Comments on Two Controversial Oriental Assassin Bug Species of the Genus Rhynocoris (Heteroptera: Reduviidae: Harpactorinae), with the Description of R. minutus sp. nov. from China †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimens and Acronyms
- CAU Entomological Museum, China Agricultural University, Beijing, China
- ZMUC Zoological Museum of University of Copenhagen, Copenhagen, Denmark
- NHMUK Natural History Museum, London, United Kingdom
- NHRS Swedish Museum of Natural History, Stockholm, Sweden
- RBINS Royal Belgian Institute of Natural Sciences, Brussels, Belgium
2.2. Taxonomy
2.3. Biological Study
3. Results
3.1. Systematics
- Genus Rhynocoris Hahn, 1834
- Rhinocoris Kolenati, 1857: 460 [20]. Unjustified emendation.
- Note: Hsiao and Ren in 1981 keyed ten species and two subspecies of Rhynocoris in China [3] but arranged all Chinese Rhynocoris species under the Neotropical generic name, Harpactor Laporte [3,19]. Most of the non-neotropical species in Harpactor belong in Rhynocoris and other genera, such as Sphedanolestes Stål, 1867 and Biasticus Stål, 1867 [1,2,3].
- The key to the Chinese species in the genus Rhynocoris Hahn, 1834
|
| – Anterior pronotal lobe smooth, without dense appressed setae and glabrous sculptures…………………………………………………………………………………………3 |
|
|
|
| – Second antennal segment shorter than third segment…………………………………………………………………………………………………………………………………..9 |
|
| – Abdomen ventrally black, or red to orange without black transversal intersegmental stripes……………………………………………………………………………………5 |
|
| – Connexivum bicolor, basal half of each segment black, apical half red………………………………………………………………………………………………………………6 |
|
| – All femora with brownish annular markings, or completely black……………………………………………………………………………………………………………………8 |
|
| – Each femur with two red annular markings, basally and medially………………………………………………………………………..Rhynocoris dauricus Kiritshenko, 1926 |
|
| – Pronotum black, sometimes lateral margin of lateral pronotal angle red; all femora completely black…………………………………Rhynocoris sibiricus (Jakovlev, 1893) |
|
| – Connexivum bicolor, red and black; body red with black markings………………………………………………………………………………………………………………..10 |
|
| – Femora black, without annular markings………………………………………………………………………………………………………………………………………………12 |
|
|
|
| – Femora completely black; sterna of abdomen red, with black intersegmental stripes……………………………………………………..Rhynocoris fuscipes (Fabricius, 1787) |
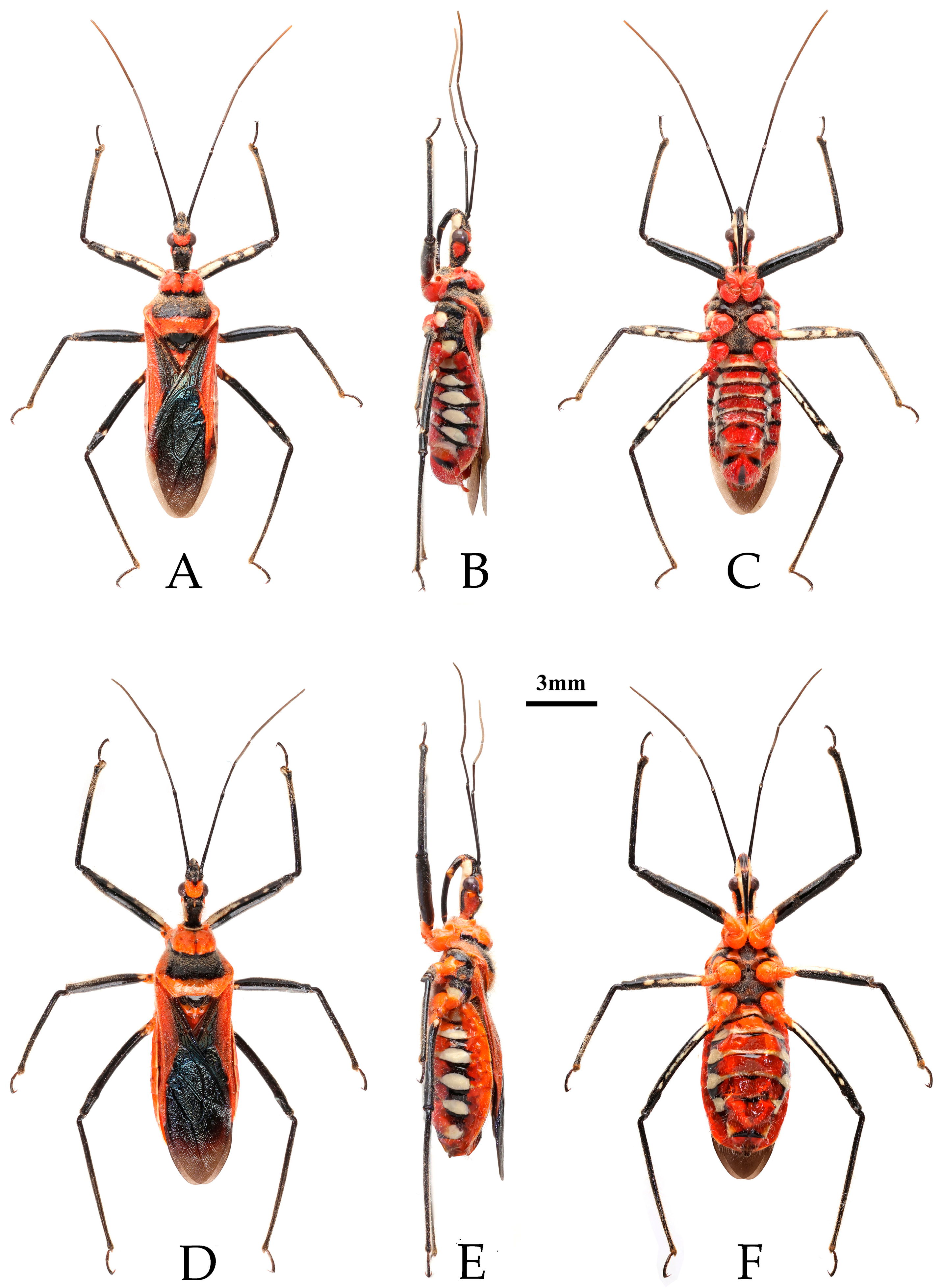
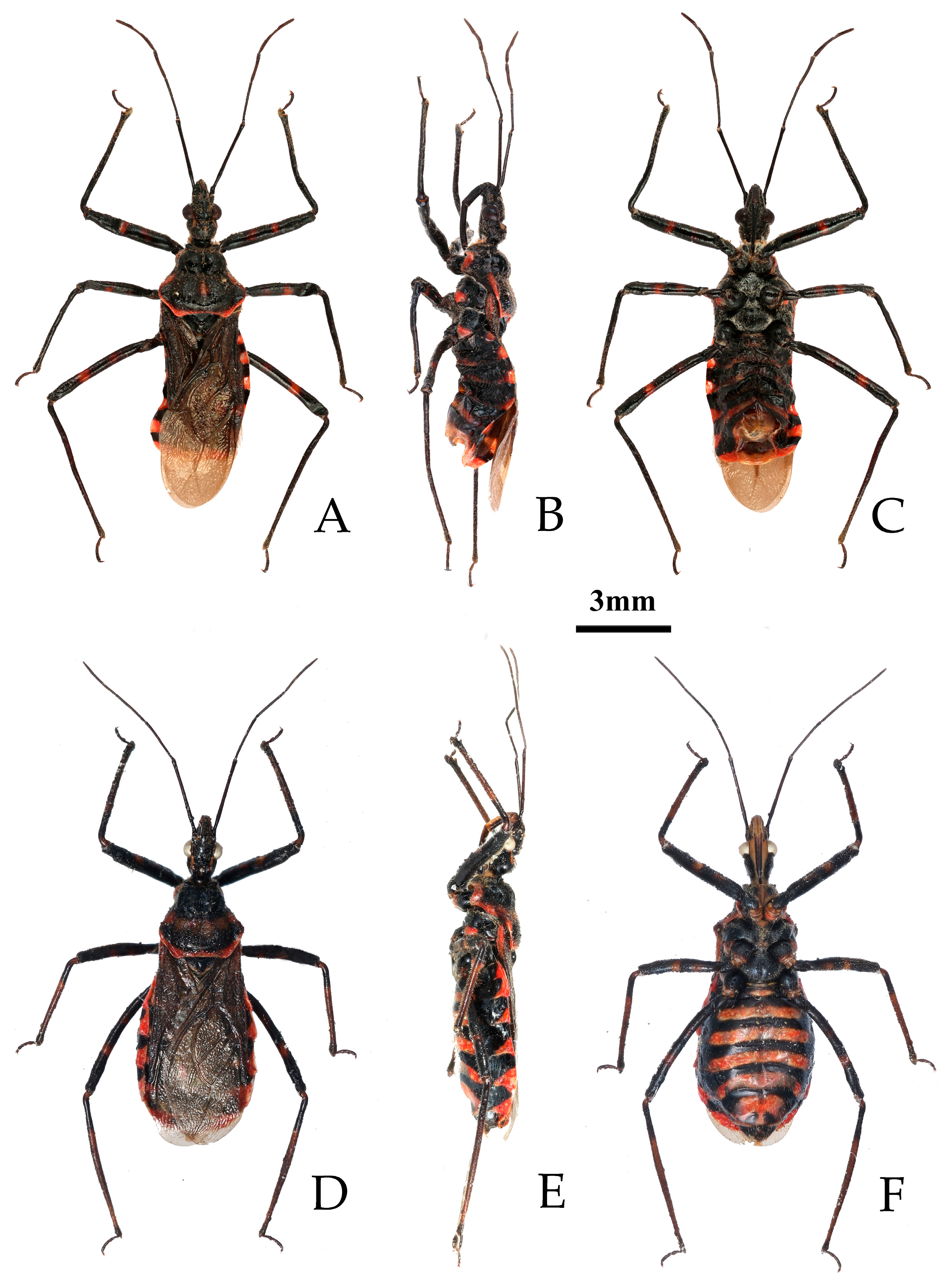
- Rhynocoris costalis (Stål, 1867)
- Chinese common name: 山彩瑞猎蝽
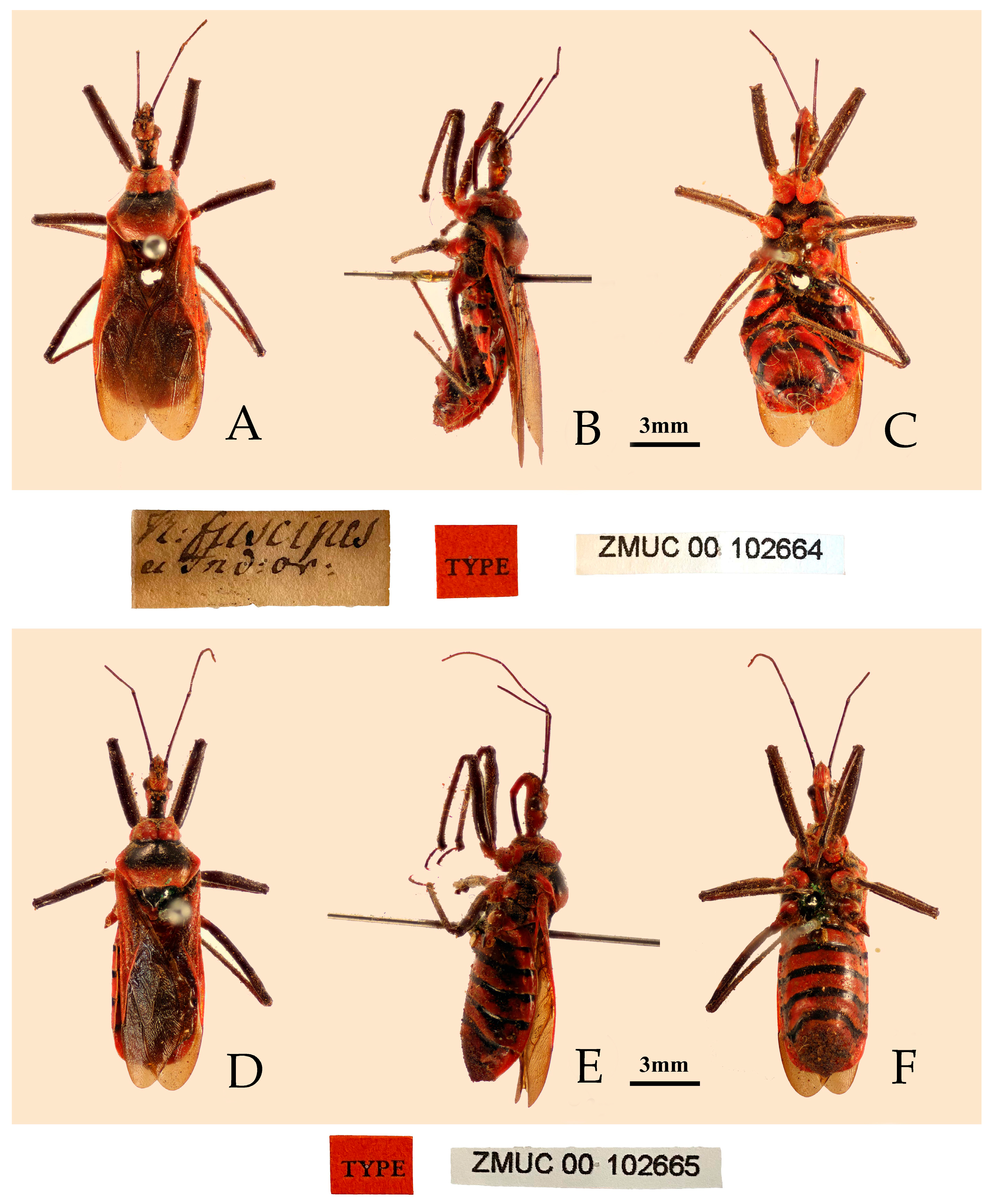
- Reduvius costalis Stål, 1867: 285 [25]. Syntype (1♀): “Bengalia”, NHRS.
- Reduvius (Reduvius) costalis: Reuter, 1883: 293 [26], as a variety of R. fuscipes.
- Harpactor costalis: Distant, 1904: 334 [27].
- Harpactor fuscipes: Hsiao and Ren, 1981: 532 [3]. Misidentification.
- Type material examined. Syntype: 1♀, Bengalia, Sundwall (NHRS) (Figure 1).

- Rhynocoris fuscipes (Fabricius, 1787)
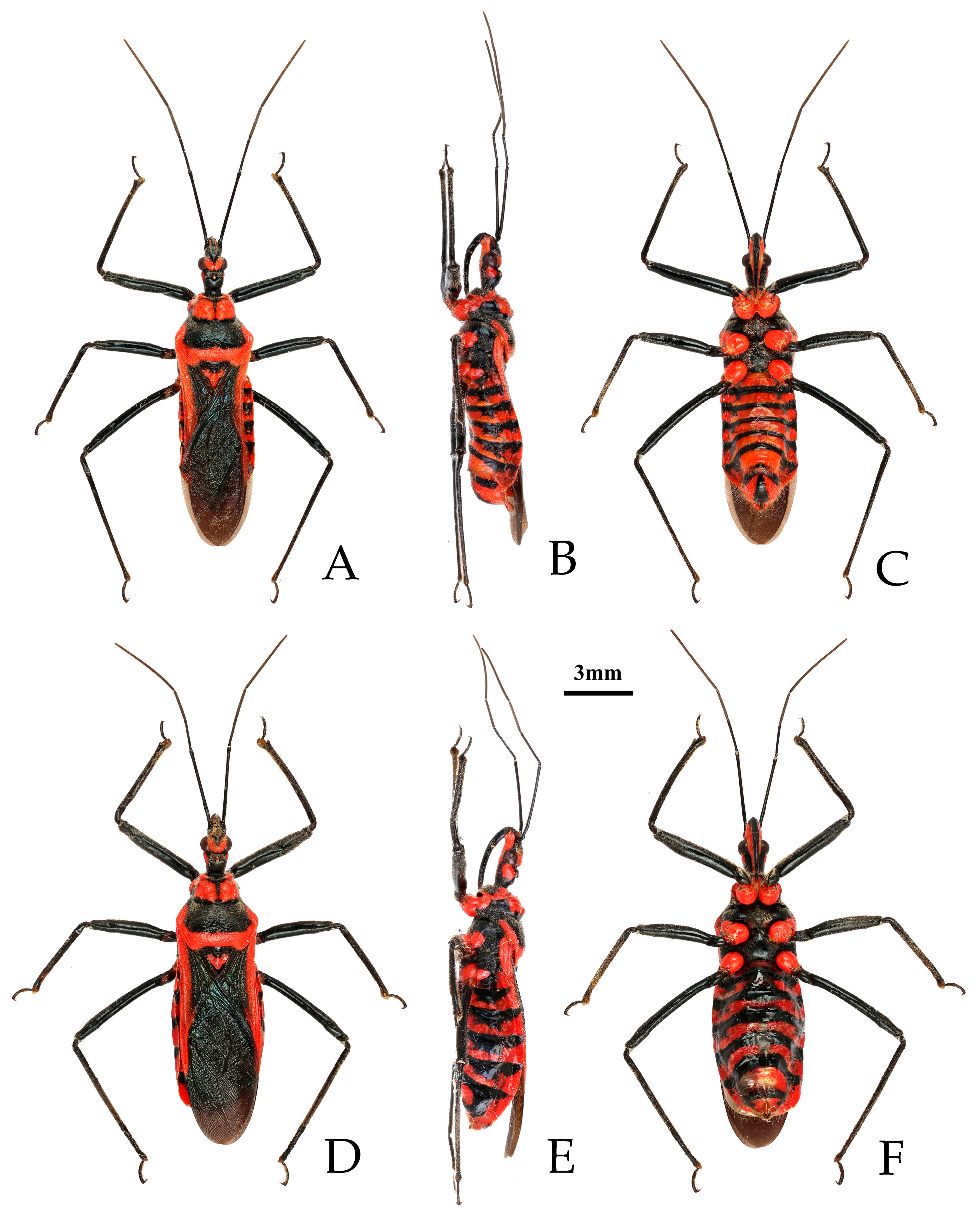
- Chinese common name: 红彩瑞猎蝽
- Reduvius fuscipes Fabricius, 1787: 312 [29]. Syntypes (2♀): “India Orientalis”, ZMUC.
- Reduvius (Reduvius) fuscipes: Stål, 1874: 39 [23].
- Harpactor fuscipes: Walker (1873: 110 [33]).
- Rhinocoris fuscipes: Bergroth, 1914: 362 [36].
- Type material examined. Syntypes: 2♀, “India Orientalis” (ZMUC) (Figure 4).
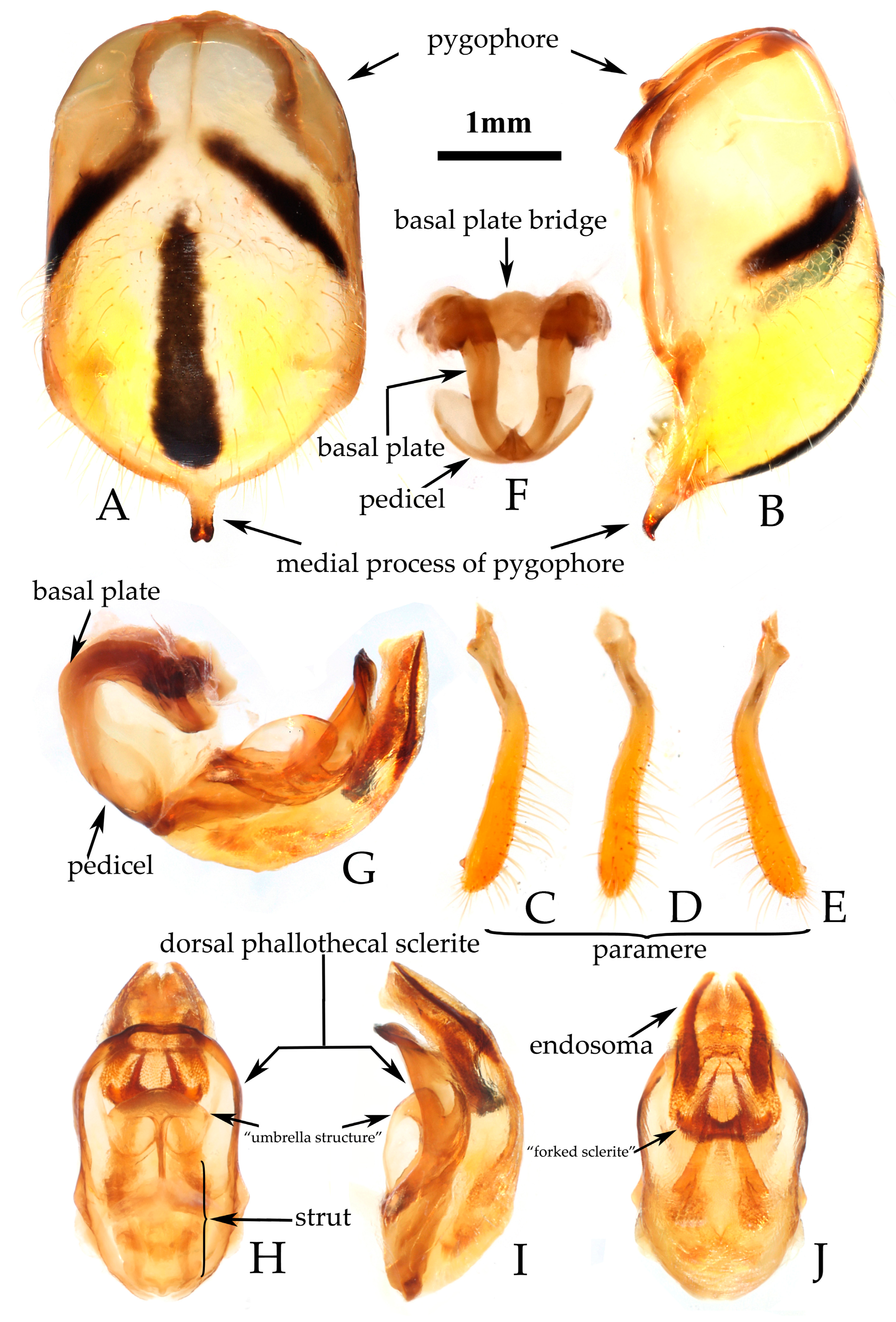
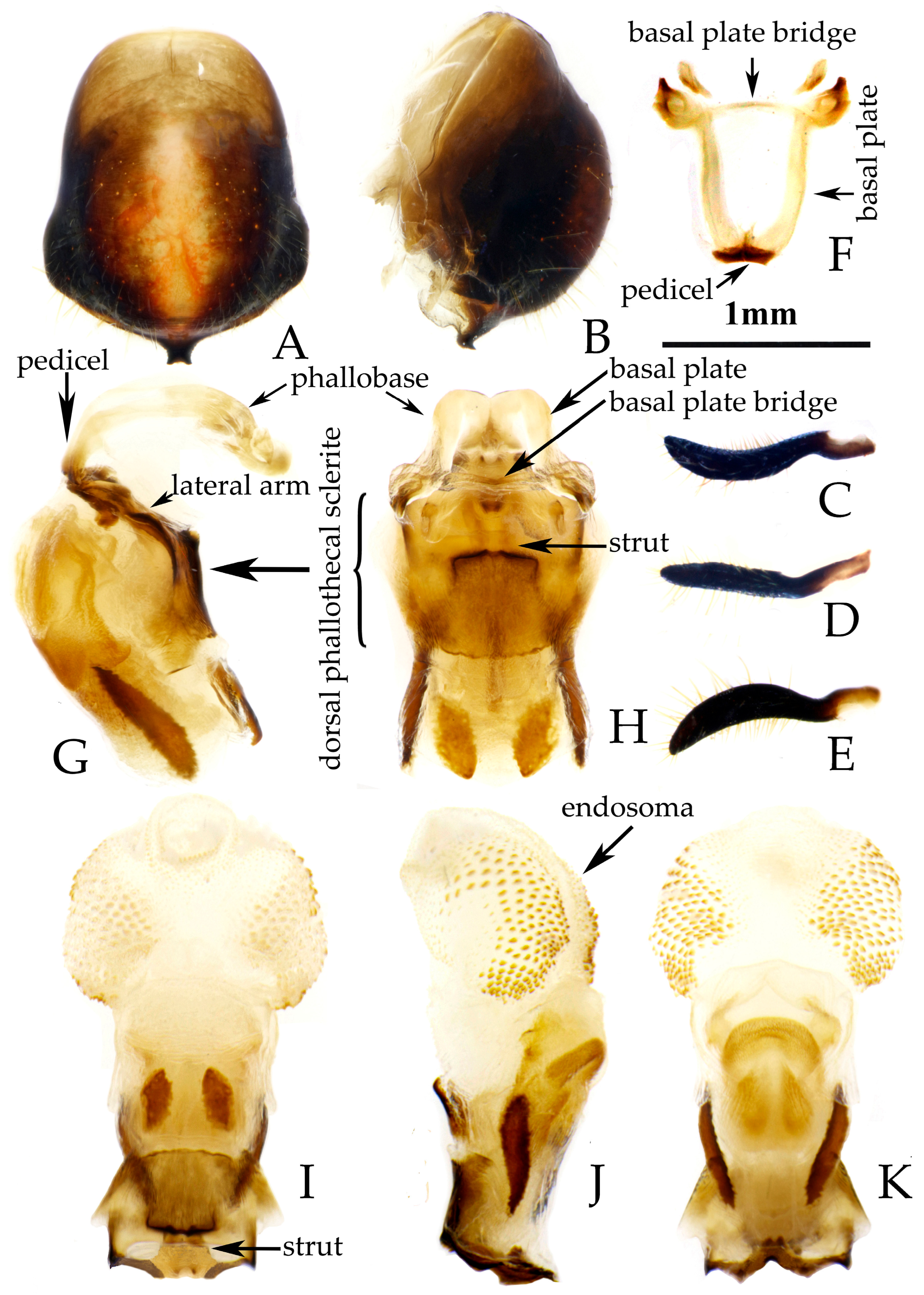
- Male genitalia. Medial process of pygophore extending backward with a narrow tip, its apical part with a central concavity (Figure 6A,B); paramere rod-shaped, basal part curved, apical part blunt and rounded, with a noticeable protrusion on inner side of subapical part (Figure 6C,D). Phallobase of phallosoma stubby, basal plate bridge subequal to basal plate in length and thickness (Figure 6E); dorsal phallothecal sclerite with a strongly sclerotized margin around it, a "W"-shaped sclerotized support structure in middle part, and a transverse sclerotized plate in sub-basal part (Figure 3F–I); struts stout, completely separated from each other (Figure 3G); endosoma with stout forked sclerite, its tips blunt and rounded (Figure 3G), and apical part with two stripe-shaped sclerites on either side (Figure 6F–I).
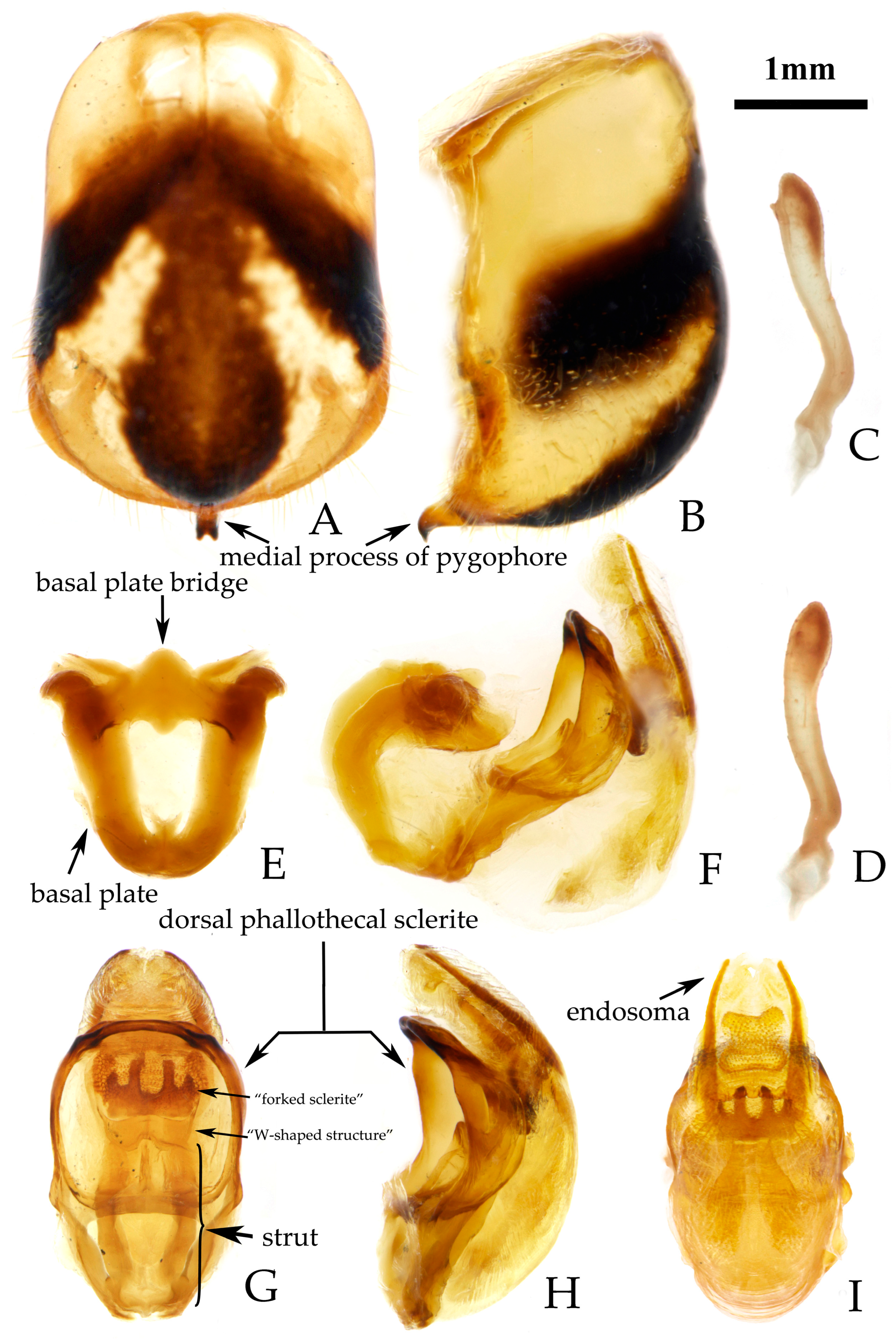
- Rhynocoris minutus Liu, Zhao & Cai sp. nov.
- Chinese common name: 小瑞猎蝽
- Type material. Holotype: ♂, China, Yunnan, Lijiang, Yulong, Yulong Snow Mountain, 27.1129° N, 100.1552° E, 3121 m, 2020-VII-7, Chen Zhuo (CAU). Paratypes: 1♂, China, Yunnan, Diqing, Shangri-la, Sanba, Haba, 27.2255° N, 100.0616° E, 3176 m, 2020-VI-19, Huang Weidong, Guo Qiuhong, Liu Liyuan and Peng Feng; 1♀, China, Sichuan, Ganzi, Daocheng, Sangdui, 29.1410° N, 100.0852° E, 4282 m, 2016-VI-16, Lin Yejie; 1♀, Yunnan, Diqing, Gezan, 3000 m, 2010-VIII-18, Cao Liangming; 1♀, China, Yunnan, Lijiang, Yulong, Baisha, Wenhai, 3099 m, 2020-VI-16, 26.9666° N, 100.1706° E, Huang Weidong; 1♀, China, Yunnan, Lijiang, Yulong, 1980-VIII-29; 1♀, China, Yunnan, Lijiang, Yulong, Ludian, Kuaihuoyuan, 3157 m, 2020-VII-3, 27.1949° N, 99.4268° E, Chen Zhuo (CAU).
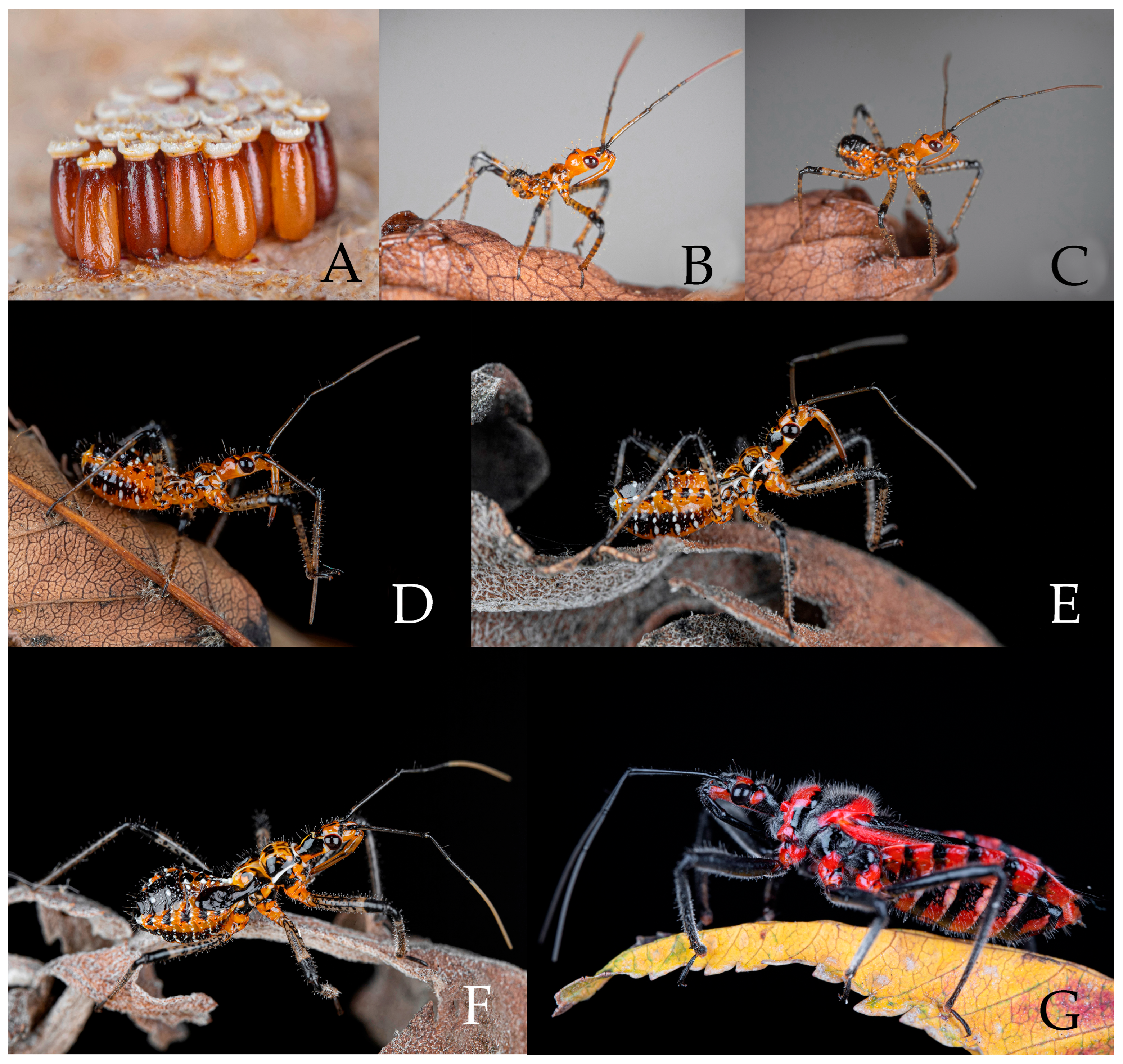
3.2. Biology
3.2.1. Rhynocoris costalis (Stål, 1867)
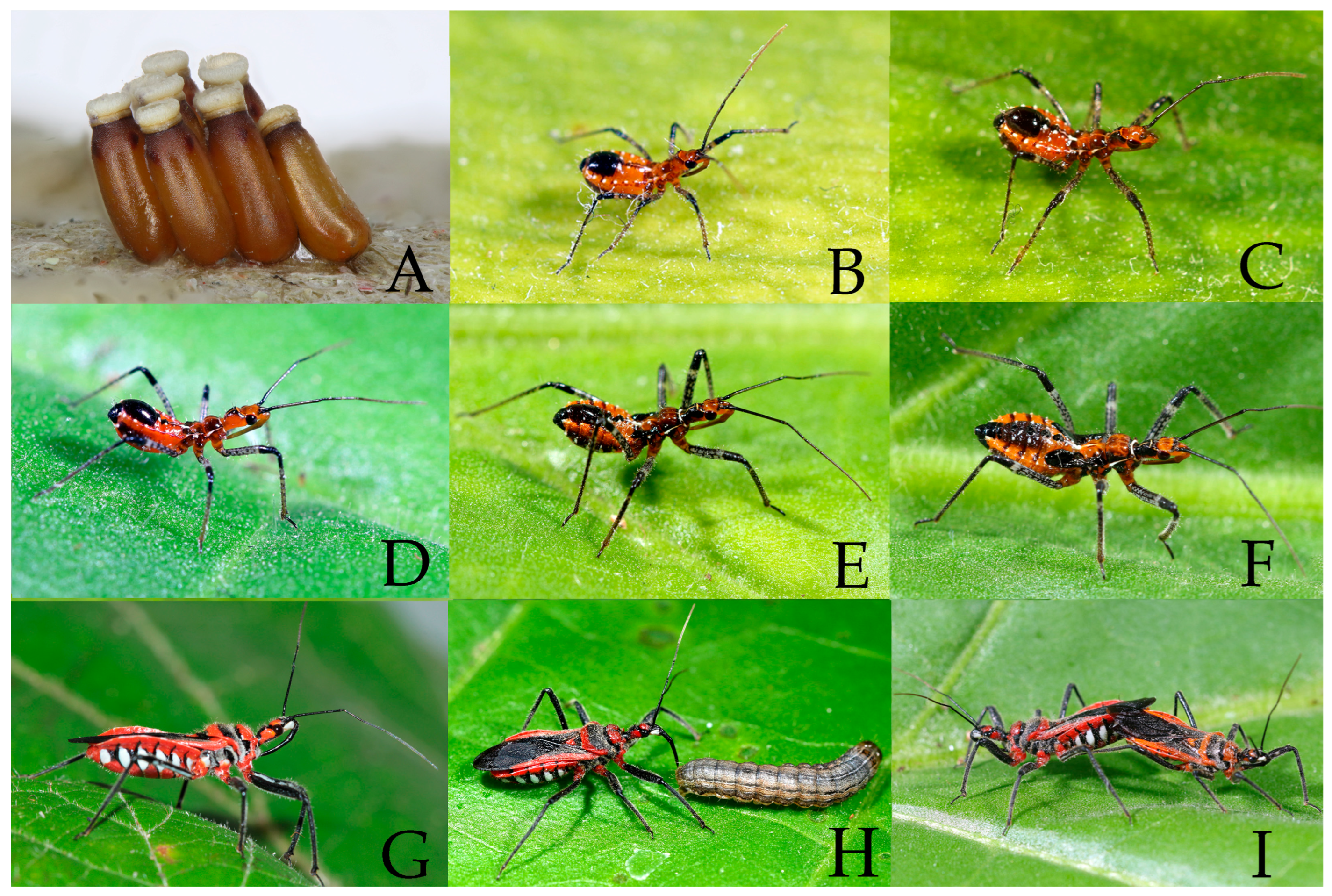
3.2.2. Rhynocoris fuscipes (Fabricius, 1787)
4. Discussion
4.1. Taxonomy Comments and the Identification of Species
4.2. Effective Reduviid Predators in the Agricultural Ecosystem
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maldonado-Capriles, J. Systematic catalogue of the Reduviidae of the world (Insecta: Heteroptera). Caribbean J. Sci. Spec. Ed. 1990, 1, i–x + 1–694. [Google Scholar]
- Putshkov, V.G.; Putshkov, P.V. Family Reduviidae Latreille, 1807, assassin-bugs. In Catalogue of the Heteroptera of the Palaearctic Region; Aukema, B., Rieger, C., Eds.; The Netherlands Entomological Society: Amsterdam, The Netherlands, 1996; Volume 2, Cimicomorpha I; pp. 148–265. [Google Scholar]
- Hsiao, T.Y.; Ren, S.Z. Reduviidae. In A Handbook for the Determination of the Chinese Hemiptera-Heteroptera (II); Hsiao, T.Y., Ren, S.Z., Zheng, L.Y., Jing, X.L., Zou, H.G., Liu, S.L., Eds.; Science Press: Beijing, China, 1981; pp. 390–538, (In Chinese with English Summary). [Google Scholar]
- Putshkov, P.V.; Moulet, P. Faune de France. 92. Hémiptères Reduviidae d’Europe Occidentale; Fédération Française des Sociétés de Sciences Naturelles: Paris, France, 2010; pp. 1–668. [Google Scholar]
- Sahayaraj, K.; Subash, N.; Allingham, R.W.; Kumar, V.; Avery, P.B.; Mehra, L.K.; McKenzie, C.L.; Osborne, L.S. Lethal and sublethal effects of three microbial biocontrol agents on Spodoptera litura and its natural predator Rhynocoris kumarii. Insects 2018, 9, 101. [Google Scholar] [CrossRef]
- Pradeep, P.; Deshmukh, S.S.; Kalleshwaraswamy, C.M.; Rajan, S.J. Biology and predation potential of the hemipteran predator, Rhynocoris marginatus (Fab., 1794) on the fall armyworm, Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae). Egypt. J. Biol. Pest Control 2022, 32, 64. [Google Scholar] [CrossRef]
- Idemudia, I.; Fening, K.O.; Agboyi, L.K.; Wilson, D.; Clottey, V.A.; Beseh, P.; Aigbedion-Atalor, P.O. First report of the predatory potential and functional response of the red flower assassin bug Rhynocoris segmentarius (Germar), a natural enemy of Spodoptera frugiperda (J.E. Smith). Biol. Control 2024, 191, 105465. [Google Scholar] [CrossRef]
- Lindberg, H. Schwedisch-chinesische wissenschaftliche Expedition nach den nordwestlicen Provinzen Chinas. Insekten. 47. Hemiptera. 2. Hemiptera Heteroptera. Ark. Zool. 1934, 27A, 1–43. [Google Scholar]
- Wu, C.F. Catalogue of Chinese Insects; Bulletin of the Fan Memorial Institute of Biology: Beijing, China, 1935; Volume II, pp. 1–634. [Google Scholar]
- China, W.E. Key to subfamilies and genera of Chinese Reduviidae with descriptions of new genera and species. Lingnan Sci. J. 1940, 19, 1–253. [Google Scholar]
- Hoffmann, W.E. Catalogue of Reduviidae of China. Lingnan Univ. Sci. Bull. 1944, 10, 1–80. [Google Scholar]
- Deng, H.B.; Lyu, Y.H.; Tian, M.Y.; Chen, Y.M.; Zhang, M.X.; Chen, W.M. Functional responses and searching efficiency of Harpactor fuscipes on predation of Myzus persicae. Acta Tabacaria Sin. 2015, 21, 74–78, (In Chinese with English Summary). [Google Scholar] [CrossRef]
- Su, X.N.; Deng, H.B.; Zhu, D.L.; Cai, Q.N.; Zhang, M.X. Study on indoor dispersive capacity and predatory behavior of Harpactor fuscipes (Fabricius) to larvae of Spodoptera litura. Acta Tabacaria Sin. 2016, 22, 111–119, (In Chinese with English Summary). [Google Scholar] [CrossRef]
- Xue, C.; Mao, J.; Xu, B.; Zhou, L.; Zhou, H.; Mao, J.; Shen, Z.; Zhang, L.; Wang, M.; Li, Y. Biological control potential of the reduviid predator Rhynocoris fuscipes (Fabricius) in managing noctuid pests: Insights on predation and prey preference. Insects 2025, 16, 224. [Google Scholar] [CrossRef]
- Davis, N.T. Contribution to the morphology and phylogeny of the Reduvioidea (Hemiptera: Heteroptera). Part III. The male and female genitalia. Ann. Entomol. Soc. Am. 1966, 59, 911–924. [Google Scholar] [CrossRef]
- Weirauch, C. Cladistic analysis of Reduviidae (Heteroptera: Cimicomorpha) based on morphological characters. Syst. Entomol. 2008, 33, 229–274. [Google Scholar] [CrossRef]
- Hahn, C.W. Die Wanzenartigen Insecten, Getreu Nach Der Natur Abgebildet Und Beschrieben; Zeh Nuremberg: Nuremberg, Germany, 1834; Volume 2, pp. 20–23. [Google Scholar]
- Kirkaldy, G.W. Bibliographical and nomenclatorial notes on Rhynchota No.1. Entomologist 1900, 33, 238–243. [Google Scholar] [CrossRef]
- Amyot, C.J.B.; Serville, J.G.A. Histoire Naturelle des Insectes, Hémiptères; Libraire Encyclopédique de Roret: Paris, France, 1843; pp. i–lxxvi + 1–675. [Google Scholar]
- Kolenati, F.A. Meletemata entomologica. Fasc. VI. Hemipterorum Heteropterorum Caucasi. Harpagocorisae, monographice dispositae. Bull. Soc. Imp. Nat. Moscou 1857, 29, 419–502. [Google Scholar]
- Stål, C. Genera reduviidarum Europae disposuit. Öfversigt Af K. Vetenskapsakademiens Förhandlingar 1872, 29, 43–48. [Google Scholar]
- Villiers, A. Faune de l’Empire Français. IX. Hémiptères Réduviidés de l’Afrique Noire; Éditions du Muséum: Paris, France, 1948; pp. 1–489. [Google Scholar]
- Stål, C. Enumeratio Hemipterorum IV. Kungliga Svenska Vetenskapsakademiens Handlingar (N.F.) 1874, 12, 1–186. [Google Scholar]
- Jeannel, R. Insectes Hémiptères, III. Henicocephalidae et Reduviidae. In Voyage Alluaud et R. Jeannel en Afrique Orientale (1911–1912); Résultats Scientifiques: Paris, France, 1919; Volume 3, pp. 133–313. [Google Scholar]
- Stål, C. Bidrag till Reduviidernas kännedom. Öfversigt Af K. Vetenskapsakdemiens Förhandlingar 1867, 23, 235–302. [Google Scholar]
- Reuter, O.M. Ad cognitionem Reduviidarum mundi antique. Acta Soc. Sci. Fenn. 1883, 12, 269–339. [Google Scholar]
- Distant, W.L. The Fauna of British India, Including Ceylon and Burma. Rhynchota 2 (Heteroptera); Taylor and Francis: London, UK, 1904; pp. 1–503. [Google Scholar] [CrossRef]
- Putshkov, V.G.; Putshkov, P.V. III. Harpactorinae. In A Catalogue of the Assassin Bugs (Heteroptera, Reduviidae) of the World; Putshkov, V.G., Putshkov, P.V., Eds.; VINITI: Moscow, Russsia, 1988; Volume 3, pp. 1–264. [Google Scholar]
- Fabricius, J.C. Mantissa insectorum sistens species nuper detectas adjectis synonymis, observationibus, descriptionibus, emendationibus. Christ. Gottlieb Proft. Hafniae 1787, 2, 1–382. [Google Scholar]
- Wolff, J.F. Abbildungen der Wanzen mit Beschreibungen. Icones Cimicum Descriptionibus Illustrate; Johann Jacob Palm: Erlangen, Germany, 1804; pp. 127–166. [Google Scholar]
- Stål, C. Till kännedomen om Reduvini. Öfversigt Af K. Vetenskapsakademiens Förhandlingar 1859, 16, 1–203. [Google Scholar]
- Lepeletier, A.L.M.; Serville, J.G.A. Encyclopédie Méthodique; Oliver, G.A., Ed.; Agasse: Paris, France, 1825; Volume 10, pp. 1–344. [Google Scholar]
- Walker, F. Catalogue of the Specimens of Hemiptera Heteroptera in the Collection of the British Museum; Trustees of the British Museum: London, UK, 1873; Part VII; pp. 1–123. [Google Scholar]
- Kirby, W.F. Catalogue of the described Hemiptera Heteroptera and Homoptera of Ceylon based on the collection formed (chiefly at Pundaloya) by Mr. E. Ernest Green. Zool. J. Linn. Soc. 1891, 24, 72–176. [Google Scholar] [CrossRef]
- Distant, W.L. Rhynchotal Notes. XVI. Heteroptera: Family Reduviidae (continued), Apiomerinae, Harpactorinae and Nabidae. Ann. Mag. Nat. Hist. Zool. Bot. Geol. 1903, 7, 203–213. [Google Scholar] [CrossRef]
- Bergroth, E.H. Sauter’s Formosa-Ausbeute: Hemiptera Heteroptera I. Aradidae, Phyrrhocoridae, Myodochidae, Tingidae, Reduviidae, Ochtheridae. Entomol. Mitt. 1914, 3, 353–364. [Google Scholar]
- You, Z.Y.; Sun, Z.; Xia, C.J.; Deng, H.B. Effect of different oviposition substrates on oviposition biology of Rhynocoris fuscipes. Tianjin Agric. Sci. 2023, 29, 53–56. Available online: http://tjnykx.paperopen.com/#/digest?ArticleID=4289 (accessed on 25 March 2023). (In Chinese with English Summary).
- Deng, H.B.; Lv, Y.H.; Qiu, M.W.; Lu, Z.; Chen, Y.M.; Tian, M.Y. Studies on the biological characteristics of predatory bug Harpactor fuscipes. Chin. Tob. Sci. 2014, 35, 109–112, (In Chinese with English Summary). [Google Scholar] [CrossRef]
- You, Z.Y.; Liu, P.P.; Pu, X.M.; Sun, Z.; Deng, H.B. Studies on the predatory functional response of Harpactor fuscipes to Agrotis ipsilon. Tianjin Agric. Sci. 2023, 29, 49–55, (In Chinese with English Summary). [Google Scholar] [CrossRef]
- You, Z.Y.; Zeng, T.; Liu, P.P.; Chen, D.X.; Pu, X.M.; Sun, Z.; Deng, H.B. Control ability of Harpactor fuscipes against Spodoptera frugiperda larvae. Environ. Entomol. 2023, 45, 1653–1664, (In Chinese with English Summary). [Google Scholar] [CrossRef]
- Durkga, S.J.; Sudarmani, D.N.P.; Sundareswari, C. Diversity and effect of meteorological factors on the reduviid bug population from selected habitats of Virudhunagar District, Tamil Nadu, India. Int. J. Entomol. Res. 2023, 8, 60–64. [Google Scholar]
- Ambrose, D.P. Bioecology of Rhinocoris fuscipes Fabr. (Reduviidae) a potential predator on insect pests. Uttar Pradesh J. Zool. 1986, 6, 36–39. Available online: https://www.researchgate.net/publication/320383562 (accessed on 13 October 2017).
- Sahayaraj, K.; Selvaraj, P. Life table characteristics of Rhynocoris fuscipes Fabricius in relation to sex ratios. Ecol. Environ. Conserv. 2003, 9, 115–119. Available online: https://www.researchgate.net/publication/253310979 (accessed on 24 April 2016).
- Ambrose, D.P.; Claver, M.A. Functional and numerical responses of the reduviid predator, Rhynocoris fuscipes F. (Het., Reduviidae) to cotton leafworm Spodoptera litura F. (Lep., Noctuidae). J. Appl. Entomol. 2009, 121, 331–336. [Google Scholar] [CrossRef]
- Sunil, V.; Sampathkumar, M.; Lydia, C.; Chiranjeevi, K.; Shanker, C. Biology, predatory potential and functional response of Rhynocoris fuscipes (Fabricius) (Hemiptera: Reduviidae) on rice brown planthopper, Nilaparvata lugens (Stål) (Homoptera: Delphacidae). J. Exp. Zool. India 2018, 21, 259–263. [Google Scholar]
- Sundareswari, C.; Sudarmani, D.N.P.; Durkga, S.J. Population dynamics of phytophagous pest Epilachna vigintioctopunctata (Coccinellidae: Coleoptera) and natural enemy Rhynocoris fuscipes Fab. on selected brinjal cultivars in Virudhunagar. Int. J. Entomol. Res. 2023, 8, 70–72. Available online: https://www.researchgate.net/publication/370648897 (accessed on 10 May 2023).
- Janzen, D.H.; Ataroff, M.; Fariñas, M.; Reyes, S.; Rincon, N.; Soler, A.; Soriano, P.; Vera, M. Changes in the arthropod community along an elevational transect in the Venezuelan Andes. Biotropica 1976, 8, 193–203. [Google Scholar] [CrossRef]
- Hodkinson, I.D. Terrestrial insects along elevation gradients: Species and community responses to altitude. Biol. Rev. Camb. Philos. Soc. 2005, 80, 489–513. [Google Scholar] [CrossRef] [PubMed]
- Baskar, A.; Ambrose, D.P.; Tirumurugaan, K.G.; Fleming, A.T. Ecotypic diversity in the assassin bug Rhynocoris fuscipes (Fabricius) (Heteroptera: Reduviidae). J. Adv. Zool. 2012, 33, 113–121. Available online: https://www.researchgate.net/publication/289842836 (accessed on 19 September 2017).
- Sahayaraj, K.; Selvaraj, P.; Nirmala, K. Biological control potential of a reduviid predator, Rhynocoris fuscipes (Fab) on three groundnut pests. Asian J. Microbiol. Biotechnol. Environ. Sci. 2002, 4, 451–455. Available online: https://www.researchgate.net/publication/294133577 (accessed on 25 June 2018).
- Tomson, M. Functional response, host stage preference and development of Rhynocoris fuscipes (Fab.) (Heteroptera: Reduviidae) for two cotton pests. J. Biopestic. 2021, 14, 12–21. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Chen, Z.; Xiong, H.; Chen, Z.; Li, H.; Zhao, P.; Cai, W. Comments on Two Controversial Oriental Assassin Bug Species of the Genus Rhynocoris (Heteroptera: Reduviidae: Harpactorinae), with the Description of R. minutus sp. nov. from China. Insects 2025, 16, 823. https://doi.org/10.3390/insects16080823
Liu H, Chen Z, Xiong H, Chen Z, Li H, Zhao P, Cai W. Comments on Two Controversial Oriental Assassin Bug Species of the Genus Rhynocoris (Heteroptera: Reduviidae: Harpactorinae), with the Description of R. minutus sp. nov. from China. Insects. 2025; 16(8):823. https://doi.org/10.3390/insects16080823
Chicago/Turabian StyleLiu, Huaiyu, Zhuo Chen, Haoyang Xiong, Zhaoyang Chen, Hu Li, Ping Zhao, and Wanzhi Cai. 2025. "Comments on Two Controversial Oriental Assassin Bug Species of the Genus Rhynocoris (Heteroptera: Reduviidae: Harpactorinae), with the Description of R. minutus sp. nov. from China" Insects 16, no. 8: 823. https://doi.org/10.3390/insects16080823
APA StyleLiu, H., Chen, Z., Xiong, H., Chen, Z., Li, H., Zhao, P., & Cai, W. (2025). Comments on Two Controversial Oriental Assassin Bug Species of the Genus Rhynocoris (Heteroptera: Reduviidae: Harpactorinae), with the Description of R. minutus sp. nov. from China. Insects, 16(8), 823. https://doi.org/10.3390/insects16080823







