Effects of Parasitism and Venom from the Endoparasitoid Brachymeria lasus on Immunity of the Host Galleria mellonella
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Collection, Rearing, and Parasitization
2.2. Venom Collection
2.3. Hemocyte Quantification, Mortality Assessment, and Cell Spreading Assay
2.4. Reactive Oxygen Species (ROS) Quantification
2.5. Intracellular Calcium Ion Detection
2.6. Caspase-3 Activity and Mitochondrial Membrane Potential (MMP) Assay
2.7. Hemocyte Encapsulation Assay
2.8. Melanization Assay
2.9. Data Analysis
3. Results
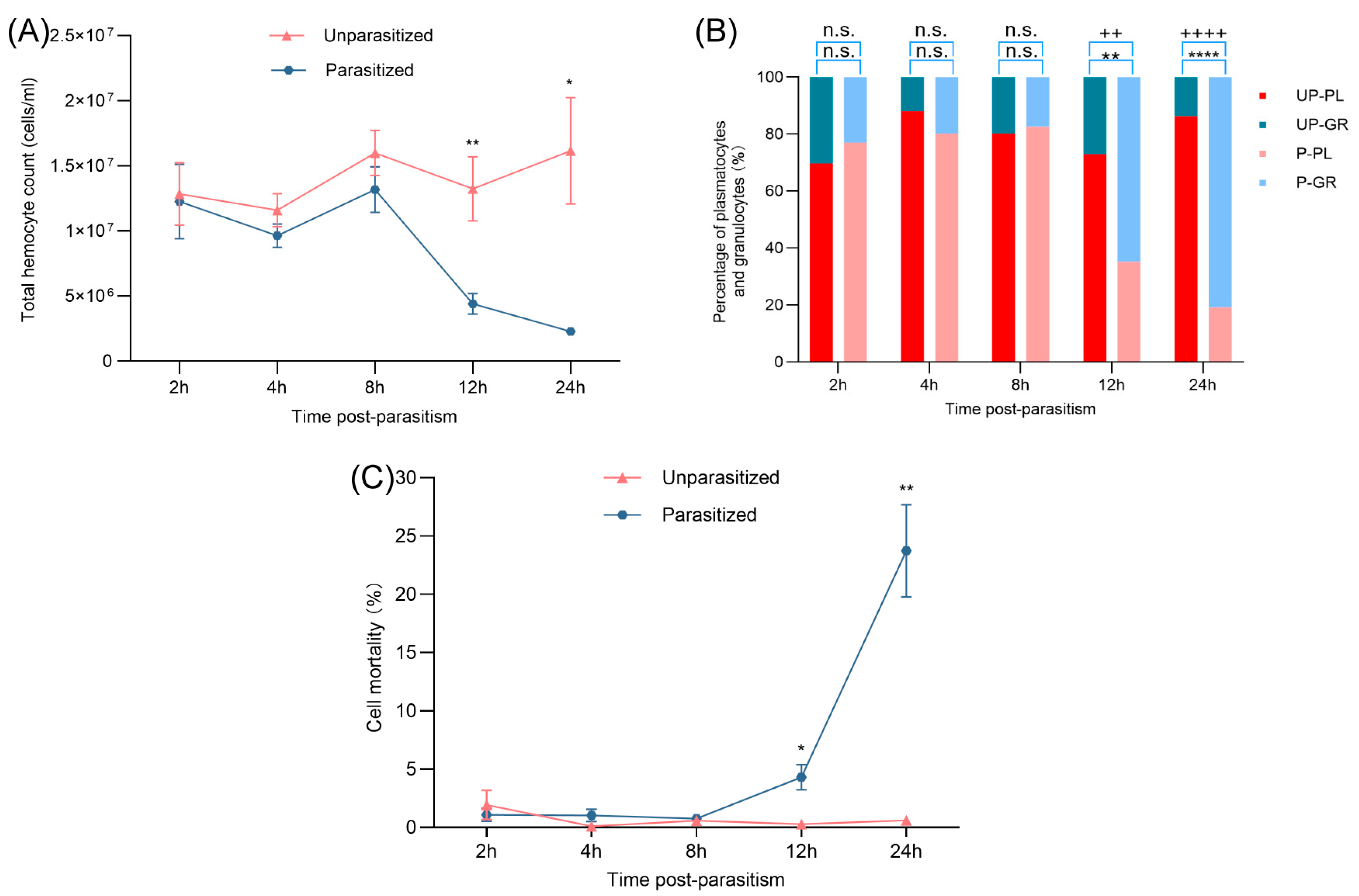
3.1. Effects of Parasitism on THCs, DHCs, and Mortality
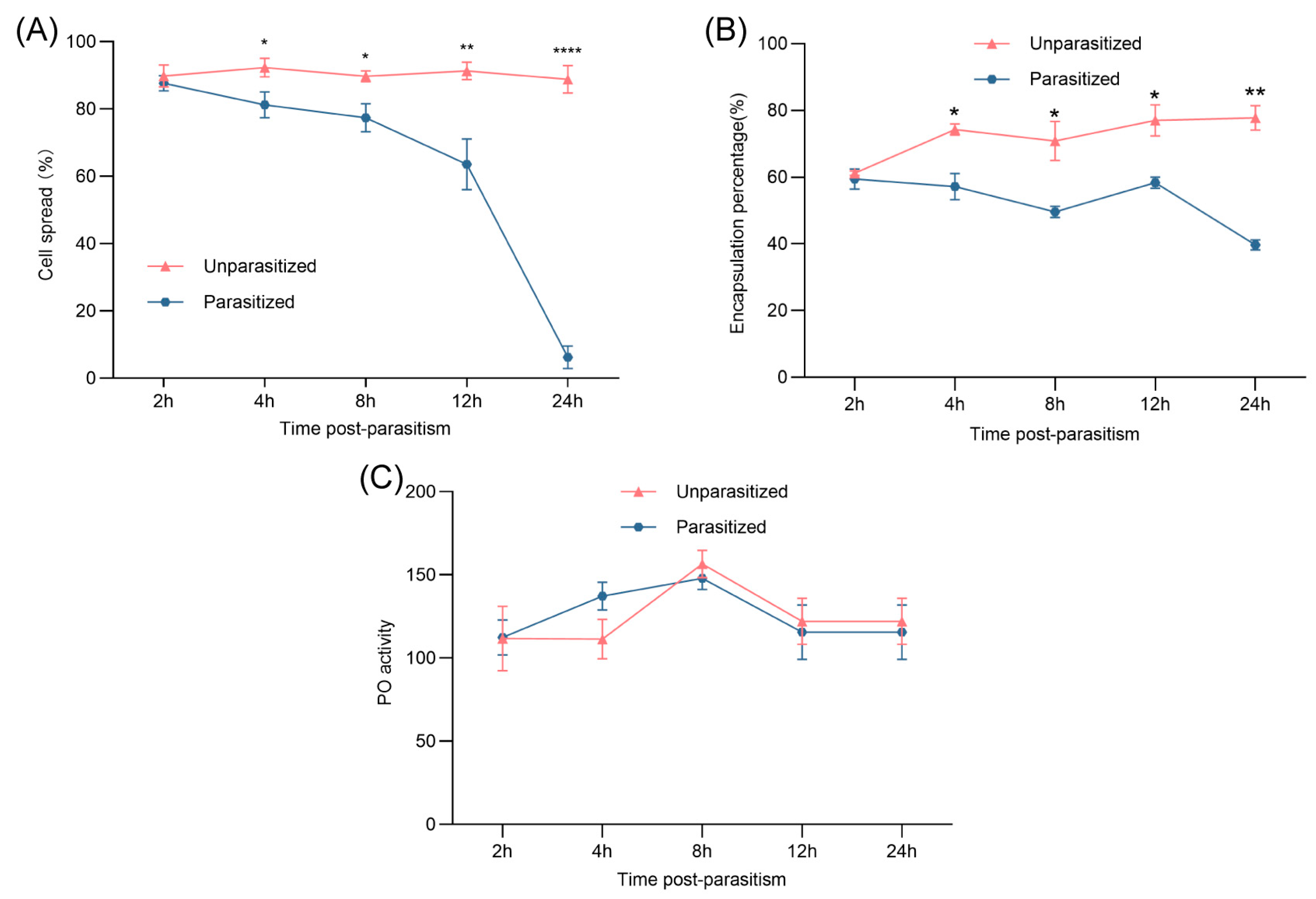
3.2. Effects of Parasitism on Hemocyte Spreading, Encapsulation, and Melanin Production
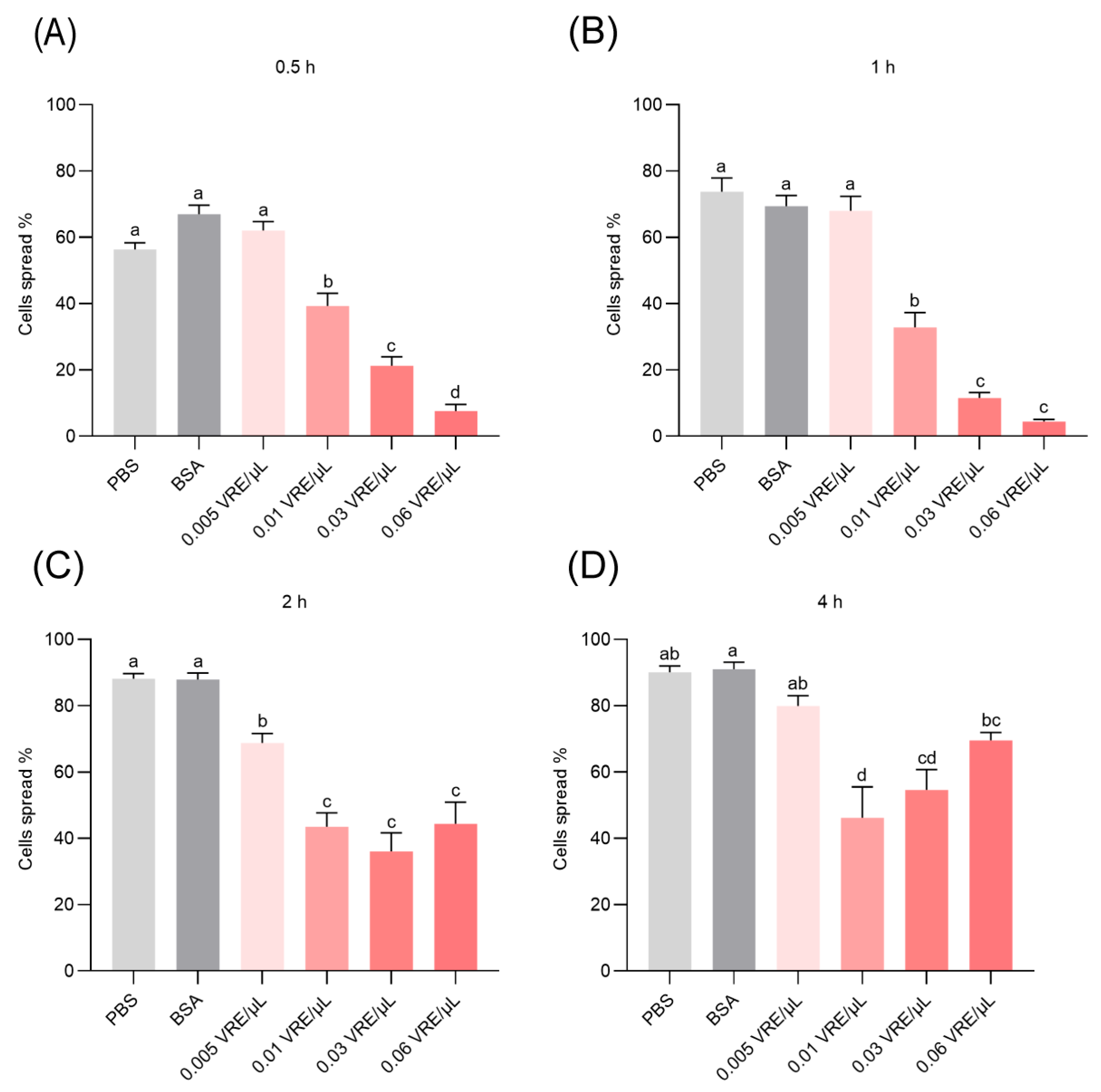
3.3. Effects of Venom on Hemocyte Spreading Behavior
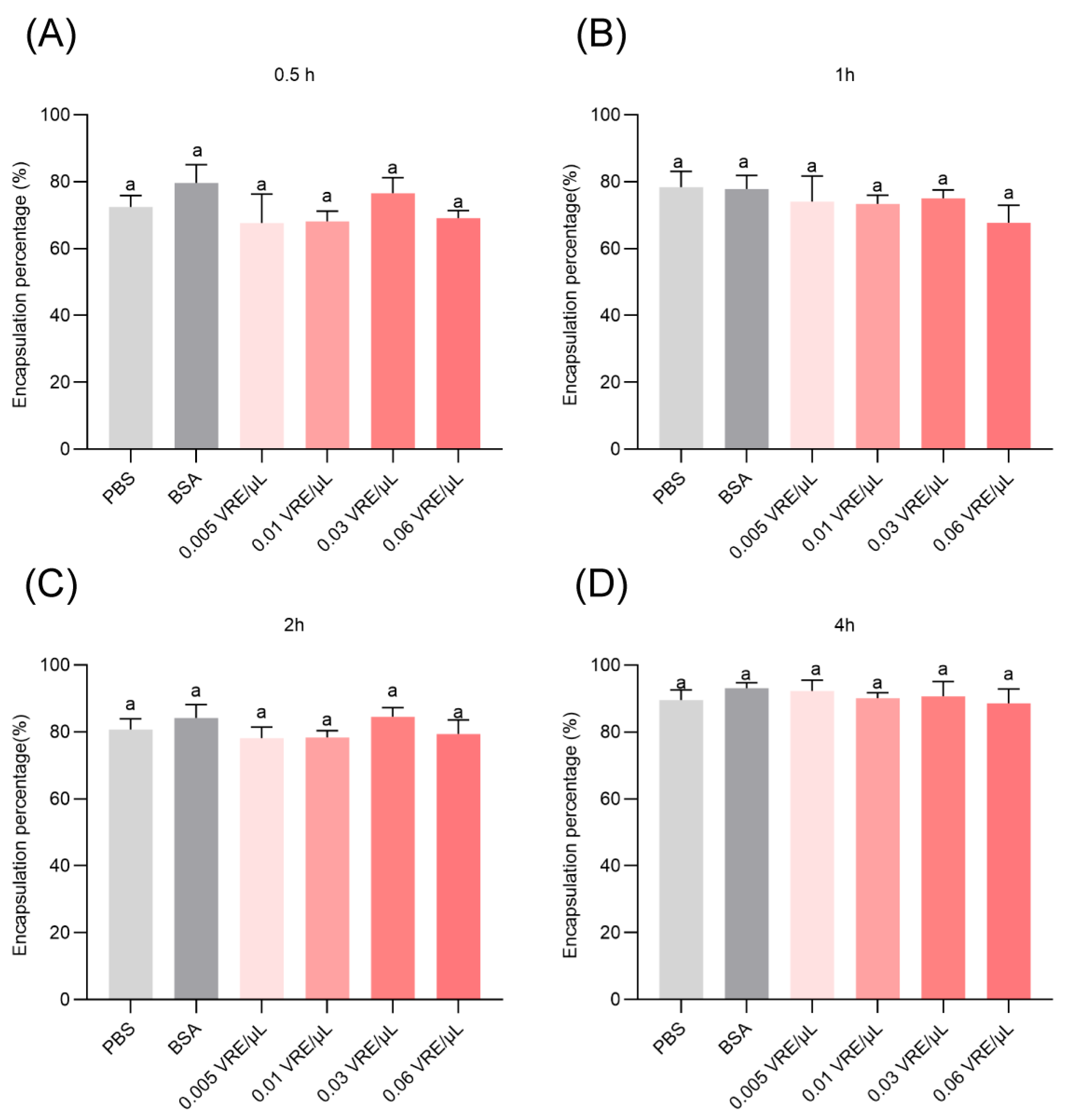
3.4. Effects of Venom on Encapsulation Response
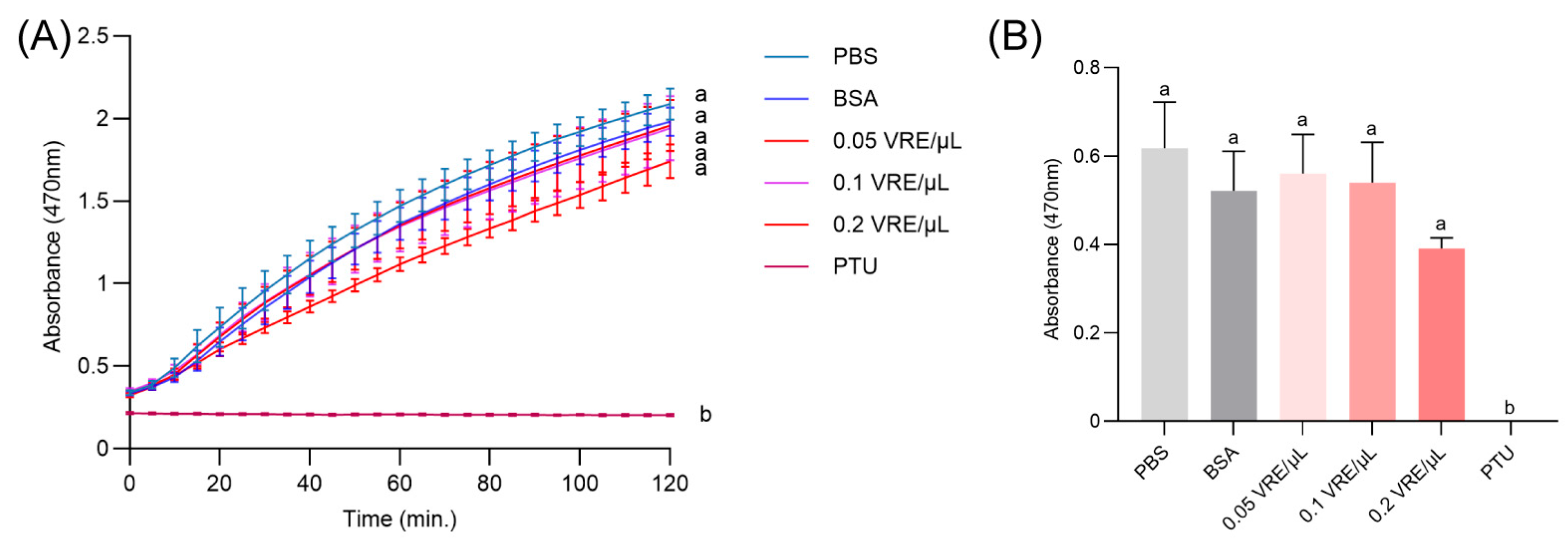
3.5. Effects of Venom on Melanin Formation
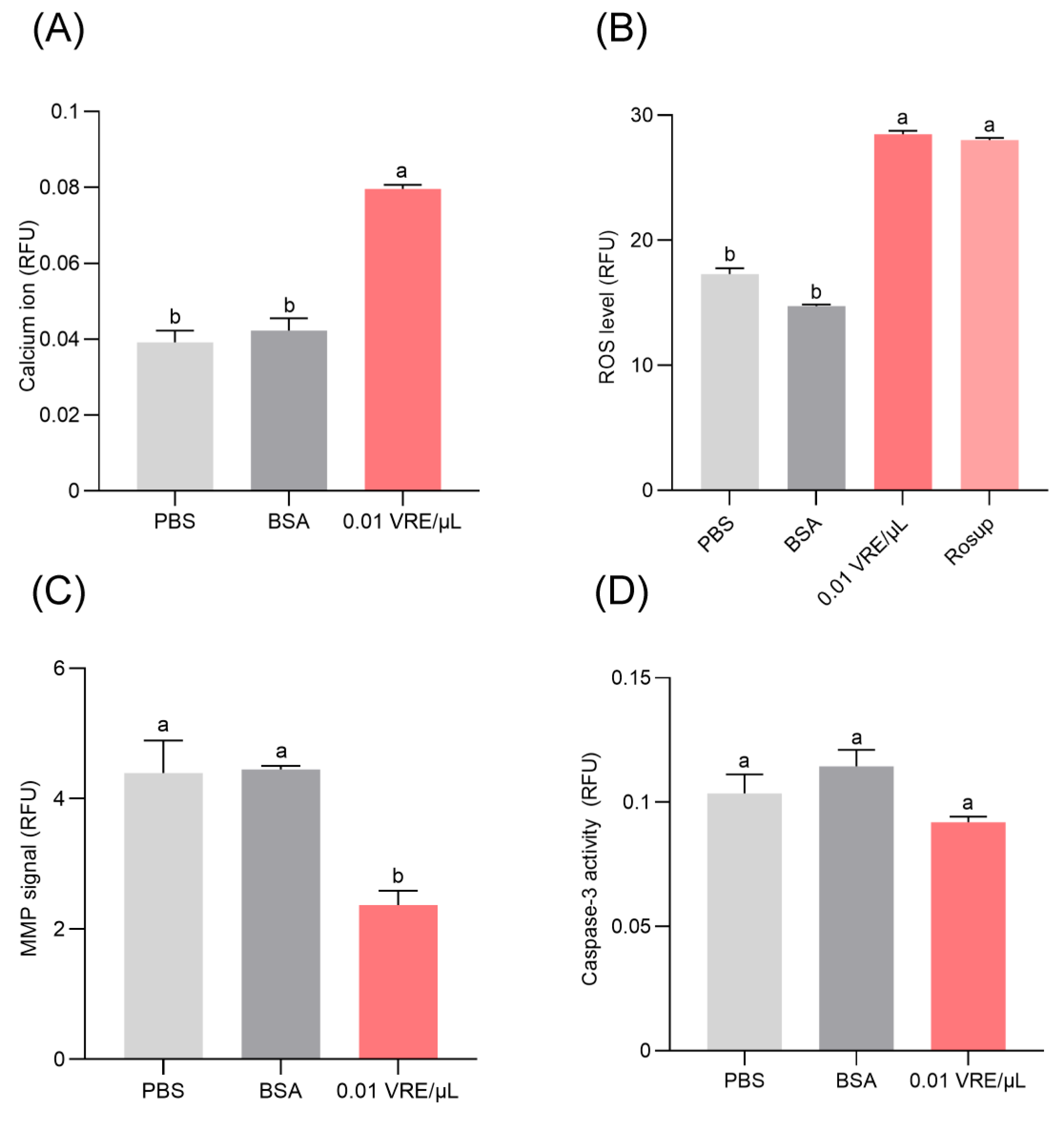
3.6. Effects of Venom on Hemocyte Death and Cell Death Mechanisms
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Condon, M.A.; Scheffer, S.J.; Lewis, M.L.; Wharton, R.; Adams, D.C.; Forbes, A.A. Lethal interactions between parasites and prey increase niche diversity in a tropical community. Science 2014, 343, 1240–1244. [Google Scholar] [CrossRef]
- Asgari, S.; Rivers, D.B. Venom proteins from endoparasitoid wasps and their role in host-parasite interactions. Annu. Rev. Entomol. 2011, 56, 313–335. [Google Scholar] [CrossRef]
- Burke, G.R.; Sharanowski, B.J. Parasitoid wasps. Curr. Biol. 2024, 34, R483–R488. [Google Scholar] [CrossRef]
- Mrinalini; Werren, J.H. Parasitoid wasps and their venoms. In Evolution of Venomous Animals and Their Toxins; Malhotra, A., Ed.; Springer: Dordrecht, The Netherlands, 2017; pp. 187–212. ISBN 978-94-007-6457-6. [Google Scholar]
- Quicke, D.L.J.; Butcher, B.A. Review of venoms of non-polydnavirus carrying ichneumonoid wasps. Biology 2021, 10, 50. [Google Scholar] [CrossRef]
- Kosman, E.S.; Yaroslavtseva, O.N.; Kryukova, N.A.; Rotskaya, U.N.; Glupov, V.V.; Kryukov, V.Y. Wax moth larvae demonstrate a high level of humoral immunity after envenomation by parasitoid Habrobracon hebetor. Entomol. Exp. Appl. 2025, 173, 351–360. [Google Scholar] [CrossRef]
- Kamiyama, T.; Shimada-Niwa, Y.; Mori, H.; Tani, N.; Takemata-Kawabata, H.; Fujii, M.; Takasu, A.; Katayama, M.; Kuwabara, T.; Seike, K.; et al. Parasitoid wasp venoms degrade Drosophila imaginal discs for successful parasitism. Sci. Adv. 2025, 11, eadq8771. [Google Scholar] [CrossRef]
- Beckage, N.E. Polydnaviruses as endocrine regulators. In Parasitoid viruses; Elsevier: Amsterdam, The Netherlands, 2012; pp. 163–168. ISBN 978-0-12-384858-1. [Google Scholar]
- Wang, Z.Z.; Ye, X.Q.; Shi, M.; Li, F.; Wang, Z.H.; Zhou, Y.N.; Gu, Q.J.; Wu, X.T.; Yin, C.L.; Guo, D.H.; et al. Parasitic insect-derived miRNAs modulate host development. Nat. Commun. 2018, 9, 2205. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Zhou, Y.N.; Yang, J.; Ye, X.Q.; Shi, M.; Huang, J.H.; Chen, X.X. Genome-wide profiling of Diadegma semiclausum Ichnovirus integration in parasitized Plutella xylostella hemocytes identifies host integration motifs and insertion sites. Front. Microbiol. 2021, 11, 608346. [Google Scholar] [CrossRef]
- Strand, M.R. Polydnavirus gene products that interact with the host immune system. In Parasitoid Viruses; Elsevier: Amsterdam, The Netherlands, 2012; pp. 149–161. ISBN 978-0-12-384858-1. [Google Scholar]
- Suzuki, M.; Tanaka, T. Virus-like particles in venom of Meteorus pulchricornis induce host hemocyte apoptosis. J. Insect Physiol. 2006, 52, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Salvia, R.; Scieuzo, C.; Grimaldi, A.; Fanti, P.; Moretta, A.; Franco, A.; Varricchio, P.; Vinson, S.B.; Falabella, P. Role of ovarian proteins secreted by Toxoneuron nigriceps (Viereck) (Hymenoptera, Braconidae) in the early suppression of host immune response. Insects 2021, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Salvia, R.; Cozzolino, F.; Scieuzo, C.; Grimaldi, A.; Franco, A.; Vinson, S.B.; Monti, M.; Falabella, P. Identification and functional characterization of Toxoneuron nigriceps ovarian proteins involved in the early suppression of host immune response. Insects 2022, 13, 144. [Google Scholar] [CrossRef]
- Strand, M.R. Teratocytes and their functions in parasitoids. Curr. Opin. Insect Sci. 2014, 6, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.P.G.; Walker, A.A.; Robinson, S.D.; King, G.F.; Rossi, G.D. Proteotranscriptomics reveals the secretory dynamics of teratocytes, regulators of parasitization by an endoparasitoid wasp. J. Insect Physiol. 2022, 139, 104395. [Google Scholar] [CrossRef]
- Wu, Z.W.; Yuan, R.Z.; Gu, Q.J.; Wu, X.T.; Gu, L.C.; Ye, X.Q.; Zhou, Y.A.; Huang, J.H.; Wang, Z.Z.; Chen, X.X. Parasitoid serpins evolve novel functions to manipulate host homeostasis. Mol. Biol. Evol. 2023, 40, msad269. [Google Scholar] [CrossRef]
- Russo, E.; Di Lelio, I.; Shi, M.; Becchimanzi, A.; Pennacchio, F. Aphidius ervi venom regulates Buchnera contribution to host nutritional suitability. J. Insect Physiol. 2023, 147, 104506. [Google Scholar] [CrossRef]
- Scieuzo, C.; Salvia, R.; Franco, A.; Pezzi, M.; Cozzolino, F.; Chicca, M.; Scapoli, C.; Vogel, H.; Monti, M.; Ferracini, C.; et al. An integrated transcriptomic and proteomic approach to identify the main Torymus sinensis venom components. Sci. Rep. 2021, 11, 5032. [Google Scholar] [CrossRef]
- Nakamatsu, Y.; Tanaka, T. Venom of ectoparasitoid, Euplectrus sp. near plathypenae (Hymenoptera: Eulophidae) regulates the physiological state of Pseudaletia separata (Lepidoptera: Noctuidae) host as a food resource. J. Insect Physiol. 2003, 49, 149–159. [Google Scholar] [CrossRef]
- Wu, C.Y.; Huang, J.M.; Zhao, Y.J.; Xu, Z.W.; Zhu, J.Y. Venom serine proteinase homolog of the ectoparasitoid Scleroderma guani impairs host phenoloxidase cascade. Toxicon 2020, 183, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.C.; Fang, Q.; Liu, Y.; Xiao, S.; Yang, L.; Wang, F.; An, C.J.; Werren, J.H.; Ye, G.Y. A venom serpin splicing isoform of the endoparasitoid wasp Pteromalus puparum suppresses host prophenoloxidase cascade by forming complexes with host hemolymph proteinases. J. Biol. Chem. 2017, 292, 1038–1051. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.L.; Chen, J.; Bai, X.; Xiong, S.J.; Ye, X.H.; Yang, Y.; Yao, H.W.; Wang, F.; Fang, Q.; Song, Q.S.; et al. Multi-omic identification of venom proteins collected from artificial hosts of a parasitoid wasp. Toxins 2023, 15, 377. [Google Scholar] [CrossRef]
- Cantori, L.V.; Garcia, A.G.; Pinto, A.D.; Godoy, W.A.C.; Parra, J.R.P. Detailed look at paralysis of hosts by the ectoparasitoid Habrobracon hebetor (Hymenoptera: Braconidae): Does more efficient paralysis mean more effective parasitism? BioControl 2022, 67, 555–562. [Google Scholar] [CrossRef]
- Zhang, X.M.; Zhang, H.J.; Liu, M.; Liu, B.; Zhang, X.F.; Ma, C.J.; Fu, T.T.; Hou, Y.M.; Tang, B.Z. Cloning and immunosuppressive properties of an acyl-activating enzyme from the venom apparatus of Tetrastichus brontispae (Hymenoptera: Eulophidae). Toxins 2019, 11, 672. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Lin, Z.; Volovych, O.; Lu, Z.Q.; Zou, Z. A RhoGAP venom protein from Microplitis mediator suppresses the cellular response of its host Helicoverpa armigera. Dev. Comp. Immunol. 2020, 108, 103675–103685. [Google Scholar] [CrossRef]
- Dani, M.P.; Richards, E.H. Cloning and expression of the gene for an insect haemocyte anti-aggregation protein (VPr3) from the venom of the endoparasitic wasp, Pimpla hypochondriaca. Arch. Insect Biochem. Physiol. 2009, 71, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Dani, M.P.; Richards, E.H. Identification, cloning, and expression of a second gene (vprI) from the venom of the endoparasitic wasp, Pimpla hypochondriaca that displays immunosuppressive activity. J. Insect Physiol. 2010, 56, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Burke, G.R.; Strand, M.R. Systematic analysis of a wasp parasitism arsenal. Mol. Ecol. 2014, 23, 890–901. [Google Scholar] [CrossRef]
- Strand, M.R. The insect cellular immune response. Insect Sci. 2008, 15, 1–14. [Google Scholar] [CrossRef]
- Zdybicka-Barabas, A.; Stączek, S.; Kunat-Budzyńska, M.; Cytryńska, M. Innate immunity in insects: The lights and shadows of phenoloxidase system activation. Int. J. Mol. Sci. 2025, 26, 1320. [Google Scholar] [CrossRef]
- Asgari, S.; Zhang, G.M.; Zareie, R.; Schmidt, O. A serine proteinase homolog venom protein from an endoparasitoid wasp inhibits melanization of the host hemolymph. Insect Biochem. Mol. Biol. 2003, 33, 1017–1024. [Google Scholar] [CrossRef]
- Zhou, L.Z.; Wang, R.J.; Lin, Z.; Shi, S.K.; Chen, C.H.; Jiang, H.B.; Zou, Z.; Lu, Z.Q. Two venom serpins from the parasitoid wasp Microplitis mediator inhibit the host prophenoloxidase activation and antimicrobial peptide synthesis. Insect Biochem. Mol. Biol. 2023, 152, 103895. [Google Scholar] [CrossRef]
- Richards, E.H.; Parkinson, N.M. Venom from the endoparasitic wasp Pimpla hypochondriaca adversely affects the morphology, viability, and immune function of hemocytes from larvae of the tomato moth, Lacanobia oleracea. J. Invert. Pathol. 2000, 76, 33–42. [Google Scholar] [CrossRef]
- Furihata, S.X.; Matsumoto, H.; Kimura, M.T.; Hayakawa, Y. Venom components of Asobara japonica impair cellular immune responses of host Drosophila melanogaster. Arch. Insect Biochem. Physiol. 2013, 83, 86–100. [Google Scholar] [CrossRef]
- Wang, J.L.; Yan, Z.C.; Xiao, S.; Wang, B.B.; Fang, Q.; Schlenke, T.; Ye, G.Y. Characterization of a cell death-inducing endonuclease-like venom protein from the parasitoid wasp Pteromalus puparum (Hymenoptera: Pteromalidae). Pest Manag. Sci. 2021, 77, 224–233. [Google Scholar] [CrossRef]
- Zhang, Z.; Ye, G.Y.; Cai, J.; Hu, C. Comparative venom toxicity between Pteromalus puparum and Nasonia vitripennis (Hymenoptera: Pteromalidae) toward the hemocytes of their natural hosts, non-target insects and cultured insect cells. Toxicon 2005, 46, 337–349. [Google Scholar] [CrossRef]
- Cai, J.; Ye, G.Y.; Hu, C. Parasitism of Pieris rapae (Lepidoptera: Pieridae) by a pupal endoparasitoid, Pteromalus puparum (Hymenoptera: Pteromalidae): Effects of parasitization and venom on host hemocytes. J. Insect Physiol. 2004, 50, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Rivers, D.B.; Ruggiero, L.; Hayes, M. The ectoparasitic wasp Nasonia vitripennis (Walker) (Hymenoptera: Pteromalidae) differentially affects cells mediating the immune response of its flesh fly host, Sarcophaga bullata Parker (Diptera: Sarcophagidae). J. Insect Physiol. 2002, 48, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Uçkan, F.; Er, A.; Ergin, E. Levels of encapsulation and melanization in Galleria mellonella (Lepidoptera: Pyralidae) parasitized and envenomated by Pimpla turionellae (Hymenoptera: Ichneumonidae). J. Appl. Entomol. 2010, 134, 718–726. [Google Scholar] [CrossRef]
- Mortimer, N.T.; Goecks, J.; Kacsoh, B.Z.; Mobley, J.A.; Bowersock, G.J.; Taylor, J.; Schlenke, T.A. Parasitoid wasp venom SERCA regulates Drosophila calcium levels and inhibits cellular immunity. Proc. Natl. Acad. Sci. USA 2013, 110, 9427–9432. [Google Scholar] [CrossRef]
- Kim-Jo, C.; Gatti, J.-L.; Poirié, M. Drosophila cellular immunity against parasitoid wasps: A complex and time-dependent process. Front. Physiol. 2019, 10, 603. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, B.B.; Qiu, L.M.; Wan, B.; Yang, Y.; Liu, M.M.; Wang, F.; Fang, Q.; Stanley, D.W.; Ye, G.Y. Functional characterization of a venom protein calreticulin in the ectoparasitoid Pachycrepoideus vindemiae. Insects 2019, 11, 29. [Google Scholar] [CrossRef]
- Kryukova, N.A.; Dubovskiy, I.M.; Chertkova, E.A.; Vorontsova, Y.L.; Slepneva, I.A.; Glupov, V.V. The Effect of Habrobracon hebetor venom on the activity of the prophenoloxidase system, the generation of reactive oxygen species and encapsulation in the haemolymph of Galleria mellonella larvae. J. Insect Physiol. 2011, 57, 796–800. [Google Scholar] [CrossRef]
- Zhang, G.M.; Schmidt, O.; Asgari, S. A calreticulin-like protein from endoparasitoid venom fluid is involved in host hemocyte inactivation. Dev. Comp. Immunol. 2006, 30, 756–764. [Google Scholar] [CrossRef]
- Eslin, P.; Prévost, G. Racing against Host’s Immunity Defenses: A likely strategy for passive evasion of encapsulation in Asobara tabida parasitoids. J. Insect Physiol. 2000, 46, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Kraaijeveld, A.R.; Alphen, J.M. Geographical variation in resistance of the parasitoid Asobara tabids against encapsulation by Drosophila melanogaster larvae: The mechanism explored. Physiol. Entomol. 1994, 19, 9–14. [Google Scholar] [CrossRef]
- Kraaijeveld, A.R.; Nowee, B.; Najem, R.W. Adaptive variation in host-selection behaviour of Asobara tabida, a parasitoid of Drosophila larvae. Funct. Ecol. 1995, 9, 113–118. [Google Scholar] [CrossRef]
- Eslin, P.; Giordanengo, P.; Fourdrain, Y.; Prévost, G. Avoidance of encapsulation in the absence of VLP by a braconid parasitoid of Drosophila larvae: An ultrastructural study. Can. J. Zool. 1996, 74, 2193–2198. [Google Scholar] [CrossRef]
- Teng, Z.W.; Xu, G.; Gan, S.Y.; Chen, X.; Fang, Q.; Ye, G.Y. Effects of the endoparasitoid Cotesia chilonis (Hymenoptera: Braconidae) parasitism, venom, and calyx fluid on cellular and humoral immunity of its host Chilo suppressalis (Lepidoptera: Crambidae) larvae. J. Insect Physiol. 2016, 85, 46–56. [Google Scholar] [CrossRef]
- Er, A.; Uçkan, F.; Rivers, D.B.; Ergin, E.; Sak, O. Effects of parasitization and envenomation by the endoparasitic wasp Pimpla turionellae (Hymenoptera: Ichneumonidae) on hemocyte numbers, morphology, and viability of its host Galleria mellonella (Lepidoptera: Pyralidae). Ann. Entomol. Soc. Am. 2010, 103, 273–282. [Google Scholar] [CrossRef]
- Tian, S.; Gu, T.Z.; Chen, C.; Zhao, X.D.; Liu, P.C.; Hao, D.J. The effects of temperature and host size on the development of Brachymeria lasus parasitising Hyphantria cunea. J. For. Res. 2021, 32, 401–407. [Google Scholar] [CrossRef]
- Husni; Kainoh, Y.; Honda, H. Effects of host pupal age on host preference and host suitability in Brachymeria lasus (Walker) (Hymenoptera: Chalcididae). Appl. Entomol. Zool. 2001, 36, 97–102. [Google Scholar] [CrossRef]
- Mao, H.X.; Kunimi, Y. Effects of temperature on the development and parasitism of Brachymeria lasus, a pupal parasitoid of Homona magnanima. Entomol. Exp. Appl. 1994, 71, 87–90. [Google Scholar] [CrossRef]
- Mao, H.X.; Kunimi, Y. Longevity and fecundity of Brachymeria lasus (Walker) (Hymenoptera: Chalcididae), a pupal parasitoid of the oriental tea tortrix, Homona magnanima Diakonoff (Lepidoptera: Tortricidae) under laboratory conditions. Appl. Entomol. Zool. 1994, 29, 237–243. [Google Scholar] [CrossRef]
- Kumar, Y.; Yadav, S. Seasonal incidence of greater wax moth, Galleria mellonella Linnaeus in Apis mellifera colonies in ecological condition of Hisar. J. Entomol. Zool. Stud. 2018, 6, 790–795. [Google Scholar]
- Sohail, M.; Aqueel, M.A.; Ellis, J.D.; Raza, A.M.; Ullah, S. Consumption, digestion, and utilization of beeswax by greater wax moths (Galleria mellonella L.). J. Apicult. Res. 2020, 59, 876–882. [Google Scholar] [CrossRef]
- García, N.L. The current situation on the international honey market. Bee World 2018, 95, 89–94. [Google Scholar] [CrossRef]
- Hosni, E.M.; Al-Khalaf, A.A.; Nasser, M.G.; Abou-Shaara, H.F.; Radwan, M.H. Modeling the potential global distribution of honeybee pest, Galleria mellonella under changing climate. Insects 2022, 13, 484. [Google Scholar] [CrossRef]
- Banfi, D.; Bianchi, T.; Mastore, M.; Brivio, M.F. Optimization of experimental infection of the animal model Galleria mellonella Linnaeus 1758 (Lepidoptera: Pyralidae) with the Gram-positive bacterium Micrococcus luteus. Insects 2024, 15, 618. [Google Scholar] [CrossRef]
- Cutuli, M.A.; Petronio, G.P.; Vergalito, F.; Magnifico, I.; Pietrangelo, L.; Venditti, N.; Di Marco, R. Galleria mellonella as a consolidated in vivo model hosts: New developments in antibacterial strategies and novel drug testing. Virulence 2019, 10, 527–541. [Google Scholar] [CrossRef] [PubMed]
- Wojda, I. Immunity of the greater wax moth Galleria mellonella. Insect Sci. 2017, 24, 342–357. [Google Scholar] [CrossRef]
- Stoepler, T.M.; Castillo, J.C.; Lill, J.T.; Eleftherianos, I. A Simple Protocol for Extracting Hemocytes from Wild Caterpillars. J. Vis. Exp. JoVE 2012, 69, e4173. [Google Scholar] [CrossRef]
- Mizerska-Dudka, M.; Andrejko, M. Galleria mellonella hemocytes destruction after infection with Pseudomonas aeruginosa. J. Basic Microbiol. 2014, 54, 232–246. [Google Scholar] [CrossRef]
- Ling, E.J.; Yu, X.Q. Cellular encapsulation and melanization are enhanced by immulectins, pattern recognition receptors from the tobacco hornworm Manduca sexta. Dev. Comp. Immunol. 2006, 30, 289–299. [Google Scholar] [CrossRef]
- Mabiala-Moundoungou, A.D.N.; Doury, G.; Eslin, P.; Cherqui, A.; Prévost, G. Deadly venom of Asobara japonica parasitoid needs ovarian antidote to regulate host physiology. J. Insect Physiol. 2010, 56, 35–41. [Google Scholar] [CrossRef]
- Labrosse, C.; Eslin, P.; Doury, G.; Drezen, J.M.; Poirié, M. Haemocyte changes in Drosophila melanogaster in response to long gland components of the parasitoid wasp Leptopilina boulardi: A Rho-GAP protein as an important factor. J. Insect Physiol. 2005, 51, 161–170. [Google Scholar] [CrossRef]
- Labrosse, C.; Stasiak, K.; Lesobre, J.; Grangeia, A.; Huguet, E.; Drezen, J.M.; Poirie, M. A RhoGAP protein as a main immune suppressive factor in the Leptopilina boulardi (Hymenoptera, Figitidae)—Drosophila melanogaster interaction. Insect Biochem. Mol. Biol. 2005, 35, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Stettler, P.; Trenczek, T.; Wyler, T.; Pfister-Wilhelm, R.; Lanzrein, B. Overview of parasitism associated effects on host haemocytes in larval parasitoids and comparison with effects of the egg-larval parasitoid Chelonus inanitus on its host Spodoptera littoralis. J. Insect Physiol. 1998, 44, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.Y.; Ofengeim, D. A guide to cell death pathways. Nat. Rev. Mol. Cell Biol. 2024, 25, 379–395. [Google Scholar] [CrossRef]
- Qian, S.E.; Long, Y.; Tan, G.L.; Li, X.G.; Xiang, B.; Tao, Y.G.; Xie, Z.Z.; Zhang, X.W. Programmed cell death: Molecular mechanisms, biological functions, diseases, and therapeutic targets. MedComm 2024, 5, e70024. [Google Scholar] [CrossRef] [PubMed]
| Treatment | Dose * | Time (Hour) ** | ||||||
|---|---|---|---|---|---|---|---|---|
| 0.5 | 1 | 2 | 4 | 8 | 12 | 24 | ||
| PBS | - | 0.16 ± 0.16 a | 0.45 ± 0.25 a | 0.41 ± 0.22 c | 0.63 ± 0.21 c | 1.88 ± 0.70 c | 1.65 ± 0.77 c | 5.65 ± 0.89 c |
| BSA | - | 1.38 ± 0.56 a | 1.17 ± 0.40 a | 1.27 ± 0.39 c | 1.15 ± 0.43 c | 1.64 ± 0.65 c | 2.65 ± 0.77 c | 6.18 ± 1.12 c |
| Venom | 0.005 | 0.40 ± 0.40 a | 0.53 ± 0.31 a | 1.32 ± 1.15 c | 1.82 ± 0.72 c | 41.28 ± 9.82 b | 86.96 ± 1.89 a | 96.57 ± 0.08 a |
| 0.01 | 0.52 ± 0.52 a | 1.26 ± 0.67 a | 1.56 ± 0.84 bc | 4.54 ± 0.51 bc | 57.60 ± 2.90 ab | 84.36 ± 1.85 ab | 92.34 ± 1.47 ab | |
| 0.03 | 0.0 ± 0.0 a | 1.36 ± 0.2 a | 2.41 ± 1.24 ab | 11.33 ± 1.1 ab | 63.26 ± 2.91 a | 81.65 ± 0.82 ab | 91.84 ± 0.41 ab | |
| 0.06 | 0.81 ± 0.46 a | 2.03 ± 1.24 a | 2.67 ± 1.81 a | 11.81 ± 3.19 a | 64.08 ± 3.45 a | 78.99 ± 1.34 b | 89.17 ± 0.64 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, L.; Yuan, B.; Song, J.; Wang, F.; Fang, Q.; Yao, H.; Ye, G. Effects of Parasitism and Venom from the Endoparasitoid Brachymeria lasus on Immunity of the Host Galleria mellonella. Insects 2025, 16, 863. https://doi.org/10.3390/insects16080863
Peng L, Yuan B, Song J, Wang F, Fang Q, Yao H, Ye G. Effects of Parasitism and Venom from the Endoparasitoid Brachymeria lasus on Immunity of the Host Galleria mellonella. Insects. 2025; 16(8):863. https://doi.org/10.3390/insects16080863
Chicago/Turabian StylePeng, Lijia, Bo Yuan, Jiqiang Song, Fang Wang, Qi Fang, Hongwei Yao, and Gongyin Ye. 2025. "Effects of Parasitism and Venom from the Endoparasitoid Brachymeria lasus on Immunity of the Host Galleria mellonella" Insects 16, no. 8: 863. https://doi.org/10.3390/insects16080863
APA StylePeng, L., Yuan, B., Song, J., Wang, F., Fang, Q., Yao, H., & Ye, G. (2025). Effects of Parasitism and Venom from the Endoparasitoid Brachymeria lasus on Immunity of the Host Galleria mellonella. Insects, 16(8), 863. https://doi.org/10.3390/insects16080863







