.C. Effects of periodical extremely high temperature on the growth, development and reproduction of sevenspotted lady beetle Coccinella septempunctata. J. Plant Protect. 2023, 50, 129–135. [Google Scholar]
- Zhou, J.H.; Luo, W.F.; Song, S.Q.; Wang, Z.H.; Zhu, X.F.; Gao, S.J.; He, W.; Xu, J.J. The impact of high-temperature stress on the growth and development of Tuta absoluta (Meyrick). Insects 2024, 15, 423. [Google Scholar] [CrossRef]
- Chen, Q.; Tian, Y.; Li, J.; Zhang, J.L.; Ren, H.; Chen, G.H.; Zhang, X.M. Effects of short-term high and low temperature on the survival rate of Trichopria drosophilae. Chin. J. Ecol. 2025, 44, 1270–1275. [Google Scholar] [CrossRef]
- Gao, J.W.; Wang, H.; Hu, Q.M.; Shi, P.L.; Shi, A.M.; Liu, Z.C. Effects of short-term high and low temperature stress on the development and reproduction of Aphidius colemani Viereck. Contemp. Hort. 2024, 47, 14–18. [Google Scholar]
- Meehl, G.A.; Tebaldi, C. More intense, more frequent, and longer lasting heat waves in the 21st century. Science 2004, 305, 994–997. [Google Scholar] [CrossRef]
- Alexander, L.V.; Zhang, X.B.; Peterson, T.C.; Caesar, J.; Vazquez-Aguirre, J.L. Global observed changes in daily climate extremes of temperature and precipitation. J. Geophys. Res-Atmos. 2006, 111, D05109. [Google Scholar] [CrossRef]
- Ma, C.S.; Ma, G.; Pincebourde, S. Survive a warming climate: Insect responses to extreme high temperatures. Annu. Rev. Entomol. 2021, 66, 163–184. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, T.; Xue, P.; Shen, G.S.; Huang, J.Y.; Zhu, J.; Wang, M.X.; Tang, S.M.; Shen, X.J. Changes in the mRNA levels and activities of carbohydrate metabolism-related enzymes in the diapause-destined, non-diapause-destined and immediately acid-treated eggs of the bivoltine silkworm (Bombyx mori). Acta Entomol. Sin. 2023, 63, 1431–1440. [Google Scholar] [CrossRef]
- Xing, D.X.; Xiao, Y.; Liao, S.T.; Li, Q.R.; Ye, M.Q.; Yang, Q. Effect of high temperature instant acid treatment to silkworm eggs on blocking Transovarial transmission of pebrine disease. Sci. Seric. 2018, 44, 100–104. [Google Scholar]
- Xia, Q.Y.; Zhou, Z.Y.; Lu, C.; Cheng, D.J.; Dai, F.Y.; Li, B.; Zhao, P.; Zha, X.F.; Cheng, T.C.; Chai, C.L.; et al. Biology Analysis Group. A draft sequence for the genome of the domesticated silkworm (Bombyx mori). Science 2004, 306, 1937–1940. [Google Scholar] [CrossRef]
- Felton, G.W.; Summers, C.B. Antioxidant systems in insects. Arch. Insect Biochem. 1995, 29, 187–197. [Google Scholar] [CrossRef]
- Jones, D.P. Radical-free biology of oxidative stress. Am. J. Physiol-Cell Ph. 2008, 295, C849–C868. [Google Scholar] [CrossRef]
- Cui, J.; Qiao, F.; Hu, Y.L.; Tian, X.Y.; Zhao, L.N.; Shi, S.S. Antioxidant responses of Megacopta cribraria (Hemiptera:Plataspidae) adults exposed to high temperature stress. J. Environ. Entomol. 2021, 43, 1244–1249. [Google Scholar]
- Zhou, Y.J.; Lu, C.D.; Shen, H.Y.; Liang, Y.; Chen, Z.H.; Zhong, J.H.; Tan, J.J.; Wang, X.Y.; Liang, G.H. Adaptation and physiological response of Dastarcus helophoroides (Fairmaire) to high temperature stress. Chin. J. Biol. Contr. 2021, 37, 1179–1188. [Google Scholar]
- Qiao, L.; Zhang, T.H.; Geng, S.B.; Jin, L.; Zhang, F.M.; Wu, Y.Q.; Pan, P.L. Effects of high temperature on lifespan, fecundity, and protective enzymes of Ectropis grisescens. Fujian J. Agr. Sci. 2023, 38, 1337–1343. [Google Scholar]
- Long, J.Z.; Guo, W.X.; Ji, M.; Song, Y.Y.; Li, L.L.; Men, X.Y.; Cui, H.Y.; Yu, Y.; Liu, C.Z. Effect of high temperature stress on mortality and protective enzymes of Encarsia formosa. Grassl. Turf. 2023, 43, 130–136. [Google Scholar]
- King, A.M.; MacRae, T.H. Insect heat shock proteins during stress and diapause. Annu. Rev. Entomol. 2015, 60, 59–75. [Google Scholar] [CrossRef]
- Haslbeck, M.; Franzmann, T.; Weinfurtner, D.; Buchner, J. Some like it hot: The structure and function of small heat-shock proteins. Nat. Struct. Mol. Biol. 2005, 12, 842–846. [Google Scholar] [CrossRef]
- Xu, Y.P.; Zheng, G.W.; Dong, S.Z.; Liu, G.F.; Yu, X.P. Molecular cloning, characterization and expression analysis of HSP60, HSP70 and HSP90 in the golden apple snail, Pomacea canaliculata. Fish. Shellfish. Immun. 2014, 41, 643–653. [Google Scholar] [CrossRef]
- Le Bourg, E.; Valenti, P.; Lucchetta, P.; Payre, F. Effects of mild heat shocks at young age on aging and longevity in Drosophila melanogaster. Biogerontology 2001, 2, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.T.; McCarroll, S.A.; Bargmann, C.I.; Fraser, A.; Kamath, R.S.; Ahringer, J.; Li, H.; Kenyon, C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 2003, 424, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Wang, J.J.; Ding, W.; Zhao, Z.M. Heat shock proteins in insects. Entomol. Knowl. 2004, 40, 16–19. [Google Scholar]
- Shao, Y.F.; Zhang, J.; Wang, Y.Y.; Jiang, L.Q.; Liu, S.; Qiu, T.H.; Fan, Z.M.; Li, M.Y. Identification of AiHSP19.3 gene and its response to high temperature and insecticide stress in Agrotis ipsilon. Plant Protect. 2024, 50, 80–87. [Google Scholar]
- Zhao, S.Y.; Liu, Y.K.; Li, H.; Li, Z.C.; Hao, D.J. Spatiotemporal patterns of five small heat shock protein genes in Hyphantria cunea in response to thermal stress. Int. J. Mol. Sci. 2023, 24, 15176. [Google Scholar] [CrossRef]
- Wei, C.P.; Jiang, J.; Li, X.R.; Zhang, Y.H.; Zhang, F.M.; Cheng, D.F.; Guo, W.C.; Liu, H. Cloning and sequence analysis of heat shock protein gene Ld-HSP60 in Leptinotarsa decemlineata (Coleoptera:Chrysomelidae) and its expression under temperature stress. Acta Entomol. Sin. 2017, 60, 523–532. [Google Scholar]
- Ma, Q.; Dong, J.H.; Li, Z.X.; Zhu, K.Y.; Cheng, W.N. Molecular characteristics and functional study of heat shock protein gene smHsp60 in Sitodiplosis mosellana (Diptera: Cecidomyidae). Acta Entomol. Sin. 2023, 66, 859–869. [Google Scholar]
- Meng, J.Y.; Yang, C.L.; Wang, H.C.; Cao, Y.; Zhang, C.Y. Molecular characterization of six heat shock protein 70 genes from Arma chinensis and their expression patterns in response to temperature stress. Cell Stress Chaperon. 2022, 27, 659–671. [Google Scholar] [CrossRef]
- Zheng, H.X.; Zhang, T.H.; Zhang, M.; Zhang, X.H.; Jia, B.L. Identification of reference genes and expression analysis of heat shock protein 70 gene in the Chinese bean weevil Callosobruchus chinensis under high temperature stress. J. Plant Protect. 2022, 49, 799–808. [Google Scholar]
- Liu, Z.H.; Zhong, J.; Yang, W.K.; Li, Q.Y.; Chen, A.L.; Dong, Z.P. Cloning of heat shock protein 90 gene in Antheraea assamensis and its expression characteristics under high or low temperature stress. Sci. Sericult. 2020, 46, 59–66. [Google Scholar]
- Wang, J.; Que, S.Q.; Liu, X.; Jin, M.; Xin, T.R.; Zou, Z.W.; Xia, B. Characteristic and expression of Hsp70 and Hsp90 genes from Tyrophagus putrescentiae and their response to thermal stress. Sci. Rep. 2021, 11, 11672. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Wang, D.M.; Ji, R. Changes in the contents of stress resistant substances in Gomphocerus sibiricus (Orthoptera: Acrididae) under high temperature stress. Acta Entomol. Sin. 2014, 57, 1155–1161. [Google Scholar]
- Jiang, S.; Li, S.; Zhang, B.; Li, H.G.; Wan, F.H.; Zheng, C.Y. Effects of extreme high temperature on survival rate, reproduction, trehalose and sorbitol of Frankliniella occidentalis. Sci. Agric. Sin. 2016, 49, 2310–2321. [Google Scholar]
- Ma, X.J.; Zhang, Z.H.; Wang, Z.; Zhang, Y.X.; Chen, J. Effects of brief exposure to high temperature on free amino acids, total sugar and trehalose of female adult Monolepta hieroglyphica (Motschulsky). Plant Protect. 2018, 44, 111–115. [Google Scholar]
- Wei, S.H.; Wu, X.Z.; Wang, Y.; Ma, Z.N.; Gao, L.Y.; Huang, W.G.; Zhang, R. Cold resistance, high temperature tolerance and resistance mechanism of grasshopper Chorthippus albonemus. J. Plant Protect. 2021, 48, 172–178. [Google Scholar]
- Dang, Y.Q.; Wang, X.Y.; Zhang, Y.L.; Wei, K.; Cao, L.M. Physiological responses of Agrilus planipennis adults to short-time high-temperature conditions. Sci. Silvae Sin. 2023, 59, 112–120. [Google Scholar]
- Zhang, J.; Liao, T.L.; Xie, K.; Miao, W.; Yu, C.L.; Song, L.W. Effect of different frequencies of high-temperature days on saccharides content in Tetranychustruncatus. J. Gansu Agric. Univ. 2023, 58, 110–116. [Google Scholar]
- Gui, H.M.; Xia, T.; Wang, S.J.; Wu, G.X.; Zhang, Z.B.; Zhu, G.Y.; Zhang, Y.K.; Gao, X. Effects of temperature on the growth, development and reproduction of Noorda blitealis Walker. J. So. Agr. 2021, 52, 3400–3407. [Google Scholar]
- Kutcherov, D. Stagewise resolution of temperature-dependent embryonic and postembryonic development in the cowpea seed beetle Callosobruchus maculatus. BMC Ecol. 2020, 20, 50. [Google Scholar] [CrossRef] [PubMed]
- Meikle, W.G.; Patt, J.M. The effects of temperature, diet, and other factors on development, survivorship, and oviposition of Aethina tumida (Coleoptera: Nitidulidae). J. Econ. Entomol. 2011, 104, 753–763. [Google Scholar] [CrossRef]
- Luo, Z.X.; Ren, L.L.; Qi, L.Y.; Zhou, S.D.; Dai, H.G. Effects of temperature on the development of Bactrocera dorsalis (Diptera: Tephritidae) population. Chin. J. Ecol. 2009, 28, 921–924. [Google Scholar]
- Xu, C.F.; Luo, D.; Yin, L.X.; Liu, X.P. Influence of temperature on the development and reproduction of the camphor sawfly, Mesoneura rufonota (Hymenoptera:Tenthredinidae). Acta Entomol. Sin. 2017, 60, 1216–1225. [Google Scholar]
- Liu, Y.H.; Yan, X.F.; Li, X.H.; Gao, F. Effects of short-term high temperature on the growth, development and reproduction of Acleris fimbriana Thunberg. Shaanxi J. Agr. Sci. 2020, 66, 4–6. [Google Scholar]
- Li, X.; Fu, Y.G.; Chen, J.Y.; Wang, J.Y.; Zhu, J.H.; Zhang, F.P. Effects of temperature and photoperiod on the development and reproduction of endoparasitoid wasp Coccophagus japonicus Compere. J. Plant Protect. 2021, 48, 848–854. [Google Scholar]
- Xiong, B.; Li, Z.J.; Tang, R.X.; Liu, X.X.; Mao, G.; Li, Z. Impacts of high temperature on the development and parasitic capacity of pine caterpillar miunte egg parasite Trichogramma dendrolimi. J. Plant Protect. 2022, 49, 637–643. [Google Scholar]
- Guo, H.Z.; Jiang, L.; Xia, Q.Y. Selection of reference genes for analysis of stress-responsive genes after challenge with viruses and temperature changes in the silkworm Bombyx mori. Mol. Genet. Genom. 2016, 291, 999–1004. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Aruda, A.M.; Baumgartner, M.F.; Reitzel, A.M.; Tarrant, A.M. Heat shock protein expression during stress and diapause in the marine copepod Calanus finmarchicus. J. Insect Physiol. 2011, 57, 665–675. [Google Scholar] [CrossRef]
- Jiang, X.F.; Zhai, H.F.; Wang, L.; Luo, L.Z.; Sappington, T.W.; Zhang, L. Cloning of the heat shock protein 90 and 70 genes from the beet armyworm, Spodoptera exigua, and expression characteristics in relation to thermal stress and development. Cell Stress Chaperon. 2012, 17, 67–80. [Google Scholar] [CrossRef]
- Xiang, M.; Hu, H.X.; Yu, F.; Ji, R.; Wang, H. Effects of brief exposure to high temperatures on HSP70 expression in oogenesis of Calliptamus italicus. Pratacult. Sci. 2017, 34, 1299–1305. [Google Scholar]
- González-Tokman, D.; Córdoba-Aguilar, A.; Dáttilo, W.; Lira-Noriega, A.; Sánchez-Guillén, R.A.; Villalobos, F. Insect responses to heat: Physiological mechanisms, evolution and ecological implications in a warming world. Biol. Rev. Camb. Philos. Soc. 2020, 95, 802–821. [Google Scholar] [CrossRef]
- Yang, Q.; Lu, Y.H. RNAi-mediated mediating of P-Hsp70 gene expression affects Propylaea quatuordecimpunctata longevity and fecundity under different temperatures. Chin. J. Appl. Entomol. 2024, 61, 9–15. [Google Scholar]
- Liu, J.P.; Liu, Y.; Li, Q.; Lu, Y.H. Heat shock protein 70 and Cathepsin B genes are involved in the thermal tolerance of Aphis gossypii. Pest Manag. Sci. 2023, 79, 2075–2086. [Google Scholar] [CrossRef]
- Jin, J.S.; Zhao, M.T.; Wang, Y.; Zhou, Z.S.; Wan, F.H.; Guo, J.Y. Induced thermotolerance and expression of three key Hsp genes (Hsp70, Hsp21, and sHsp21) and their roles in the high temperature tolerance of Agasicles hygrophila. Front. Physiol. 2020, 10, 1593. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, S.R.; Jeong, C.R.; Park, J.W.; Cho, S.S.; Kim, S.J. A review on the processing of functional proteins or peptides derived from fish by-products and their industrial applications. Heliyon 2023, 9, e14188. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.H.; Zhang, L.L.; Zhang, M.; Liu, Z.G.; Zheng, L.; Sun, L.L.; Sun, Z. Physiological mechanism of mitigation of high temperature stress by peptide-chelated iron application in cherry radish seedling. J. Plant Nutr. Fertil. 2019, 25, 1393–1400. [Google Scholar]
- Ozawa, H.; Miyazawa, T.; Burdeos, G.C.; Miyazawa, T. Biological functions of antioxidant dipeptides. J. Nutr. Sci. Vitaminol. 2022, 68, 162–171. [Google Scholar] [CrossRef]
- Zou, J.B.; Sang, W.T.; Wang, F.; Yang, S.Q.; Zhang, T.; Zeng, N. Protective effect of Periplaneta americana extract on mice with ethanol-induced acute gastric ulcer. Chin. Trad. Pat. Med. 2016, 38, 2325–2331. [Google Scholar]
- Sun, R.X.; Tang, Y.P.; Yang, L.L.; Fu, R.; Wang, J.Y.; Sui, S.Y. Inhibition of SPPA pretreatment on H2O2-induced oxidative stress and apoptosis in human ovarian granulosa cells. Shandong Med. J. 2023, 63, 1–4+14. [Google Scholar]
- Wang, W.Y.; Mejia, E.G.D. A New frontier in soy bioactive peptides that may prevent age-related chronic diseases. Compr. Rev. Food. Sci. F. 2005, 4, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Chen, F.; Wang, X.; Ji, B.P.; Wu, Y.N. Isolation and identification of antioxidative peptides from porcine collagen hydrolysate by consecutive chromatography and electrospray ionization–mass spectrometry. Food Chem. 2007, 102, 1135–1143. [Google Scholar] [CrossRef]
- Alemán, A.; Giménez, B.; Pérez-Santin, E.; Gómez-Guillén, M.C.; Montero, P. Contribution of Leu and Hyp residues to antioxidant and ACE-inhibitory activities of peptide sequences isolated from squid gelatin hydrolysate. Food Chem. 2011, 125, 334–341. [Google Scholar] [CrossRef]
- Nollen, E.A.; Morimoto, R.I. Chaperoning signaling pathways:molecular chaperones as stress-sensing ‘heat shock’ proteins. J. Cell Sci. 2002, 115, 2809–2816. [Google Scholar] [CrossRef]
- Wang, F.; She, X.X.; Sun, B.J.; Du, J.C.; Zhang, J.W.; Ren, D.F.; Lu, J. Study on the optimized extraction and in vitro antioxidant activities of γ-Linolenic acid from Spirulina platensis. Sci. Technol. Food Ind. 2014, 35, 68–72. [Google Scholar]
- You, Y.; Zhang, W.J.; Xu, R.; Zhou, Y.F.; Lv, Y.K.; Zhang, H.; Wu, G.C.; Wang, X.G. Research progress on the enrichment of γ-linolenic acid and its physiological functions. Sci. Technol. Cereals Oils Foods 2024, 32, 101–108. [Google Scholar]
- Ogawa, H.; Gomi, T.; Takusagawa, F.; Fujioka, M. Structure, function and physiological role of glycine N-methyltransferase. Int. J. Biochem. Cell Biol. 1998, 30, 13–26. [Google Scholar] [CrossRef]
- Haubrich, D.R.; Gerber, N.H. Choline dehydrogenase: Assay, properties and inhibitors. Biochem. Pharmacol. 1998, 30, 2993–3000. [Google Scholar] [CrossRef]
- Arumugam, M.K.; Paal, M.C.; Donohue, T.M.; Ganesan, M.; Osna, N.A.; Kharbanda, K.K. Beneficial effects of betaine: A comprehensive review. Biology 2021, 10, 456. [Google Scholar] [CrossRef] [PubMed]



| Gene ID | log2Fold Change | KEGG Annotation | ||
|---|---|---|---|---|
| CK vs. CG | CK vs. GW | CG vs. GW | ||
| BMSK0007389 | 2.88 | 4.47 | 1.59 | glycine N-methyltransferase |
| BMSK0014190 | 2.10 | 3.53 | 1.43 | choline dehydrogenase |
| BMSK0006069 | 1.29 | 2.02 | 0.74 | heat shock protein 68 isoform X1 |
| BMSK0012507 | 4.05 | 5.52 | 1.49 | heat shock protein 68 |
| BMSK0015592 | 2.74 | 2.90 | 0.17 | heat shock protein 19.9 |
| BMSK0015594 | 2.77 | 3.37 | 0.62 | heat shock protein 20.8 |
| BMSK0015595 | 2.83 | 3.78 | 0.97 | heat shock protein 20.4 |
| BMSK0015668 | 2.59 | 3.60 | 1.03 | heat shock protein 70 A1 |
| BMSK0015669 | 3.70 | 5.67 | 1.99 | heat shock protein 70 |
| BMSK0015670 | 3.88 | 5.04 | 1.17 | heat shock protein 70 |
| BMSK0015671 | 3.88 | 5.44 | 1.58 | heat shock protein 68 |
| BMSK0015711 | 2.25 | 2.94 | 0.70 | heat shock protein 23.7 precursor |
| Metabolite ID | log2Fold Change | Compound Annotations | ||
|---|---|---|---|---|
| CK vs. CG | CK vs. GW | CG vs. GW | ||
| MW0158501 | 0.85 | 2.09 | 1.24 | Tyr-Ile-His |
| MW0110745 | 0.87 | 1.80 | 0.93 | 2-Hexadecanoylthio-1-ethylphosphorylcholine |
| MW0014926 | 0.74 | 1.65 | 0.91 | N-(6-Aminohexanoyl)-6-aminohexanoate |
| MW0154890 | 0.25 | 1.08 | 0.83 | Pantetheine |
| MW0109139 | 1.87 | 2.58 | 0.70 | Phenylalanyltyrosine |
| MW0158567 | 0.65 | 1.20 | 0.55 | Tyr-Phe-Glu-Lys |
| MEDP1869 | 0.47 | 0.97 | 0.50 | Histidylleucine |
| MW0052451 | −2.01 | −4.43 | −2.42 | Docosahexaenoic Acid-d5 |
| MEDP1388 | −0.53 | −2.65 | −2.12 | Carnitine C18:3-OH |
| MEDL02638 | −0.39 | −2.26 | −1.87 | Methylephedrine |
| MW0015407 | −1.88 | −3.72 | −1.84 | (9S,10S,12Z)-9,10-Dihydroxy-12-octadecenoic acid |
| MW0052740 | −1.05 | −2.50 | −1.45 | Ethyl tetradecanoate |
| MW0012192 | −0.96 | −2.38 | −1.43 | (9Z,12S,13R)-12,13-Dihydroxy-9-octadecenoic acid |
| MW0116986 | −1.17 | −2.24 | −1.07 | Cetylpyridinium |
| MW0006389 | −1.31 | −2.34 | −1.03 | Bisphenol A diglycidyl ether |
| MW0150223 | −1.70 | −2.31 | −0.61 | Glu-Val-Tyr-Asp |
| MW0144237 | −0.80 | −1.39 | −0.60 | Ac-DEVD-CHO |
| MW0144166 | −0.86 | −1.38 | −0.52 | 9-keto Fluprostenol |
| MW0139561 | −1.53 | −2.05 | −0.52 | Resveratrol-3-O-sulfate |
| MW0052998 | −3.56 | −2.73 | 0.83 | Gamma-Linolenic Acid |
| MW0069424 | −0.99 | −0.09 | 0.90 | Triglyceride |
| Gene (ID) | Compounds (ID) | Coefficient Rate | p Value |
|---|---|---|---|
| Hsp68 (BMSK0012507) | Tyr-Ile-His (MW0158501) | 0.84 | 5.58 × 10−4 |
| Phenylalanyltyrosine (MW0109139) | 0.84 | 5.86 × 10−4 | |
| Tyr-Phe-Glu-Lys (MW0158567) | 0.96 | 5.36 × 10−7 | |
| Histidylleucine (MEDP1869) | 0.96 | 1.27 × 10−6 | |
| Hsp70 A1 (BMSK0015668) | Tyr-Ile-His (MW0158501) | 0.85 | 4.35 × 10−4 |
| Phenylalanyltyrosine (MW0109139) | 0.89 | 1.25 × 10−4 | |
| Tyr-Phe-Glu-Lys (MW0158567) | 0.98 | 6.11 × 10−9 | |
| Histidylleucine (MEDP1869) | 0.96 | 7.14 × 10−7 | |
| Hsp70 (BMSK0015669) | Tyr-Ile-His (MW0158501) | 0.84 | 6.10 × 10−4 |
| Tyr-Phe-Glu-Lys (MW0158567) | 0.92 | 1.66 × 10−5 | |
| Histidylleucine (MEDP1869) | 0.92 | 2.42 × 10−5 | |
| Hsp70 (BMSK0015670) | Tyr-Ile-His (MW0158501) | 0.88 | 1.50 × 10−4 |
| Phenylalanyltyrosine (MW0109139) | 0.90 | 8.01 × 10−5 | |
| Tyr-Phe-Glu-Lys (MW0158567) | 0.97 | 2.43 × 10−7 | |
| Histidylleucine (MEDP1869) | 0.92 | 2.94 × 10−5 | |
| Hsp68 (BMSK0015671) | Tyr-Ile-His (MW0158501) | 0.88 | 1.87 × 10−4 |
| Phenylalanyltyrosine (MW0109139) | 0.84 | 6.58 × 10−4 | |
| Tyr-Phe-Glu-Lys (MW0158567) | 0.94 | 3.85 × 10−6 | |
| Histidylleucine (MEDP1869) | 0.90 | 6.00 × 10−5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Y.; Li, Q.; Sun, Z.; Fu, B.; Yang, Q.; Jiang, M.; Zhang, W.; Huang, X.; Xing, D. Transcriptomics and Metabolomics Combined to Analyze the Response Mechanism of Silkworm Eggs to High-Temperature Stress. Insects 2025, 16, 862. https://doi.org/10.3390/insects16080862
Xiao Y, Li Q, Sun Z, Fu B, Yang Q, Jiang M, Zhang W, Huang X, Xing D. Transcriptomics and Metabolomics Combined to Analyze the Response Mechanism of Silkworm Eggs to High-Temperature Stress. Insects. 2025; 16(8):862. https://doi.org/10.3390/insects16080862
Chicago/Turabian StyleXiao, Yang, Qingrong Li, Zhenbo Sun, Bing Fu, Qiong Yang, Mangui Jiang, Weilong Zhang, Xuhua Huang, and Dongxu Xing. 2025. "Transcriptomics and Metabolomics Combined to Analyze the Response Mechanism of Silkworm Eggs to High-Temperature Stress" Insects 16, no. 8: 862. https://doi.org/10.3390/insects16080862
APA StyleXiao, Y., Li, Q., Sun, Z., Fu, B., Yang, Q., Jiang, M., Zhang, W., Huang, X., & Xing, D. (2025). Transcriptomics and Metabolomics Combined to Analyze the Response Mechanism of Silkworm Eggs to High-Temperature Stress. Insects, 16(8), 862. https://doi.org/10.3390/insects16080862





