Multigenerational Heat Selection Enhancing Thermal Acclimation and Transcriptional Response of Hsps to Heat Stress in Spodoptera frugiperda Male Adults
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Multigenerational Heat Selection
2.3. Survival Test of the Heat-Acclimated Adults Exposed to Heat Stress
2.4. Effect of Heat Acclimation on the Transcription of Adult Males
2.4.1. Treating and Sampling
2.4.2. Sequencing and Assembly
2.4.3. Differential Expression Analysis and Functional Enrichment
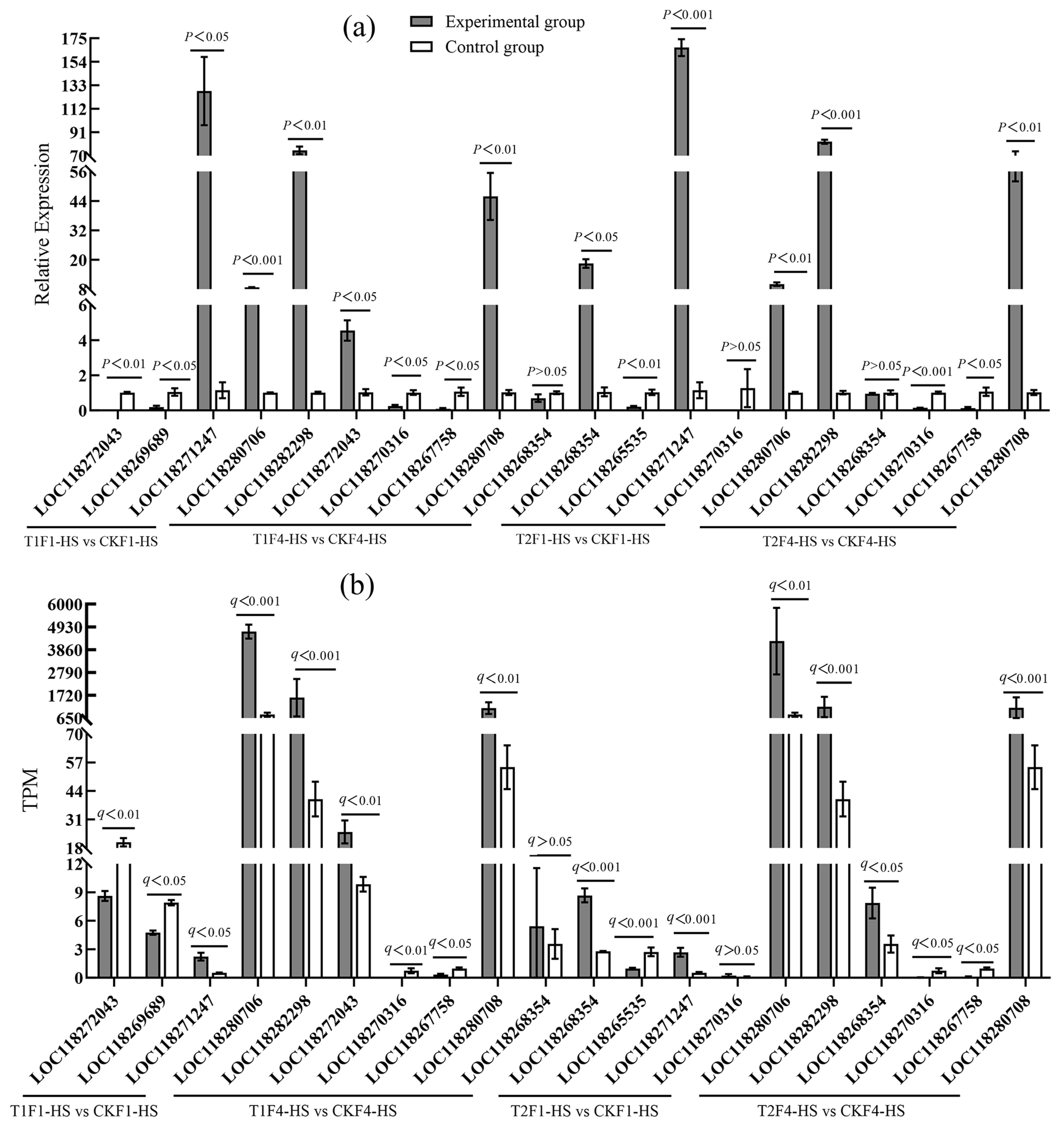
2.4.4. Validation of RNA-Seq
2.5. Statistics
3. Results
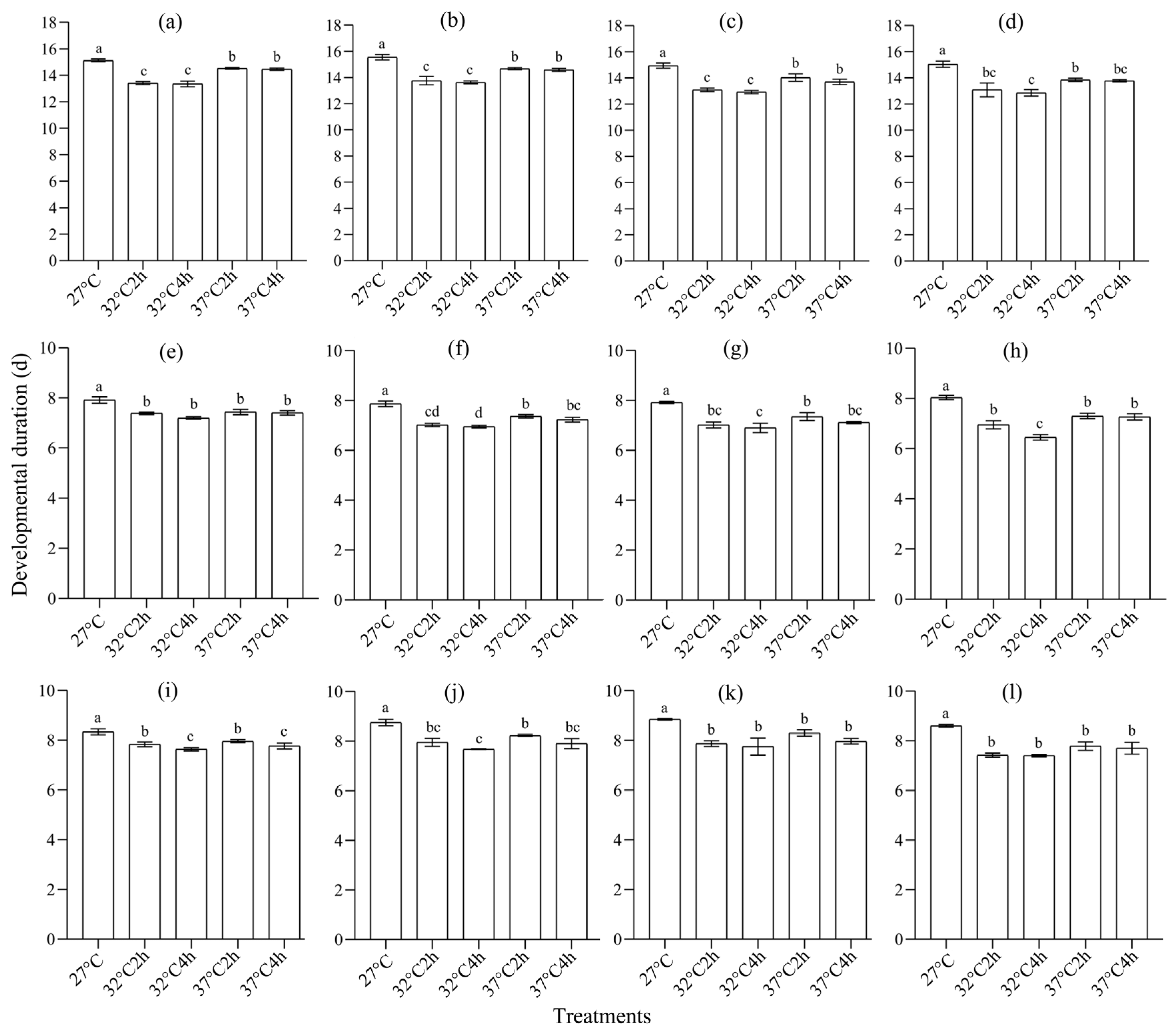
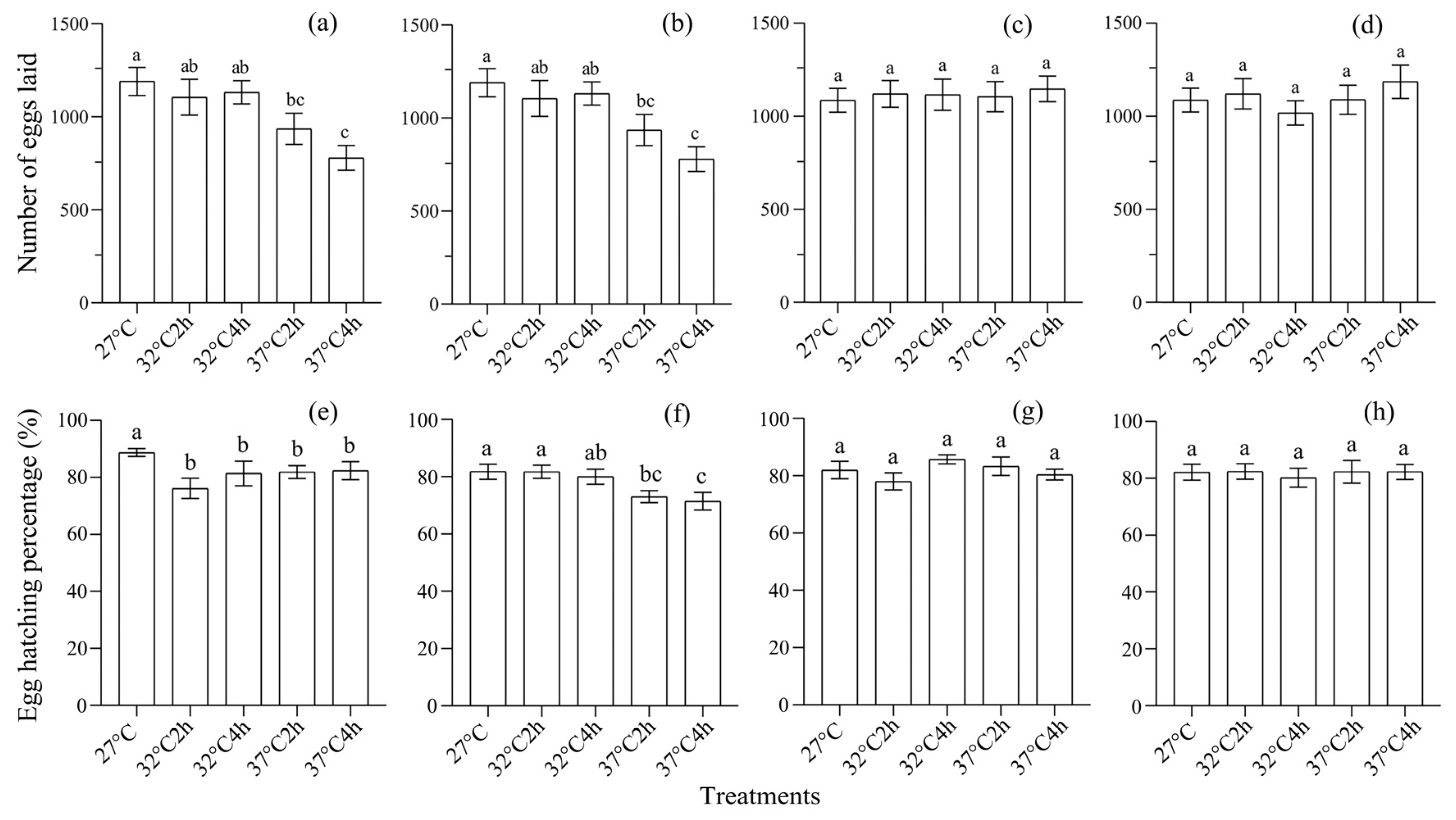
3.1. Effect of Heat Selection on Development and Reproduction
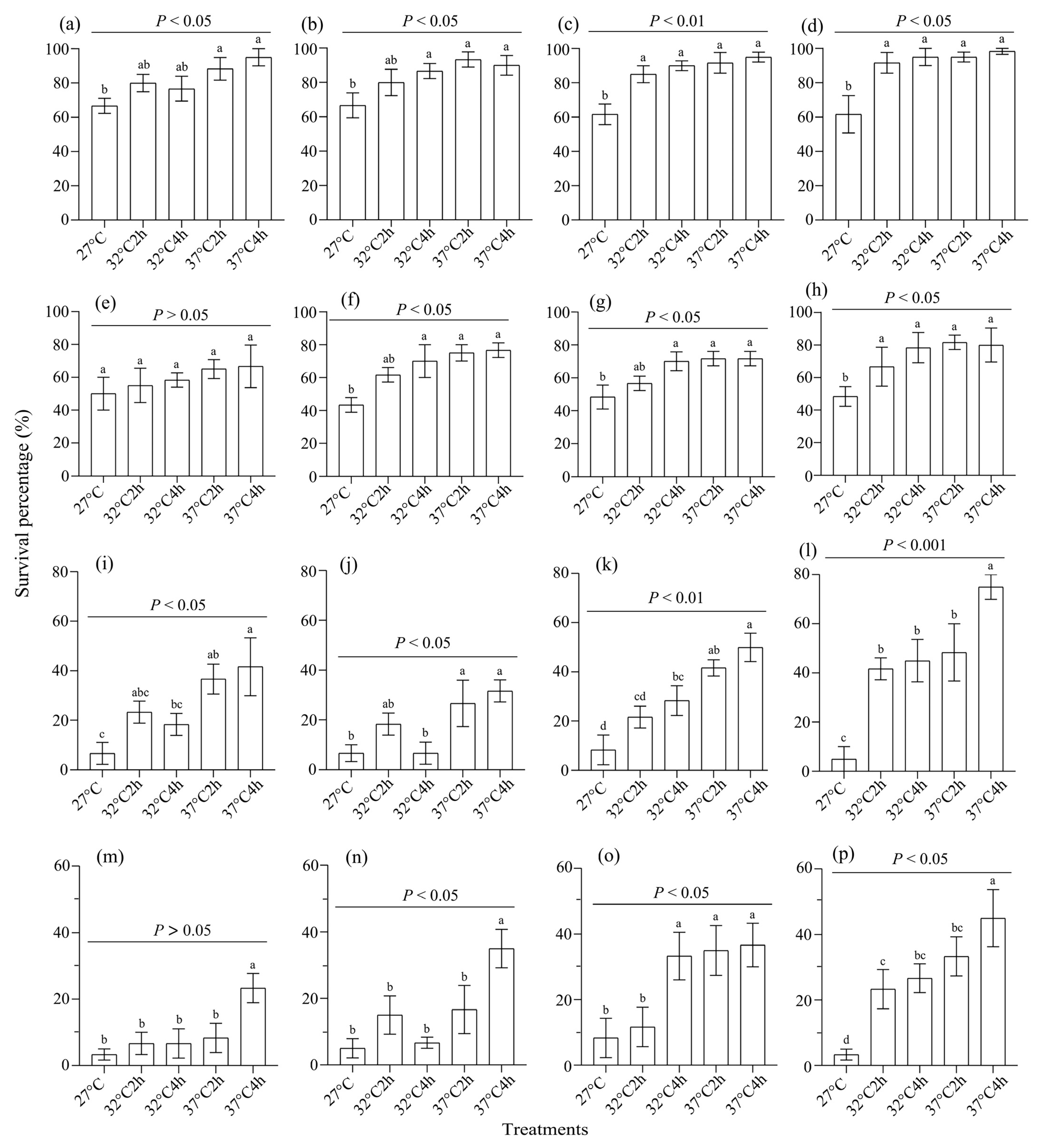
3.2. Survival of Heat-Acclimated Adults Under Extreme Temperature
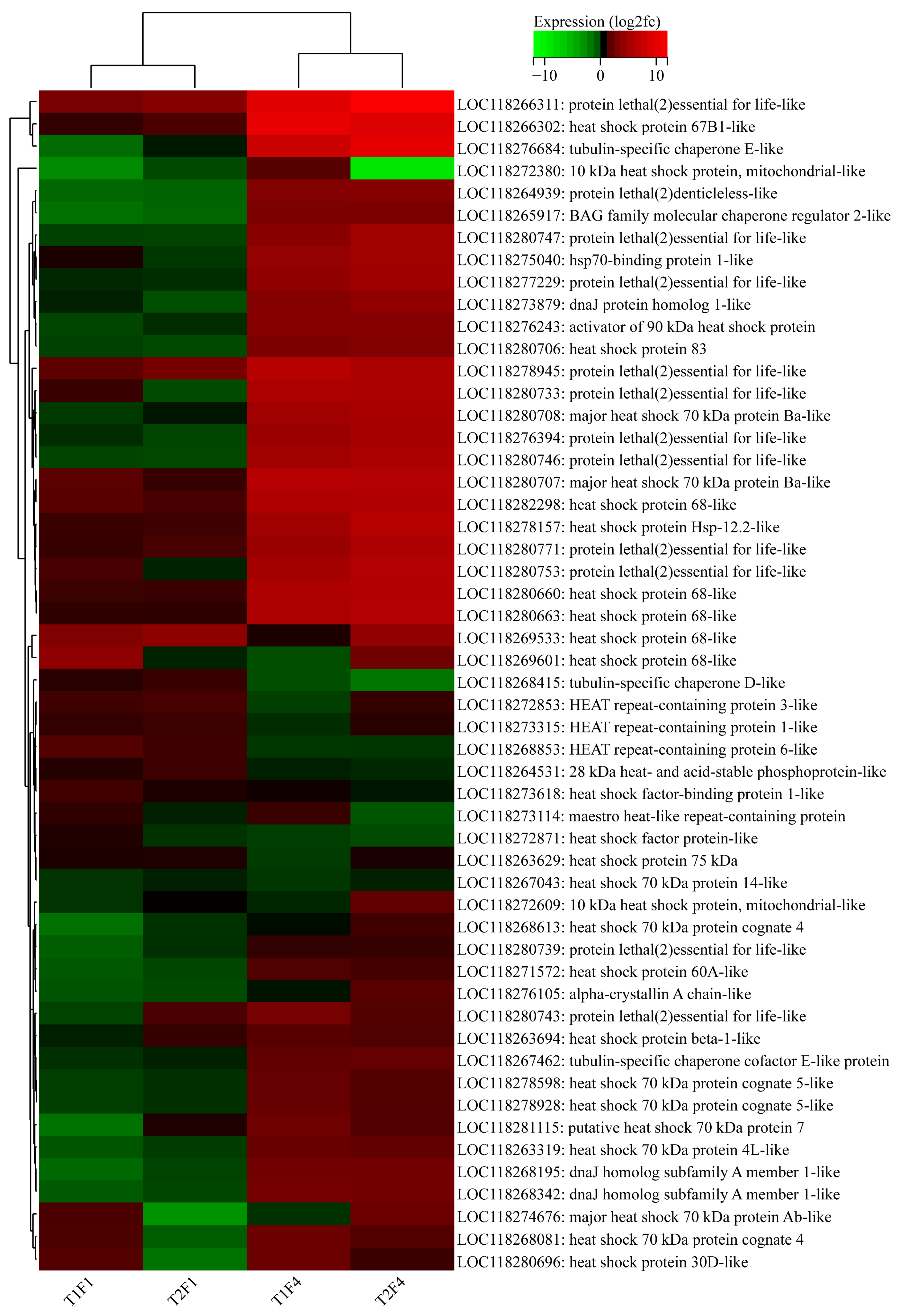
3.3. Effect of Heat Selection and Heat Stress on the Transcription of Male Adults
3.3.1. RNA Sequencing and Assembly
3.3.2. Overview of Transcriptional Changes
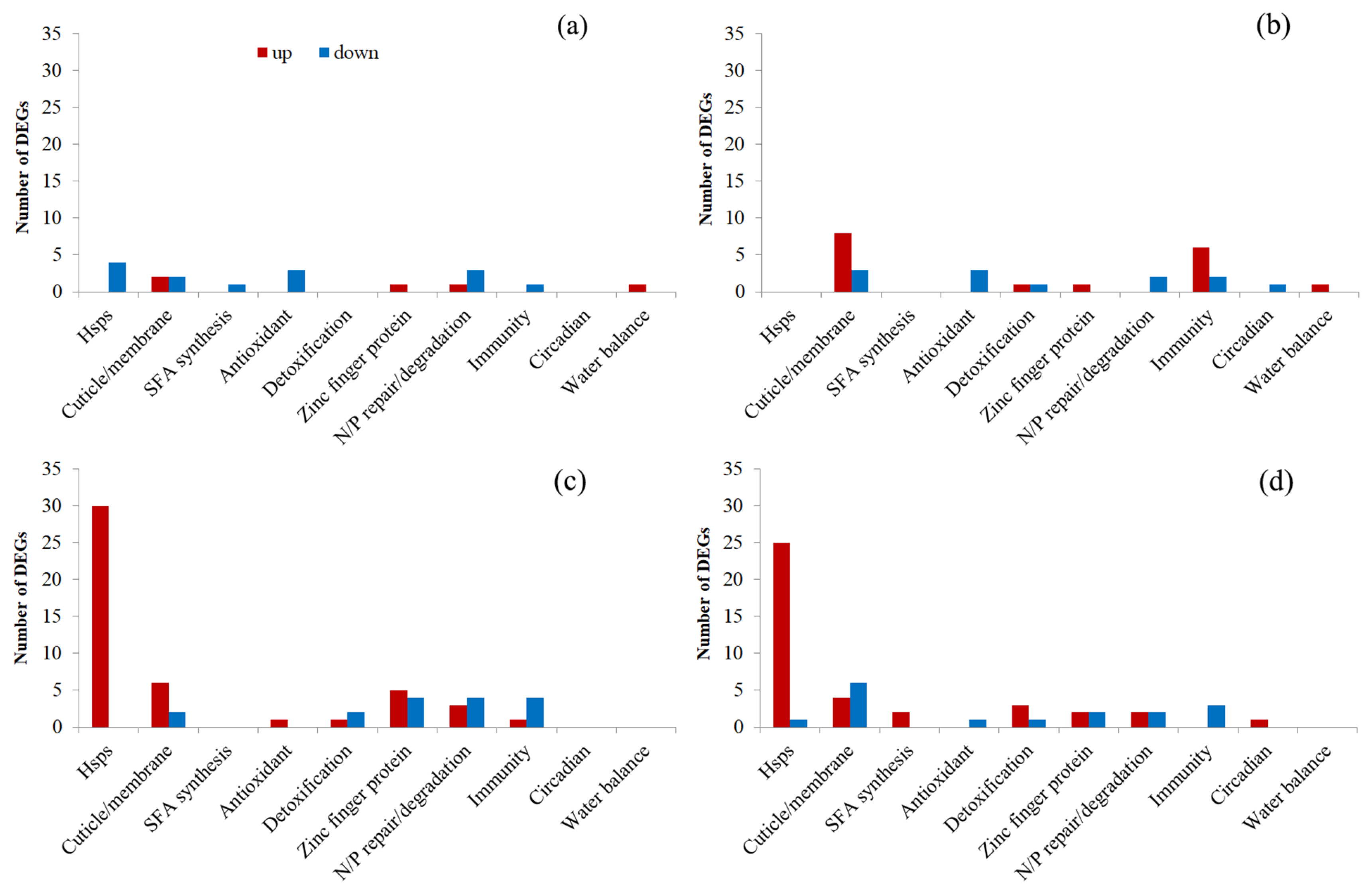
3.3.3. Transcriptional Changes in the First Generation
3.3.4. Transcriptional Changes in the Fourth Generation
3.4. Validation of RNA-Seq Data
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arnell, N.W.; Lowe, J.A.; Challinor, A.J.; Osborn, T.J. Global and regional impacts of climate change at different levels of global temperature increase. Clim. Change 2019, 155, 377–391. [Google Scholar] [CrossRef]
- Zanne, A.E.; Flores-Moreno, H.; Powell, J.R.; Cornwell, W.K.; Dalling, J.W.; Austin, A.T.; Classen, A.T.; Eggleton, P.; Okada, K.-I.; Parr, C.L.; et al. Termite sensitivity to temperature affects global wood decay rates. Science 2022, 377, 1440–1443. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Hothorn, T.; Yuan, Y.; Seibold, S.; Mitesser, O.; Rothacher, J.; Freund, J.; Wild, C.; Wolz, M.; Menzel, A. Weather explains the decline and rise of insect biomass over 34 years. Nature 2024, 628, 349–354. [Google Scholar] [CrossRef]
- Sorensen, J.G.; Kristensen, T.N.; Overgaard, J. Evolutionary and ecological patterns of thermal acclimation capacity in Drosophila: Is it important for keeping up with climate change? Curr. Opin. Insect Sci. 2016, 17, 98–104. [Google Scholar] [CrossRef]
- Rubalcaba, J.G.; Olalla-Tárraga, M.Á. The biogeography of thermal risk for terrestrial ectotherms: Scaling of thermal tolerance with body size and latitude. J. Anim. Ecol. 2020, 89, 1277–1285. [Google Scholar] [CrossRef]
- Nega, A. Climate change impacts on agriculture: A review of plant diseases and insect pests in Ethiopia and East Africa, with adaptation and mitigation strategies. Adv. Agric. 2025, 2025, 5606701. [Google Scholar] [CrossRef]
- Lombardo, J.A.; Elkinton, J.S. Environmental adaptation in an asexual invasive insect. Ecol. Evol. 2017, 7, 5123–5130. [Google Scholar] [CrossRef] [PubMed]
- Jarošík, V.; Kenis, M.; Honěk, A.; Skuhrovec, J.; Pyšek, P. Invasive insects differ from non-invasive in their thermal requirements. PLoS ONE 2015, 10, e0131072. [Google Scholar] [CrossRef]
- Mutamiswa, R.; Mbande, A.; Nyamukondiwa, C.; Chidawanyika, F. Thermal adaptation in Lepidoptera under shifting environments: Mechanisms, patterns, and consequences. Phytoparasitica 2023, 51, 929–955. [Google Scholar] [CrossRef]
- Ma, C.-S.; Ma, G.; Pincebourde, S. Survive a warming climate: Insect responses to extreme high temperatures. Annu. Rev. Entomol. 2021, 66, 163–184. [Google Scholar] [CrossRef] [PubMed]
- Quan, P.Q.; Guo, P.L.; He, J.; Liu, X.D. Heat-stress memory enhances the acclimation of a migratory insect pest to global warming. Mol. Ecol. 2024, 33, e17493. [Google Scholar] [CrossRef]
- Mpofu, P.; Cuthbert, R.N.; Machekano, H.; Nyamukondiwa, C. Transgenerational responses to heat and fasting acclimation in the Angoumois grain moth. J. Stored Prod. Res. 2022, 97, 101979. [Google Scholar] [CrossRef]
- Quan, P.Q.; Li, J.R.; Liu, X.D. Glucose dehydrogenases-mediated acclimation of an important rice pest to global warming. Int. J. Mol. Sci. 2023, 24, 10146. [Google Scholar] [CrossRef] [PubMed]
- Chidawanyika, F.; Terblanche, J.S. Rapid thermal responses and thermal tolerance in adult codling moth Cydia pomonella (Lepidoptera: Tortricidae). J. Insect Physiol. 2011, 57, 108–117. [Google Scholar] [CrossRef]
- Shen, Y.; Gong, Y.-J.; Gu, J.; Huang, L.-H.; Feng, Q.-L. Physiological effect of mild thermal stress and its induction of gene expression in the common cutworm, Spodoptera litura. J. Insect Physiol. 2014, 61, 34–41. [Google Scholar] [CrossRef]
- Mbande, A.; Mutamiswa, R.; Chidawanyika, F. Thermal tolerance in Spodoptera frugiperda: Influence of age, sex, and mating status. Sci. Afr. 2023, 22, e01911. [Google Scholar] [CrossRef]
- Phungula, S.M.; Krüger, K.; Nofemela, R.S.; Weldon, C.W. Developmental diet, life stage and thermal acclimation affect thermal tolerance of the fall armyworm, Spodoptera frugiperda. Physiol. Entomol. 2023, 48, 122–131. [Google Scholar] [CrossRef]
- Gu, L.-L.; Li, M.-Z.; Wang, G.-R.; Liu, X.-D. Multigenerational heat acclimation increases thermal tolerance and expression levels of Hsp70 and Hsp90 in the rice leaf folder larvae. J. Therm. Biol. 2019, 81, 103–109. [Google Scholar] [CrossRef]
- Foucault, Q.; Wieser, A.; Waldvogel, A.M.; Feldmeyer, B.; Pfenninger, M. Rapid adaptation to high temperatures in Chironomus riparius. Ecol. Evol. 2018, 8, 12780–12789. [Google Scholar] [CrossRef]
- Lü, Z.-C.; Wang, Y.-M.; Zhu, S.-G.; Yu, H.; Guo, J.-Y.; Wan, F.-H. Trade-offs between survival, longevity, and reproduction, and variation of survival tolerance in Mediterranean Bemisia tabaci after temperature stress. J. Insect Sci. 2014, 14, 124. [Google Scholar] [CrossRef][Green Version]
- Verma, N.; Giri, S.K.; Singh, G.; Gill, R.; Kumar, A. Epigenetic regulation of heat and cold stress responses in crop plants. Plant Gene 2022, 29, 100351. [Google Scholar] [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and its basic function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef]
- Peña, M.V.D.L.; Piskobulu, V.; Murgatroyd, C.; Hager, R. DNA methylation patterns respond to thermal stress in the viviparous cockroach Diploptera punctata. Epigenetics 2021, 16, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Tokman, D.; Cordoba-Aguilar, A.; Dattilo, W.; Lira-Noriega, A.; Sanchez-Guillen, R.A.; Villalobos, F. Insect responses to heat: Physiological mechanisms, evolution and ecological implications in a warming world. Biol. Rev. 2020, 95, 802–821. [Google Scholar] [CrossRef]
- Hartl, F.U.; Hayer-Hartl, M. Molecular chaperones in the cytosol: From nascent chain to folded protein. Science 2002, 295, 1852–1858. [Google Scholar] [CrossRef] [PubMed]
- Nover, L.; Scharf, K.D. Heat stress proteins and transcription factors. Cell. Mol. Life Sci. 1997, 53, 80–103. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Wang, W.; Liang, G.; Lu, Y. Characterizing three heat shock protein 70 genes of Aphis gossypii and their expression in response to temperature and insecticide stress. J. Agric. Food Chem. 2025, 73, 2842–2852. [Google Scholar] [CrossRef]
- Lu, K.; Chen, X.; Liu, W.; Zhou, Q. Characterization of heat shock cognate protein 70 gene and its differential expression in response to thermal stress between two wing morphs of Nilaparvata lugens (Stål). Comp. Biochem. Physiol. A 2016, 199, 47–53. [Google Scholar] [CrossRef]
- Lu, K.; Chen, X.; Liu, W.; Zhou, Q. Identification of a heat shock protein 90 gene involved in resistance to temperature stress in two wing-morphs of Nilaparvata lugens (Stål). Comp. Biochem. Physiol. A 2016, 197, 1–8. [Google Scholar] [CrossRef]
- Hu, J.T.; Chen, B.C.; Li, Z.H. Thermal plasticity is related to the hardening response of heat shock protein expression in two Bactrocera fruit flies. J. Insect Physiol. 2014, 67, 105–113. [Google Scholar] [CrossRef]
- Tao, Y.D.; Liu, Y.; Wan, X.S.; Xu, J.; Fu, D.Y.; Zhang, J.Z. High and low temperatures differentially affect survival, reproduction, and gene transcription in male and female moths of Spodoptera frugiperda. Insects 2023, 14, 958. [Google Scholar] [CrossRef]
- Gong, W.; Lubawy, J.; Marciniak, P.; Smagghe, G.; Słocińska, M.; Liu, D.; Liu, T.; Gui, S. Transcriptome and neuroendocrinome responses to environmental stress in the model and pest insect Spodoptera frugiperda. Int. J. Mol. Sci. 2025, 26, 691. [Google Scholar] [CrossRef] [PubMed]
- Woon, J.S.; Boyle, M.J.W.; Ewers, R.M.; Chung, A.; Eggleton, P. Termite environmental tolerances are more linked to desiccation than temperature in modified tropical forests. Insect Soc. 2019, 66, 57–64. [Google Scholar] [CrossRef]
- Chen, J.; Rashid, T.; Feng, G. A comparative study between Solenopsis invicta and Solenopsis richteri on tolerance to heat and desiccation stresses. PLoS ONE 2014, 9, e96842. [Google Scholar] [CrossRef]
- Johnson, R.A. Water loss in desert ants: Caste variation and the effect of cuticle abrasion. Physiol. Entomol. 2000, 25, 48–53. [Google Scholar] [CrossRef]
- Ferveur, J.-F.; Cortot, J.; Rihani, K.; Cobb, M.; Everaerts, C. Desiccation resistance: Effect of cuticular hydrocarbons and water content in Drosophila melanogaster adults. PeerJ 2018, 6, e4318. [Google Scholar] [CrossRef]
- Edney, E.B. Water Balance in Land Arthropods; Springer: Berlin, Germany, 1977. [Google Scholar]
- Guo, P.L.; Guo, Z.Q.; Liu, X.D. Cuticular protein genes involve heat acclimation of insect larvae under global warming. Insect Mol. Biol. 2022, 31, 519–532. [Google Scholar] [CrossRef]
- Khalil, S.; El-Gamal, S.; Ibrahim, S.; Elateek, S. Characterization, expression analysis and RNAi-mediated knockdown of two aquaporin genes in the cotton leafworm, Spodoptera littoralis (Lepidoptera: Noctuidae). Eur. J. Entomol. 2023, 120, 15–25. [Google Scholar] [CrossRef]
- Goto, S.G.; Philip, B.N.; Teets, N.M.; Kawarasaki, Y.; Lee, R.E., Jr.; Denlinger, D.L. Functional characterization of an aquaporin in the Antarctic midge Belgica antarctica. J. Insect Physiol. 2011, 57, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Li, X.; Shen, J.; Du, Y.; Xu, K.; Jiang, Y. Transcriptomic analysis reveals Apis mellifera adaptations to high temperature and high humidity. Ecotoxicol. Environ. Saf. 2019, 184, 109599. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, H.; Sokabe, T.; Kohno, K.; Tominaga, M.; Kadowaki, T. Evolutionary conservation and changes in insect TRP channels. BMC Evol. Biol. 2009, 9, 228. [Google Scholar] [CrossRef]
- Hori, S.; Tateyama, M.; Shirai, T.; Kubo, Y.; Saitoh, O. Two single-point mutations in Ankyrin Repeat one drastically change the threshold temperature of TRPV1. Nat. Commun. 2023, 14, 2415. [Google Scholar] [CrossRef]
- Ali, A.; Rashid, M.A.; Huang, Q.Y.; Wong, C.; Lei, C.L. Response of antioxidant enzymes in Mythimna separata (Lepidoptera: Noctuidae) exposed to thermal stress. Bull. Entomol. Res. 2017, 107, 382–390. [Google Scholar] [CrossRef]
- Wyckhuys, K.A.G.; Akutse, K.S.; Amalin, D.M.; Araj, S.-E.; Barrera, G.; Beltran, M.J.B.; Ben Fekih, I.; Calatayud, P.-A.; Cicero, L.; Cokola, M.C.; et al. Global scientific progress and shortfalls in biological control of the fall armyworm Spodoptera frugiperda. Biol. Control 2024, 191, 105460. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Liu, J.; Xie, M.; Li, Y.H.; Yang, J.J.; Zhang, M.L.; Qiu, K. Observation on law of diffusion damage of Spodoptera frugiperdain in China in 2019. Plant Prot. 2019, 45, 10–19. [Google Scholar] [CrossRef]
- Qin, Y.; Yang, D.; Kang, D.; Zhao, Z.; Zhao, Z.; Yang, P.; Li, Z. Potential economic loss assessment of maize industry caused by fall armyworm (Spodoptera frugiperda) in China. Plant Prot. 2020, 46, 69–73. [Google Scholar] [CrossRef]
- Tambo, J.A.; Kansiime, M.K.; Mugambi, I.; Rwomushana, I.; Kenis, M.; Day, R.K.; Lamontagne-Godwin, J. Understanding smallholders’ responses to fall armyworm (Spodoptera frugiperda) invasion: Evidence from five African countries. Sci. Total Environ. 2020, 740, 140015. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wyckhuys, K.A.G.; Jia, X.; Nie, F.; Wu, K. Fall armyworm invasion heightens pesticide expenditure among Chinese smallholder farmers. J. Environ. Manag. 2021, 282, 111949. [Google Scholar] [CrossRef]
- Kenis, M.; Benelli, G.; Biondi, A.; Calatayud, P.A.; Day, R.; Desneux, N.; Harrison, R.D.; Kriticos, D.; Rwomushana, I.; van den Berg, J.; et al. Invasiveness, biology, ecology, and management of the fall armyworm, Spodoptera frugiperda. Entomol. Gen. 2023, 43, 187–241. [Google Scholar] [CrossRef]
- Wu, T.; Cao, D.-H.; Liu, Y.; Yu, H.; Fu, D.-Y.; Ye, H.; Xu, J. Mating-induced common and sex-specific behavioral, transcriptional changes in the moth fall armyworm (Spodoptera frugiperda, Noctuidae, Lepidoptera) in laboratory. Insects 2023, 14, 209. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.-J.; Zhou, J.-C.; Zhu, K.-H.; Zhang, Z.-T.; Dong, H. A simple method for identifiying sexuality of Spodoptera frugiperda (J. E. Smith) pupae and adults. Plant Prot. 2019, 45, 96–98. [Google Scholar] [CrossRef]
- Xiao, H.; Ye, X.; Xu, H.; Mei, Y.; Yang, Y.; Chen, X.; Yang, Y.; Liu, T.; Yu, Y.; Yang, W.; et al. The genetic adaptations of fall armyworm Spodoptera frugiperda facilitated its rapid global dispersal and invasion. Mol. Ecol. Resour. 2020, 20, 1050–1068. [Google Scholar] [CrossRef]
- Storey, J.D. The positive false discovery rate: A Bayesian interpretation and the q-value. Ann. Stat. 2003, 31, 2013–2035. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Hogan, J.A.; Baraloto, C.; Ficken, C.; Clark, M.D.; Weston, D.J.; Warren, J.M. The physiological acclimation and growth response of Populus trichocarpa to warming. Physiol. Plant. 2021, 173, 1008–1029. [Google Scholar] [CrossRef]
- Geerts, A.N.; Vanoverbeke, J.; Vanschoenwinkel, B.; Van Doorslaer, W.; Feuchtmayr, H.; Atkinson, D.; Moss, B.; Davidson, T.A.; Sayer, C.D.; De Meester, L. Rapid evolution of thermal tolerance in the water flea Daphnia. Nat. Clim. Change 2015, 5, 665–668. [Google Scholar] [CrossRef]
- Luo, L.L.; Yang, G.M.; Long, L.; Wang, X.H.; Sing, H.Z.; Yi, T.C.; Luo, X.D.; Liu, M. Effects of temperature on the development and reproduction of the greater wax moth, Galleria mellonella. J. Plant Prot. 2022, 49, 644–653. [Google Scholar] [CrossRef]
- Wang, B.Y.; Huang, T.F.; Ma, Y.; Tang, B.J.; Zhou, Q.; Zhang, G.R. Biological and ecological characteristics of a thermotolerant strain of Anagrus nilaparvatae. China J. Biol. Control 2021, 37, 692–700. [Google Scholar] [CrossRef]
- Saggere, R.M.S.; Lee, C.W.J.; Chan, I.C.W.; Durnford, D.G.; Nedelcu, A.M. A life-history trade-off gene with antagonistic pleiotropic effects on reproduction and survival in limiting environments. Proc. Biol. Sci. 2022, 289, 20212669. [Google Scholar] [CrossRef]
- Yu, H.; Shi, M.-R.; Xu, J.; Chen, P.; Liu, J.-H. Mating-induced trade-offs upon egg production versus fertilization and offspring’s survival in a sawfly with facultative parthenogenesis. Insects 2021, 12, 693. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Song, X.Q.; Yu, H.; Fu, D.Y.; Xu, J.; Ye, H. Mating-induced differential expression in genes related to reproduction and immunity in Spodoptera litura (Lepidoptera: Noctuidae) female moths. J. Insect Sci. 2020, 20, 10. [Google Scholar] [CrossRef] [PubMed]
- Schwenke, R.; Lazzaro, B.; Wolfner, M. Reproduction–immunity trade-offs in insects. Annu. Rev. Entomol. 2016, 61, 239–256. [Google Scholar] [CrossRef] [PubMed]
- Walsh, B.S.; Parratt, S.R.; Mannion, N.L.M.; Snook, R.R.; Bretman, A.; Price, T.A.R. Plastic responses of survival and fertility following heat stress in pupal and adult Drosophila virilis. Ecol. Evol. 2021, 11, 18238–18247. [Google Scholar] [CrossRef]
- Lü, J.; Zhang, H. The effect of acclimation to sublethal temperature on subsequent susceptibility of Sitophilus zeamais Mostchulsky (Coleoptera: Curculionidae) to high temperatures. PLoS ONE 2016, 11, e0159400. [Google Scholar] [CrossRef]
- Yilmaz, A.R. Evolutionary Responses of Arthropods to the Novel Selective Pressures of Urbanization. Ph.D. Thesis, Case Western Reserve University, Cleveland, OH, USA, 2022. [Google Scholar]
- Tanaka, K.; Murata, K. Genetic basis underlying rapid evolution of an introduced insect Ophraella communa (Coleoptera: Chrysomelidae): Heritability of photoperiodic response. Environ. Entomol. 2017, 46, 167–173. [Google Scholar] [CrossRef]
- Batz, Z.A.; Clemento, A.J.; Fritzenwanker, J.; Ring, T.J.; Garza, J.C.; Armbruster, P.A. Rapid adaptive evolution of the diapause program during range expansion of an invasive mosquito. Evolution 2020, 74, 1451–1465. [Google Scholar] [CrossRef]
- Boardman, L.; Lockwood, J.L.; Angilletta, M.J.; Krause, J.S.; Lau, J.A.; Loik, M.E.; Simberloff, D.; Thawley, C.J.; Meyerson, L.A. The future of invasion science needs physiology. Bioscience 2022, 72, 1014–1019. [Google Scholar] [CrossRef]
- Smith, L.A.; Lesley, T.L. Increased duration of extreme thermal events negatively affects cold acclimation ability in a high-latitude, freshwater ectotherm (Ischnura elegans; Odonata: Coenagrionidae). Eur. J. Entomol. 2020, 117, 93–100. [Google Scholar] [CrossRef]
- Carbonell, J.A.; Wang, Y.-J.; Stoks, R. Evolution of cold tolerance and thermal plasticity in life history, behaviour and physiology during a poleward range expansion. J. Anim. Ecol. 2021, 90, 1666–1677. [Google Scholar] [CrossRef] [PubMed]
- Grigaltchik, V.S.; Ward, A.J.; Seebacher, F. Thermal acclimation of interactions: Differential responses to temperature change alter predator-prey relationship. Proc. Biol. Sci. 2012, 279, 4058–4064. [Google Scholar] [CrossRef]
- Porras, M.F.; Agudelo-Cantero, G.A.; Santiago-Martínez, M.G.; Navas, C.A.; Loeschcke, V.; Sørensen, J.G.; Rajotte, E.G. Fungal infections lead to shifts in thermal tolerance and voluntary exposure to extreme temperatures in both prey and predator insects. Sci. Rep. 2021, 11, 21710. [Google Scholar] [CrossRef] [PubMed]
- Quan, P.Q.; Li, M.Z.; Wang, G.R.; Gu, L.L.; Liu, X.D. Comparative transcriptome analysis of the rice leaf folder (Cnaphalocrocis medinalis) to heat acclimation. BMC Genom. 2020, 21, 450. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.B.; Li, Y.Y.; Huang, J.; Chu, W.Q.; Wang, Z.Y.; Liu, H. Comparative transcriptome and proteome analysis of heat acclimation in predatory mite Neoseiulus barkeri. Front. Physiol. 2020, 11, 426. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, L.; Liu, Q.; Xing, G.; Kuai, X.; Sun, J.; Yin, X.; Wang, J.; Zhang, L.; He, F. E3 ubiquitin ligase SIAH1 mediates ubiquitination and degradation of TRB3. Cell. Signal. 2008, 20, 942–948. [Google Scholar] [CrossRef]
- Thakur, S.; Jindal, V.; Choi, M.Y. CAPA neuropeptide and its receptor in insects: A mini review. Arch. Insect Biochem. Physiol. 2025, 118, e70061. [Google Scholar] [CrossRef]
- O’Donnell, M.J. A perspective on insect water balance. J. Exp. Biol. 2022, 225, jeb242358. [Google Scholar] [CrossRef]
- Waters, J.S.; Harrison, J.F. Insect Metabolic Rates. In Metabolic Ecology: A Scaling Approach; Sibly, R.M., Brown, J.H., Astrid, K.-B., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 2012; pp. 198–211. [Google Scholar]
- Frederich, M.; Portner, H.O. Oxygen limitation of thermal tolerance defined by cardiac and ventilatory performance in spider crab, Maja squinado. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2000, 279, R1531–R1538. [Google Scholar] [CrossRef] [PubMed]
- Storey, K.B.; Storey, J.M. Metabolic rate depression in animals: Transcriptional and translational controls. Biol. Rev. 2004, 79, 207–233. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.-X.; Zhao, Q.-Y.; Song, Y.; Yu, G.-Y.; Fu, W.; Xu, J. Multigenerational Heat Selection Enhancing Thermal Acclimation and Transcriptional Response of Hsps to Heat Stress in Spodoptera frugiperda Male Adults. Insects 2025, 16, 860. https://doi.org/10.3390/insects16080860
Zhang Z-X, Zhao Q-Y, Song Y, Yu G-Y, Fu W, Xu J. Multigenerational Heat Selection Enhancing Thermal Acclimation and Transcriptional Response of Hsps to Heat Stress in Spodoptera frugiperda Male Adults. Insects. 2025; 16(8):860. https://doi.org/10.3390/insects16080860
Chicago/Turabian StyleZhang, Zhi-Xiao, Qing-Yi Zhao, Yu Song, Guo-Yun Yu, Wen Fu, and Jin Xu. 2025. "Multigenerational Heat Selection Enhancing Thermal Acclimation and Transcriptional Response of Hsps to Heat Stress in Spodoptera frugiperda Male Adults" Insects 16, no. 8: 860. https://doi.org/10.3390/insects16080860
APA StyleZhang, Z.-X., Zhao, Q.-Y., Song, Y., Yu, G.-Y., Fu, W., & Xu, J. (2025). Multigenerational Heat Selection Enhancing Thermal Acclimation and Transcriptional Response of Hsps to Heat Stress in Spodoptera frugiperda Male Adults. Insects, 16(8), 860. https://doi.org/10.3390/insects16080860




