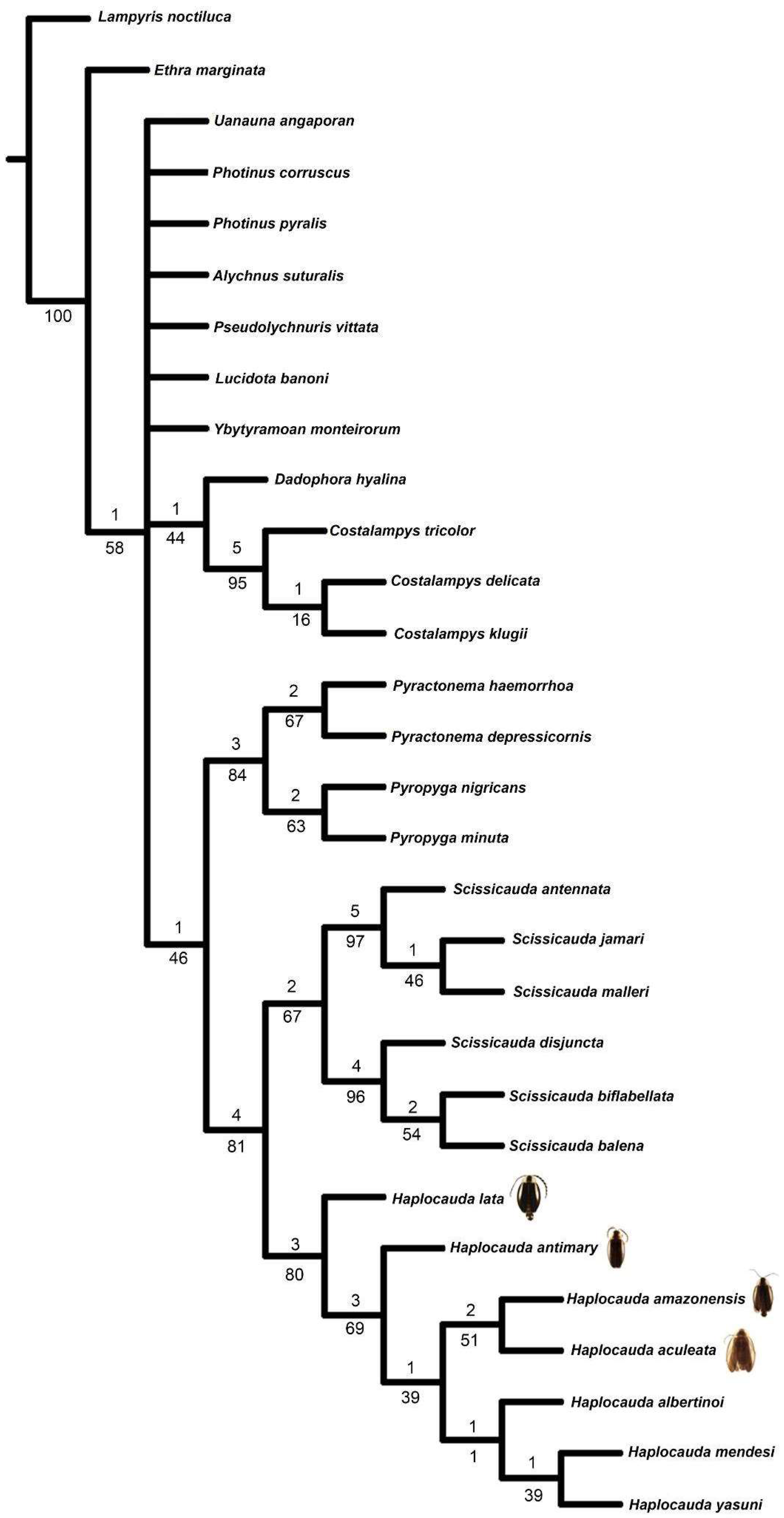
3.2. Taxonomy
Lampyridae Rafinesque, 1815
Lampyrinae Rafinesque, 1815
Photinini Olivier, 1907
Haplocauda Silveira, Lima, and McHugh, 2022
Type species: Haplocauda albertinoi Silveira, Lima, and McHugh, 2022, by original designation.
Diagnosis. Antennae serrate, without branches (
Figure 4G,N,U,Bb), tibial spurs 1-2-2 (1-2-1 in
H. yasuni Silveira, Lima, and McHugh, 2022 and
H. amazonensis sp. nov.).
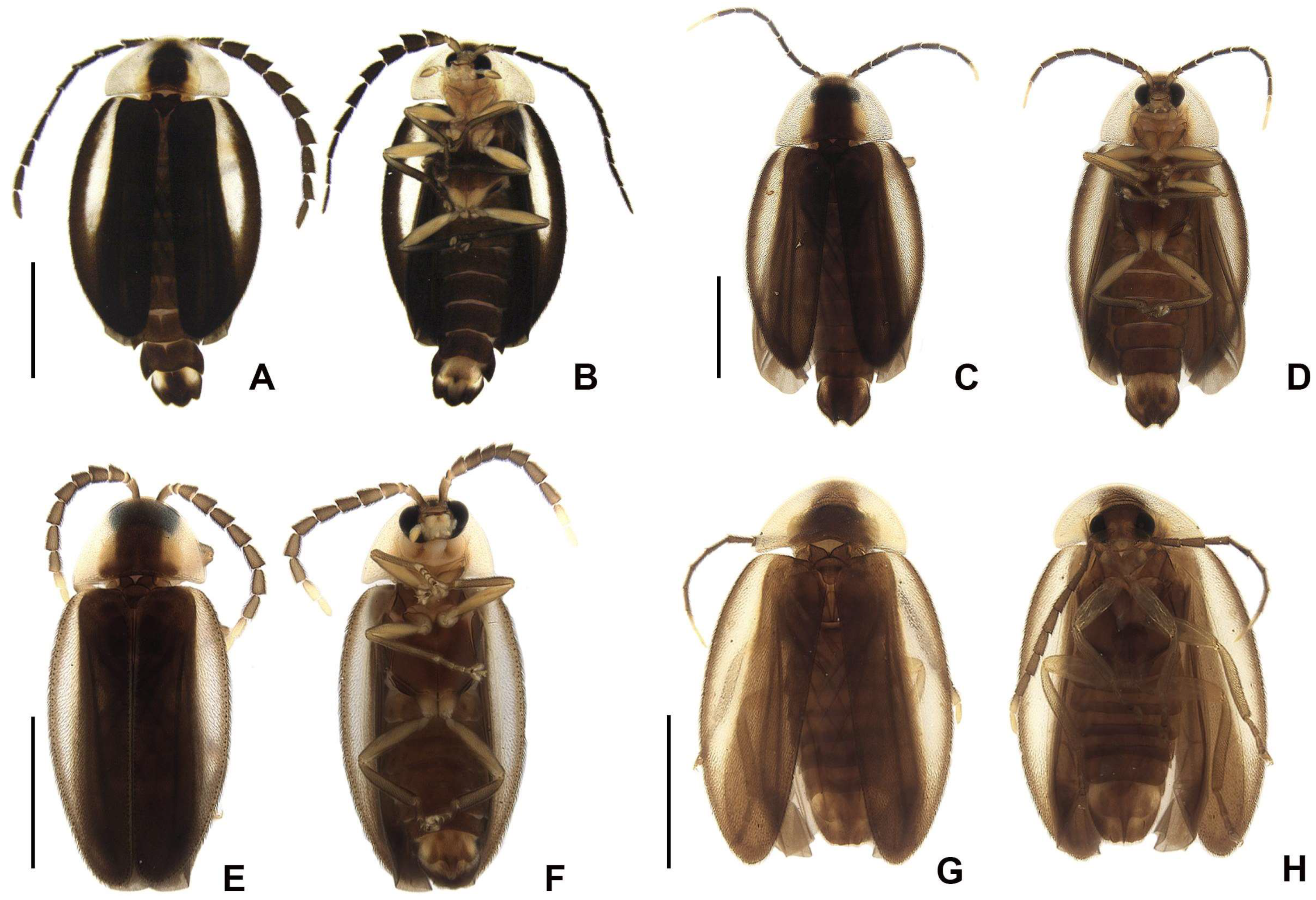
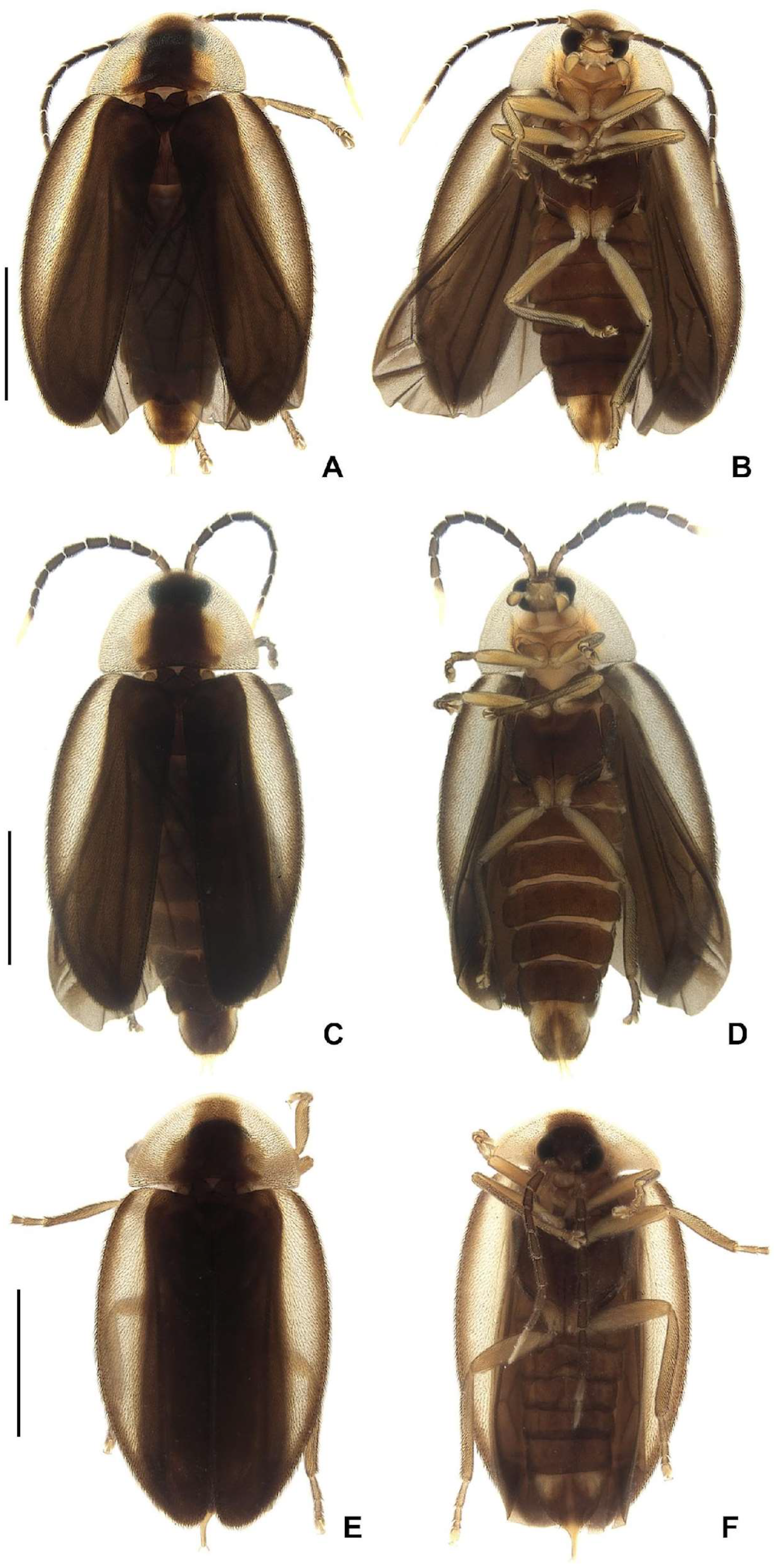
Color pattern (
Figure 3): body overall brown, except for light-brown pronotal lateral expansions, and a longitudinal light-brown lateral stripe on elytron (outlined in brown or light brown); antennomeres IX–XI sometimes creamy white; legs light brown, sometimes with brown tibia and tarsus; pygidium sometimes with translucent lateral spots; sternum VIII with translucent lateral spots.
Male: anterior claw of pro- and mesolegs with basal teeth; pygidium with anterior margin slightly or strongly emarginated, with apically blunt anterior projections (absent in
H. lata sp. nov. and
H. antimary sp. nov.), posterior margin without a median indentation, posterior angles acute or rounded; sternum VIII at least 2× longer than VII, with a posterior projection of variable length and width (absent in
Scissicauda); sternum IX with lateral rods basally fused and posteriorly thickened, posterior margin of sternum IX rounded (emarginated in
H. lata sp. nov.) with an acute projection (absent in
H. lata sp. nov.,
H. aculeata sp. nov. and
H. antimary sp. nov.).
Female: pygidium with posterior margin truncated or rounded; sternum VIII as long as wide and spiculum ventrale long and slender, as long as 3/4 or 2/3 sternum, with posterior margin slightly or deeply emarginate; internal genitalia with a large and somewhat rounded spermatophore-digesting gland and a lump-like spermatheca, bursa copulatrix with paired elongated and weakly sclerotized plates, with a very long accessory gland.
Redescription
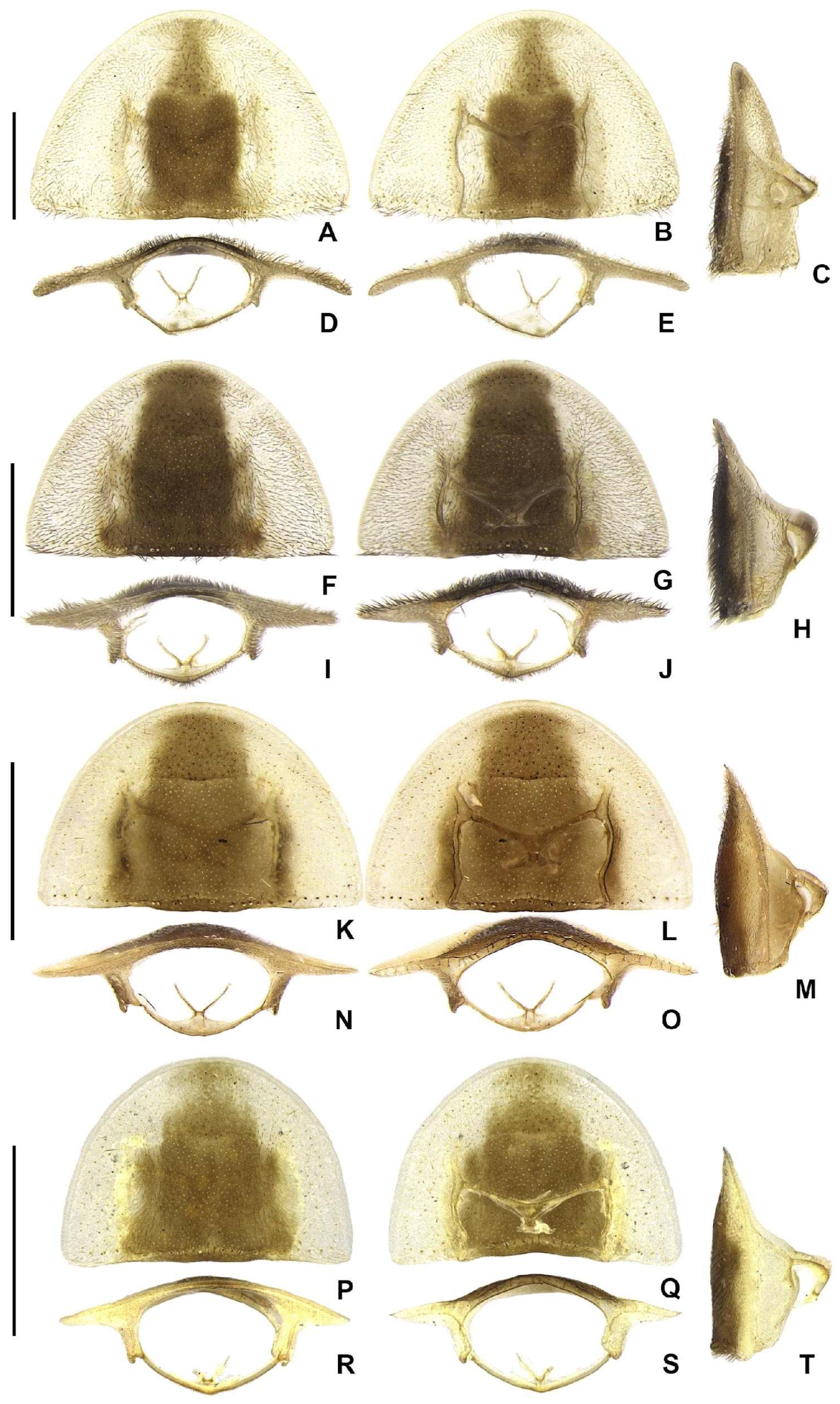
Head entirely covered by pronotum when retracted (
Figure 4A–F,H–M,O–T,V–Aa). Head capsule about 1.5–2× as wide as long, lateral margins slightly convergent posteriorly (
Figure 4B); vertex somewhat convex. Antennal sockets reniform, slightly wider than distance between sockets, antennifer process conspicuous (
Figure 4C). Antenna 11-segmented, scape constricted proximally, pedicel almost as long as wide and constricted basally, antennomeres III–X variably serrated and without lamellae, without upright bristles, antennomeres IX– or X–XI brown or creamy white, apical antennomere about as long as the subapical (
Figure 4G,N,U,Bb). Frontoclypeus very wide, slightly curved (
Figure 4C). Labrum connected to frontoclypeus by a membranous suture (
Figure 4C); nearly 4× as wide as long, anterior margin evanescent (
Figure 4A,C). Mandibles largely overlapping, long and slender, evenly arcuate, apex acute, without internal tooth, external margin sparsely setose in basal 1/2 (
Figure 4A,C). Maxilla weakly sclerotized (
Figure 4I); stipe about 2× as long as wide, posterior margins truncated, palpi 4-segmented, palpomere III subtriangular, IV lanceolate, with internal margin covered with minute, dense bristles, almost 2–3× longer than III (
Figure 4C). Labium weakly sclerotized (
Figure 4B); mentum completely divided sagittally, submentum sclerotized and bearing bristles, subcordiform or subrectangular, elongated, palpi 3-segmented, palpomere III securiform (
Figure 4B). Gular sutures almost indistinct, gular bar transverse, nearly 3× as as wide as long (
Figure 4B). Occiput piriform, 1/3 narrower than head capsule (
Figure 4F).
Thorax with pronotum semilunar, with posterior angle rounded, disc subquadrate in dorsal view, almost flat in lateral view (
Figure 5C,H,M,T), regularly punctured, punctures small and pubescent, with a line of distinct deep marginal punctures (
Figure 5A,F,K,P); pronotal expansions well developed, anterior expansion maximal length almost 1/2 as long as disc, posterior expansions almost straight (
Figure 5B); slightly wider than distance between elytral humeri (
Figure 3). Hypomeron short in lateral view (width of lateral expansion of pronotum at least 1.5–2× greater than hypomeron depth) (
Figure 5E). Prosternum 4 as wide as its major length, slightly narrowed parasagittally (
Figure 5C). Proendosternite elongated, about as long as distance between the apices of proendosternite arms (
Figure 5B,G,L,Q). Mesoscutellum with posterior margin rounded (
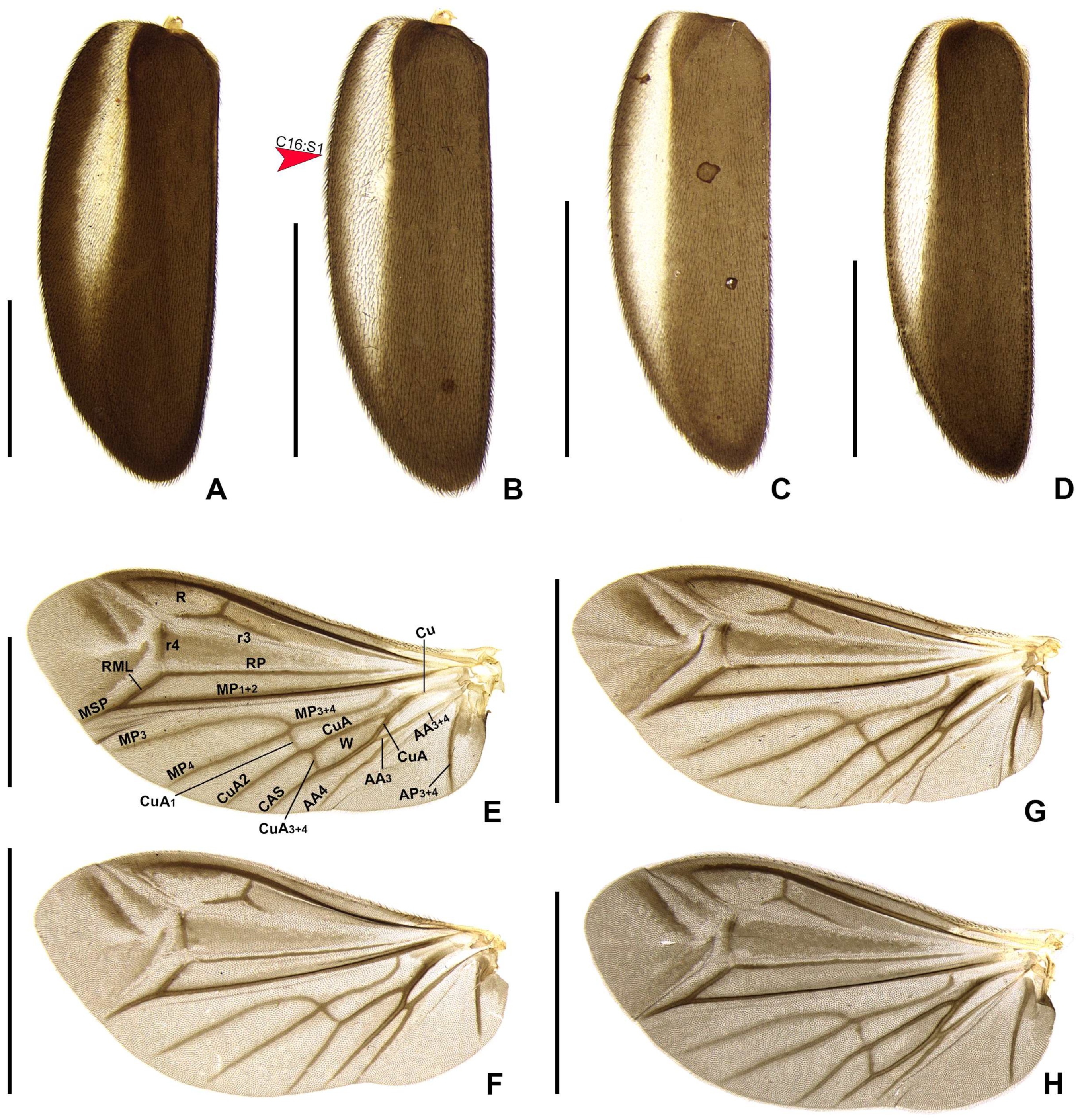
Figure 3). Elytron ellipsoid, almost 2.6–3.5× as long as wide, pubescent, with a row of conspicuous punctures surrounding sutural and lateral margins (
Figure 6A–D). Hind wing well developed, posterior margin slightly sinuate, 2× as long as wide; CuA2 present, mp-cu crossvein present; RP + MP1 + 2 3/4 r4 length, almost reaching distal margin, J indistinct (
Figure 6E–H). Profemur about as long as protibia, meso and metafemora slightly shorter than respective tibiae. Tibial spur formula: protibia 1 (only posterior), mesotibia 2, metatibia 1 (only anterior) or 2. Male with anterior claw of pro and mesothoracic legs with basal tooth. Tarsomere I 2× longer than II, II 2× longer than III, III subequal in length to IV, IV bilobed, lobes reaching 2/3 V length. Metendosternum spatulate, 2 as long as wide, median projection acute anteriad, with two lateral laminae.
Abdomen with tergum I with anterior margin membranous, laterotergite membranous, nearly rounded, with sparse bristles; spiracle obliquely oriented on the thorax. Terga II–VII with posterolateral angles more produced and acute posteriorly, posterior margins more bisinuate. Sterna II–VIII visible. Spiracles dorsal, at about 1/2 sterna lengths.
Male. Sternum VIII 2× longer than VII, with a long and wide posterior projection (
Figure 7A,G,P,W), with “larval” lanterns elongated. Pygidium with anterior margin strongly emarginated, with thick, sinuate, and apically blunt anterior projections; posterior margin without a median indentation; posterolateral angles acute (
Figure 7B,J,Q,X). Syntergite medially divided, bearing bristles posteriorly, not anteriorly fused to sternum IX, 1/3 shorter than sternum IX, connate to sternum IX along its length (
Figure 7C,K,R,Y). Sternum IX with lateral rods basally fused and posteriorly thickened, posterior margin of sternum IX with an acute projection (
Figure 7K). Aedeagus with phallobase bilaterally symmetrical, sides abruptly convergent basally; dorsal plate of phallus basally fused to parameres, apically indented and with arms apically curved and approximate; parameres sinuate, nearly 1/5 longer than dorsal plate of phallus, lacking ventral rods; ventral plate rudimentary, restricted to a sclerotized piece outlining the opening of the ejaculatory duct or I-shaped, as long as 1/2 dorsal plate of phallus (in
H. amazonensis sp. nov. and
H. antimary sp. nov.) (
Figure 7D–F,M–O,T–V,Aa–Cc).
Female. Overall similar to male (
Figure 8), except for the following traits. Metatibia with two spurs. Pygidium with posterior margin truncate (
Figure 9E) or rounded (
Figure 9H). Sternum VIII as long as wide, spiculum ventrale long and slender, 3/4 sternum length, with posterior margin moderately indented (
Figure 9D) or deeply indented (
Figure 9A,G). Internal genitalia with a large and somewhat rounded spermatophore-digesting gland and a lump-like (i.e., lacking a pedicel) spermatheca, bursa copulatrix with paired elongate, weakly sclerotized plates, or with eight irregularly shaped well-sclerotized plates [
12] with a very long accessory gland (
Figure 9J–L). Ovipositor (
Figure 9C,F,I) with paraproctal valve 3× longer than paraproct core; coxites convergent posteriorly, well developed but weakly sclerotized; styli minute, sclerotized; proctiger plate entire, elongate, weakly sclerotized.
Distribution. Amazon Rainforest, South America (
Figure 10).
Remarks. Haplocauda was classified in Photinini (Lampyridae: Lampyrinae) in the original description. Many taxonomic works of South American fireflies considered Photinini as valid, only recently synonymized with Lucidotini ([
25] but made widely known via [
1]). Among the Lucidotini included in our matrix,
Haplocauda was found closely related to other genera of Lampyrinae:
Pyractonema Solier, 1849 (Lucidotina),
Pyropyga Motschulsky, 1853 (Photinina) and
Scissicauda McDermott, 1964 (Photinina). This group has been called the “
Scissicauda lineage” and might warrant a higher taxonomic rank recognition, like subtribe, in the future.
Haplocauda can be distinguished from Scissicauda, Pyractonema and Pyropyga by the median projection of sternum VIII, syntergite asymmetrical in length (except in H. lata sp. nov.), sternum IX with a pointed projection on the posterior margin. These characteristics, along with the presence of a tooth on the anterior tarsal claw of the pro- and mesoleg (present in all Haplocauda and Scissicauda) and the pygidium with anterior blunt projections (except in H. lata sp. nov. and H. antimary sp. nov., see discussion below), are also useful to differentiate Haplocauda from Lucidota, a genus with superficially similar species (like Lucidota mellicula Olivier 1907) which lacks a clear diagnosis and is taxonomically poorly delimited.
The median projection of the posterior margin of sternum VIII is the most diverse feature among
Haplocauda species. In the original description of the genus, the posterior margin of sternum VIII with a long and wide posterior projection was sufficient to describe this structure, despite the clear differences in length and shape. However, here we describe species that present this narrow and extremely short (
H. lata sp. nov.) or long and extremely narrow (
H. aculeata sp. nov.) projection (
Figure 7A,P).
The tentatively associated females of
Haplocauda are morphologically similar to the females of
Scissicauda, and the recent discovery of new species in these two genera has overlapped some characteristics that differentiated the females of these groups, such as the shape of the pygidium [
5,
13]. The females of
Haplocauda can be distinguished from
Scissicauda by having only one spur of protibia (two spurs in
Scissicauda).
Haplocauda lata Zeballos and Silveira sp. nov.
urn:lsid:zoobank.org:act:02C7915C-0F6F-4B07-8BAE-44EE14996F6E
Diagnostic description. Antennomeres entirely brown (
Figure 4G). Pygidium brown with anterior corners mostly light brown to translucent (
Figure 7B). Sternum VIII mostly light brown to translucent, with projection well sclerotized (
Figure 7A).
Male. Metatibia with two spurs. Pygidium with anterior blunt projections absent, central 1/3 indented, margin posterior bisinuate (
Figure 7B). Sternum VIII with posterior projection short, as long as 1/5 the width of sternum VIII, basally as wide as 1/5 sternum width (
Figure 7A). Sternum IX with margin posterior emarginate, acute projection absent (
Figure 7C).
Female (
Figure 8A,B). Pygidium 1.5× as long as wide, with anterior margin emarginated, posterior margin truncated (
Figure 9B). Sternum VIII with spiculum ventrale almost 1/3 shorter than sternum, posterior margin strongly indented (
Figure 9A). Internal genitalia with spermatophore-digesting gland larger than spermatheca, bursa copulatrix with plate irregularly shaped, well sclerotized (
Figure 9J). Ovipositor with valvifers free, twisted basally, 2× longer than coxite; coxites convergent posteriorly, divided in distinct proximal and distal plates, both well developed but weakly sclerotized; styli minute, sclerotized; proctiger plate weakly sclerotized (
Figure 9C).
Immature stages unknown.
Etymology. The species epithet lata is a Latin adjective meaning “wide” or “broad.” It refers to the total width of the elytra, which is noticeably greater in this species compared to its congeners. Adjectival name.
Type material. HOLOTYPE (1♂, dissected, INPA), Brasil, Rondônia, Porto Velho,, ESEC Morro 3 Irmãos, vi.2017,, 9°00′09″ S—64°32′40″ W, Malaise,, V. S. Silva & J. A. Rafael- Rede BIA// Holotype Haplocauda lata [tracing paper, handwritten]. PARATYPES (27♂ and 10♀). (3♂, one dissected, alcohol, INPA), Brasil, AM, Tapauã,, Rio Ipixuna, Porto Coutinho,, 13–18.x.2013, Terra- Firme,, Malaise, D. M. M. Mendes Leg// Paratype Haplocauda lata [tracing paper, handwritten]. (2♂, alcohol, INPA),Brasil, AM, Careiro Castanho,, Br 319 Km 181, Sítio S. Paulo,, 4°12′48″ S—60°49′04″ W,, 16–31.xii.2016. Malaise G.,, J.A. Rafael & F. F. Xavier F°// Paratype Haplocauda lata [tracing paper, handwritten]. (1♀, alcohol), same, except for the date, 14–27.iii.2011// Paratype Haplocauda lata [tracing paper, handwritten]. (2♂, alcohol), same, except for the date, 16–31.i.2017// Paratype Haplocauda lata [tracing paper, handwritten]. (1♂, 1♀, alcohol), BR-AM, Tapauã, Rio,, Ipixuna, Porto Coutinho,, 08–13.x.2013. Terra Firme,, Malaise. D. M. M. Mendes,, leg.// INPA-COL,, 001572// Paratype Haplocauda lata [tracing paper, handwritten]. (1♂ and 1♀, alcohol, INPA), BR-AM, Ipixuna, Porto,, Coutinho,, 13–18.x.2013,, Terra Firme. Malaise,, D. M. M. Mendes leg.// INPA-COL,, 001648// Paratype Haplocauda lata [tracing paper, handwritten] (3♂, 1♀ pinned, UFMT), Brasil, Amazonas, Careiro,, Castanho, PPBIO, 03°40′42″,, S, 60°19′41″ W, 11–13.xii.,, 2013, malaise, J.A.Rafael,, J.T.Camara & F.F.Xavier F°// Paratype Haplocauda lata [yellow label]. (1♂, pinned, MZUSP), BR. AM. Manaus ZF-03,, BR 174 Km 41 Res. 1401,, Gavião,, 02°24′09″ S/59°49′45″ W// 16–31/X/1995,, Rocha e Silva, L.E.F. col.// 0061942// Malaise// Paratype Haplocauda lata [yellow label]. (1♂, pinned, MZUSP), the same as above except for the following label 0061938// Paratype Haplocauda lata [yellow label]. (1♂, pinned, MZUSP), the same as above except for the following label 0061939// Paratype Haplocauda lata [yellow label]. (1♂, pinned, MZUSP), the same as above except for the following label 0061934// Paratype Haplocauda lata [yellow label]. (1♂, pinned, MZUSP), the same as above except for the following label 0061941// Paratype Haplocauda lata [yellow label]. (1♂, pinned, MZUSP), the same as above except for the following label 0061937// Paratype Haplocauda lata [yellow label]. (1♀, pinned, MZUSP), the same as above except for the following label 0061921// Suspensa// Paratype Haplocauda lata [yellow label]. (1♂, pinned, INPA), Brasil, AM, Resex Urini,, Rio Urini, Lago 3 bocas,, 01°34′56″ S, 62°58′28″ W,, 14–22.vii.2004// Luz mista de,, mercúrio + BLB (lençol),, A. Silva F. & L. Aquino// INPA-COL,, 001194// Paratype Haplocauda lata [yellow label]. (1♂, pinned, INPA), BRASIL, AM. Parque,, Nacional do Jaú, Rio Urini,, 01°40′31″ S-61°46′34″ W,, 20–23.xi.1996. Malaise,, A. Henriques & J. Vidal/ INPA-COL,, 001242// Paratype Haplocauda lata [yellow label]. (1♂, pinned, DZUP), BR-AM, Autaz Mirin,, 15–30.i.1995; Bindá,, Arm Malaise// INPA-COL,, 001357// Paratype Haplocauda lata [yellow label]. (1♂, pinned, DZUP), BR-AM, Autaz Mirin,, 01–15.i.1995.,, Bindá col.// INPA-COL,, 001358// Paratype Haplocauda lata [yellow label]. (1♂, pinned, DZUP), the same as above except for the following label INPA-COL,, 001356// Paratype Haplocauda lata [yellow label]. (1♂, pinned, DZUP), the same as above except for the following label INPA-CO,, 001359// Paratype Haplocauda lata [yellow label]. (1♂, pinned, DZUP), the same as above except for the following label INPA-COL,, 001360// Paratype Haplocauda lata [yellow label]. (1♂, pinned, DZUP), the same as above except for the following label INPA-COL,, 001361// Paratype Haplocauda lata [yellow label]. (1♂, pinned, DZUP), the same as above except for the following label INPA-COL,, 001362// Paratype Haplocauda lata [yellow label]. (1♀, pinned, INPA), BRASIL, AM, Resex Urini,, Rio Urini, Lago 03 Bocas,, 01°34′13″ S, 62°58′54″ W,, 14–28.vii.2004// Arm. Malaise terra firme,, M.L.Oliveira, A. Silva F.,, L. Aquino leg.// Paratype Haplocauda lata [yellow label]. (2♀, pinned, INPA), BRASIL-PA-Melgaço,, Caxiuanã-ECFPn,, 23.iii.1998,, O. Silveira, J. Pena col.// Coleoptera: Polyphaga,, Elateriformia: Cantharoidea:,, Lampyridae,, Incorp: 22/iii/2002// Paratype Haplocauda lata [yellow label]. (1♀, pinned, INPA), BRASIL-PA-Melgaço,, Caxiuanã-ECFPn,, 23.iii.1998,, O. Silveira, J. Pena col.// Arm. S-Malaise,, SME 1,, ECFPn,, 23.iii.1998// Coleoptera: Polyphaga,, Elateriformia: Cantharoidea:,, Lampyridae,, Incorp: 22/iii/2002// Paratype Haplocauda lata [yellow label]. (1♂, pinned, INPA), BRASIL-PA-Melgaço,, Caxiuanã-ECFPn,, 23.iii.1998,, O. Silveira, J. Pena col.// Arm. S-Malaise,, SME 4,, Igarapé Curuazinho,, 23–28.iii.1998// Coleoptera: Polyphaga,, Elateriformia: Cantharoidea:,, Lampyridae,, Incorp: 22/iii/2002// Paratype Haplocauda lata [yellow label]. (1♀, pinned, INPA), BRASIL-PA-Melgaço,, Caxiuanã-ECFPn,, 23.iii.1998,, O. Silveira, J. Pena col.// Arm. S-Malaise,, SME 5,, ECFPn,, 20–29.iii.1998// Coleoptera: Polyphaga,, Elateriformia: Cantharoidea:,, Lampyridae,, Incorp: 22/iii/2002// Paratype Haplocauda lata [yellow label].
Distribution. Brazil: Amazonas: Careiro Castanho, Manaus, São Gabriel da Cachoeira, Tapauá, Resex Rio Unini; Pará: Melgaço; Rondônia: Porto Velho (Type locality) (
Figure 10).
Remarks. Haplocauda lata
sp. nov. is one of the largest species in the genus, along with
H. albertinoi Silveira, Lima, and McHugh, 2022, and the second species described with the antenna entirely brown (the other one is
H. mendesi Silveira, Lima, and McHugh, 2022). This species is unique among its congenerics due to the extremely short median projection of sternum VIII, wide elytra, pygidium with anterior margin emarginate, with anterior blunt projections absent and sternum IX with an emarginate posterior margin (
Figure 7B,C). The species studied in this work were collected by Malaise and light traps. It is the most widely distributed species to date, being found in three Brazilian states: Amazonas, Pará and Rondônia.
Haplocauda amazonensis Zeballos and Silveira sp. nov.
urna:lsid:zoobank.org:act:02C7915C-0F6F-4B07–8BAE-44EE14996F6E
Diagnostic description. Antennomeres X–XI creamy white (
Figure 4N). Pygidium brown to dark brown with anterior blunt projections mostly light brown to translucent (
Figure 7J). Sternum VIII with anterior corners light brown to translucent, with two rounded brown parasagittal spots, with projection well sclerotized.
Male. Metatibia with one spur. Pygidium with anterior blunt projections well developed, subparallel-sided and with inner margin almost straight, posterior margin with central 1/3 slightly emarginated (
Figure 7J). Sternum VIII with posterior projection basally as wide as ¼ sternum width, posterior projection with two basal apophyses (
Figure 7G–I, blue arrows), acute, almost reaching the posterior margin of the pygidium (
Figure 7G). Sternum IX with acute projection present, 1/5 longer than syntergite (
Figure 7K).
Female (
Figure 8C,D). Pygidium 1.3× as long as wide, with anterior margin emarginated, posterior margin almost straight (
Figure 9E). Sternum VIII with spiculum ventrale almost 1/3 shorter than sternum, posterior margin strongly indented (
Figure 9D). Internal genitalia with spermatophore-digesting gland larger than spermatheca, bursa copulatrix with plate irregularly shaped, well sclerotized (
Figure 9K). Ovipositor with valvifers free, twisted basally, 2× longer than coxite; coxites convergent posteriorly, divided in distinct proximal and distal plates, both well developed but weakly sclerotized; styli minute, sclerotized; proctiger plate weakly sclerotized (
Figure 9F).
Etymology. “Amazonensis” is a Portuguese gentilic meaning “born in Amazonas,” referring to the species’ origin in the Brazilian state of Amazonas, where Portuguese is the official language. Adjectival name.
Type material. HOLOTYPE (1♂, dissected, INPA), BR- AM, Manaus,, Reserva Ducke,, 14.ii—06.iii.2007,, Platô Norte/Sul,, Arm. Malaise,, G. Freitas & M. Feitosa cols// Holotype Haplocauda amazonensis [tracing paper, handwritten]. PARATYPES (16♂ and 14♀). (1♀, alcohol, INPA), BR- AM, Ipixuna Porto,, Coutinho,, 13–18.x.2013,, Terra Firme. Malaise,, D. M. M. Mendes leg.// Paratype Haplocauda amazonensis [tracing paper, handwritten]. (1♂, alcohol, INPA), BR- AM, Manaus, Res. Campina,, Br 174 Km 43, 27.iii-08.iv.2009,, Malaise. B. B. Souza; C. O. Kiest; G.,, Freitas; J. J. Mendes; R. Freitas,, Silva// Paratype Haplocauda amazonensis [tracing paper, handwritten]. (1♂, alcohol, INPA), Brasil- Am, Careiro Castanho,, Br 319 Km 181, Sítio São,, Paulo 4°12′48″ S—60°49′04″ W,, 16–31.xii.2016. Malaise G.,, J. A. Rafael & F. F. Xavier F°// Paratype Haplocauda amazonensis [tracing paper, handwritten]. (1♂, alcohol, INPA), BR- AM, Manaus, Reserva,, Ducke,, Platô Leste/Oeste,, 16–30.xi.2006. Malaise,, Freitas; J. Vidal; G. Freitas cols.// Paratype Haplocauda amazonensis [tracing paper, handwritten]. (1♂, alcohol, INPA), BRASIL, AM, Manaus, Reserva,, Biológica de Cuieiras ZF-2,, S 02°35′21″ W60°06′55″, 06–09.viii,, 2013. Agudelo, A.; Maldaner, C.,, Malaise [the trap was handwritten]// Paratype Haplocauda amazonensis [tracing paper, handwritten]. (1♂, alcohol, INPA), BR- AM, Manaus, ZF2,, Km 14 2°35′21″ S–,, 60°06′55″ W, 17–31,, viii.2016. Malaise,, peq. Igarapé perto,, J. A. Rafael & F. F. Xavier F°// Paratype Haplocauda amazonensis [tracing paper, handwritten]. (1♂, alcohol), BR- AM, Manaus, Reserva,, Ducke. Platô Norte/Sul,, 09–20.x.2006. Malaise,, J. Vidal; R. Ale-Rocha; G. Freitas cols.// Paratype Haplocauda amazonensis [tracing paper, handwritten]. (4♂, alcohol), Brasil, AM, Manaus,, Reserva Ducke,, CMT1, v.1995// Paratype Haplocauda amazonensis [tracing paper, handwritten]. (1♂, pinned, UFMT), BRASIL, Amazonas, Careiro,, Castanho, PPBio, 03°40′42″,, S. 60°19′41″ W, 11–13. Xii,, 2013, malaise. J. A. Rafael,, J.T.Camara & F. F. Xavier F°.// Paratype Haplocauda amazonensis [tracing paper, handwritten]. (1♂, pinned, UFMT), BRASIL, Amazonas, Careiro,, Castanho, BR 319, Km-181,, Sítio S. Paulo, 4°12′48″ S,, 60°49′04″ W, 15–30. Vi,, 2016,, malaise. J. Rafael, F. Xavier F°.// Paratype Haplocauda amazonensis [tracing paper, handwritten]. (1♂, pinned, MZUSP), Brasil Amazonas,, Am. 010. Km. 25,, Reserva Ducke// Emergence trap// 9-xi-1977,, J. Arias// Paratype Haplocauda amazonensis [tracing paper, handwritten]. (1♂, 1♀, pinned, MZUSP), BR-AM, Manaus, Reserva,, Ducke 31.09.1996,, Ulisses Luiz// Paratype Haplocauda amazonensis [tracing paper, handwritten]. (1♂, 1♀, pinned, MZUSP), BRASIL, AM, Manaus,, Rod. Am 010 Km-26,, Res. Ducke ix.2001, J. F.,, Vidal, Malaise, mata.// Paratype Haplocauda amazonensis [tracing paper, handwritten]. (1♂, pinned, DZUP), BRASIL-AM, Manaus,, Reserva Florestal Ducke,, Igarapé Bolívia 28.ii.2003,, J.M.F. Ribeiro col.// Paratype Haplocauda amazonensis [tracing paper, handwritten]. (1♀, pinned, MZUSP), BRASIL, Amazonas,, Reserva F Ducke,, Igarapé Tinga. Malaise,, 05–16.viii.2004,, Henriques, A. leg// Paratype Haplocauda amazonensis [tracing paper, handwritten]. (1♀, pinned, DZUP), BRASIL-AM, Manaus,, Reserva Florestal Ducke,, Igarapé Ipiranga iv.2003,, J.M.F. Ribeiro col.// Paratype Haplocauda amazonensis [tracing paper, handwritten]. (1♀, pinned, DZUP), BRASIL-AM, Manaus,, Reserva Floprestal Ducke,, Igarapé Uberê xii.2002,, J.M.F. Ribeiro, Jailson Vidal & J. Vidal col.// Paratype Haplocauda amazonensis [tracing paper, handwritten]. (2♀, pinned, DZUP), BR-AM, Manaus, Reserva,, Ducke 16.09.1996,, Ulisses Luiz// Paratype Haplocauda amazonensis [tracing paper, handwritten]. (2♀, pinned, UFMT), BRASIL: Amazonas, Ma-,, naus, ZF2 Km14,, 02°35′21″ S, 60°06′55″ W, 16-,, 30.ix.2016, malaise igarapé,, J.A. Rafael & F. F. Xavier F°.// Paratype Haplocauda amazonensis [tracing paper, handwritten]. (1♀, pinned, UFMT), BRASIL: Amazonas, Careiro,, Castanho, BR319, Km181,, Sítio S. Paulo, 4°12′48″ S,, 60°49′04″ W, 05–17.x.2016,, malaise, J. Rafael, F. Xavier F°// Paratype Haplocauda amazonensis [tracing paper, handwritten]. (1♀, pinned, UFMT), BRASIL: Amazonas, Ma-,, naus, ZF2Km14 tower,, 02°35′21″ S, 60°06′55″ W,, 01–15.ii.2017, malaise,, J.A. Rafael & F. F. Xavier F°.// Paratype Haplocauda amazonensis [tracing paper, handwritten]. (1♀, pinned, INPA), BRASIL-AM, Manaus,, Reserva Florestal Ducke,, Igarapé Ipiranga v.2003,, Malaise. J.M.F. Ribeiro col Paratype Haplocauda amazonensis [tracing paper, handwritten]. (1♀, pinned, INPA), BRASIL: Am,, Reserva Ducke,, 26 Km NE Manaus,, Barbosa, M. G. V.// Plot A,, Malaise 5,, Mai. 1995// Paratype Haplocauda amazonensis [tracing paper, handwritten].
Distribution. Brazil: Amazonas: Manaus (Type locality) and Careiro Castanho (
Figure 10).
Remarks. Haplocauda amazonensis sp. nov. is the only species in this genus that has two basal apophyses on the posterior projection of Sternum VIII (
Figure 7G–I, blue arrows), anterior margin of the pygidium U-shaped and bearing blunt anterior projections (as in
H.
mendesi Silveira, Lima, and McHugh, 2022). The syntergite of this species is strongly asymmetric, as in
H.
antimary sp.
nov. (
Figure 7L). The specimens studied in this work were collected using Malaise traps.
Haplocauda aculeata Zeballos and Silveira sp. nov.
urn:lsid:zoobank.org:act:EA44E79F-4C8D-4A65-A6EA-9136306F5B58
Diagnostic description. Antennomeres X–XI creamy white (
Figure 4U). Pygidium brown with anterior corners mostly light brown to translucent (
Figure 7Q). Sternum VIII with anterior corners light brown to translucent, with projection well sclerotized.
Male. Metatibia with two spurs. Pygidium with anterior blunt projections present, posterior margin with central 1/3 almost straight (
Figure 7Q). Sternum VIII with posterior projection basally as wide as 1/4 sternum width, acuminate, reaching the posterior margin of the pygidium (
Figure 7P). Sternum IX with acute projection absent, almost 1/3 longer than syntergite (
Figure 7R).
Female (
Figure 8E,F). Pygidium 1.6× as long as wide, with anterior margin emarginate, posterior margin rounded (
Figure 9H). Sternum VIII with spiculum ventrale almost 1/3 shorter than sternum, posterior margin moderately indented (
Figure 9G). Internal genitalia with spermatophore-digesting gland larger than spermatheca, bursa copulatrix without sclerotized plate (
Figure 9L). Ovipositor with valvifers free, twisted basally, almost 3× longer than coxite; coxites convergent posteriorly, divided in distinct proximal and distal plates, both well developed but weakly sclerotized; styli minute, sclerotized; proctiger plate weakly sclerotized (
Figure 9I).
Etymology. “Aculeata” is a Latin adjective meaning “sting-bearing” and refers to the prominent median projection of sternum VIII on the male abdomen. Adjectival name.
Type material. HOLOTYPE (1♂, alcohol, INPA), BR, AM, Reserva Ducke,, 22.xi- 8.xii. 2014,, Malaise. Silva Neto,, A. M. Mendes// Holotype Haplocauda aculeata [tracing paper, handwritten]. PARATYPES (23♂ and 8♀). (1♂, alcohol, INPA), Brasil- AM,, P. N. do Jaú,, Lago Miratucu,, 24–25.vii.93,, L. S. Aquino leg,, em Pensilvânia// Paratype Haplocauda aculeata [tracing paper, handwritten]. (7♂, 3♀, alcohol, INPA), BR, AM, Pq. N. do Jaú,, 29.vii- viii. 2001,, 0154275 S, 613510W,, Arm. Malaise,, Campinarana baixa,, Henriques & Vidal// Paratype Haplocauda aculeata [tracing paper, handwritten]. (1♂, alcohol), BRASIL, AM,, Pq. Nacional do,, Jaú 28.vii- viii.2001,, 01°54′27″ S; 61°35′10″ W,, Malaise. Campinarana,, Baixa Henriques & Vidal// Paratype Haplocauda aculeata [tracing paper, handwritten]. (1♂,1♀, alcohol), BR, AM, Pq. N. do Jaú,, 29.vii- viii. 2001,, 0154275 S, 613510W,, Arm. Malaise,, Campinarana baixa,, Henriques & Vidal// Paratype Haplocauda aculeata [tracing paper, handwritten]. (1♀, pinned, UFMT), BRASIL. EST. DO AMAZONAS,, Mun. São Gabriel da Cachoeira,, Querari 2° Pelotão de Fronteira,, (2° PEF) 01°05′N/ 69°51″ W// 05/IV- 27/V/ 1993,, Motta, C.S. Ferreira, R.L., Vidal J.,, & Matteo, B. col// Malaise// 0065672// Paratype Haplocauda aculeata [yellow label]. (1♀, pinned, MZUSP), BRASIL AM QUERARI,, São Gabriel da Cachoeira,, 2° Pel. Esp. De Fronteira,, 01°05′N/ 69°51″ W// 05/4- 27/V/ 1993,, Vidal, J; Ferreira, RLM col.// Malaise// 0065686// Paratype Haplocauda aculeata [yellow label]. (7♂, 1♀, pinned, MZUSP [
3], DZUP[
5]), BRASIL, Amazonas,, Parque Nacional do Jaú,, 29.vii—08.viii.2001,, 015446S, 613523W// Arm. Malaise,, Transição Campina,, Campinarana baixa,, Henriques & Vidal// Paratype Haplocauda aculeata [yellow label]. (1♂, 1♀, pinned, UFMT), BRASIL, Amazonas,, Parque Nacional do Jaú,, Campinarana Alta,, 01°53′42″ S; 61°35′10″ W,, Armadilha Malaise// 08 a 16.iv.2001,, Henriques & Vidal leg// Paratype Haplocauda aculeata [yellow label]. (1♂, pinned, UFMT), BRASIL AM QUERARI,, São Gabriel da Cachoeira,, 2° Pel. Esp. De Fronteira,, 01°05′N/ 69°51″ W// 05/4- 27/V/ 1993,, Vidal, J; Ferreira, RLM col.// Malaise// 0065704// Paratype Haplocauda aculeata [yellow label]. (1♂, pinned, INPA), BR-AM, Pq. Nacional do,, Jaú, Seringal. 27.iv-03.v.,, 1995. Arm. Malaise,, J. A. Rafael & J. Vidal// Paratype Haplocauda aculeata [yellow label]. (1♂, pinned, INPA), BR-AM, Parnajaú-Mata,, 19.vii-09.viii.2001. Malaise,, Igarapé. Terra Firme,, A. Henriques & Vidal// Paratype Haplocauda aculeata [yellow label]. (1♂, pinned, INPA), BRASIL, Amazonas, Pq. N.,, Jaú, Rio Carabinani,, Boa vista, 020105S-,, 613219W, 29–31.vii.1995// Arm. Malaise, J. A.,, Rafael & J. Vidal// Paratype Haplocauda aculeata [yellow label]. (1♂, pinned, INPA), BR, AM, Parque Nacional,, do Jaú, Rio Unini,, 20–24.vi.1996 Malaise,, A.L. Henriques, J. Vidal &,, F. L. Oliveira// Arm. Malaise, J. A.,, Rafael & J. Vidal// Paratype Haplocauda aculeata [yellow label].
Distribution. Brazil: Amazonas: Novo Airão (Type locality), São Gabriel da Cachoeira and Manaus (
Figure 10).
Remarks. Haplocauda aculeata sp. nov. is the only species that has sternum VIII with a long and narrow posterior projection (short in
H.
lata sp. nov.) (characteristic that inspired the name of the species) and pygidium with anterior margin C-shaped, with anterior blunt projections present (absent in
H.
antimary sp. nov.) (
Figure 7P,Q). The species studied in this work were collected using Malaise traps.
Haplocauda antimary Zeballos and Silveira sp. nov.
urn:lsid:zoobank.org:act:5E8B859A-A590-40F3-947F-54816EA30E74
Diagnostic description. Antennomeres X–XI creamy white (
Figure 4Bb). Pygidium brown with anterior corners mostly light brown to translucent (
Figure 7Y). Sternum VIII with anterior corners light brown to translucent, with projection well sclerotized.
Male. Metatibia with two spurs. Pygidium with anterior blunt projections absent, anterior margin emarginate, posterior margin with central 1/3 almost straight (
Figure 7Y). Sternum VIII with posterior projection basally as wide as 1/5 sternum width, almost 3× longer than sternum VIII length, not reaching the posterior margin of the pygidium, apex rounded (
Figure 7W). Sternum IX with posterior margin rounded, without acute projection, as long as aedeagus (
Figure 7Z).
Female and immature stages unknown.
Etymology. The specific epithet refers to the Antimary State Forest, located in the State of Acre, where the type specimens were collected. Noun in apposition.
Material Type. HOLOTYPE (1♂, dissected, alcohol, INPA), BRASIL, Acre, Bujari, FES Antimary,, 9°20′01″ S- 68°19′17″ W, 3.viii-8.ix.,, 2016,, Malaise grande. E. F. Morato & J. A. Rafael cols—Rede BIA// Haplocauda antimary [tracing paper, handwritten]. PARATYPES (3♂). (1♂, dissected, alcohol, INPA), same as holotype. (2♂, alcohol, INPA), BRASIL, Acre,, 22–26.ix.2020,, pensilvânia UV// Paratype Haplocauda antimary [tracing paper, handwritten].
Distribution.
Brazil: Acre: Bujari (
Figure 10).
Remarks. Haplocauda antimary sp. nov. is similar to
H.
mendesi Silveira, Lima, and McHugh, 2022 in that it presents sternum VIII with a short and rounded posterior projection. Unlike
H.
mendesi, H.
antimary sp. nov. presents the pygidium with an anterior margin trisinuose, without anterior blunt projections (
Figure 7Y). The specimens studied in this work were collected using Malaise and Pennsylvania traps.
Haplocauda mendesi Silveira, Lima, and McHugh, 2022
New record. (1♂, one Pinned, UFMT), Brasil: Mato Grosso, Alta,, Floresta, P. E. Cristalino, 9°,, 32′45″ S, 55°54′57″ W, FIT,, 15–19.ix.2019, R. A. Azevedo. Plot A FIT 4–2 (1500)// Halocauda mendesi.
Key to Haplocauda Species Based on Males (Modified from Silveira et al., 2022)
- 1
Sternum VIII up to 1.5× wider than long (excluding the median projection of posterior margin); sternum IX with posterior margin rounded, with or without a projection (
Figure 7).................................................................................................................................
2- 1′
Sternum VIII almost 2× wider than long (excluding the median projection of posterior margin); sternum IX with posterior margin emarginate (
Figure 7A–F).........................................................................................................................
H. lata sp. nov.- 2
Pygidium with anterior margin bearing blunt projections……………...........................3
- 2′
Pygidium without anterior blunt projections on the anterior margin (
Figure 7Y)................................................................................................................
H. antimary sp. nov.- 3
Sternum VIII with posterior projection acute (
Figure 7A,G,P) ……………...……...…..
4- 3′
Sternum VIII with posterior projection rounded (
Figure 7W); ………………...…….…
6- 4
Sternum IX with an acute projection (
Figure 7K); metatibia with one spur …………
5- 4′
Sternum IX without an acute projection (
Figure 7R); metatibia with two spurs ….....................…...…………..........................……..….........................
H. aculeata sp. nov.- 5
Sternum VIII with posterior projection stout, almost 1/3 as wide as sternum, abruptly curved dorsally near apex......................H. yasuni Silveira, Lima, and McHugh, 2022.
- 5′
Sternum VIII with posterior projection slender, 1/4 as wide as sternum, not curved dorsally near apex (
Figure 7G)…………...…..............................
H. amazonensis sp. nov.- 6
Sternum VIII with posterior projection reaching the posterior margin of pygidium; pygidium with central 1/3 almost straight…………...…...............................……….........................……….........................……….........................………..................H. albertinoi Silveira, Lima, and McHugh, 2022.
- 6′
Sternum VIII with posterior projection not reaching the posterior margin of pygidium; pygidium with central 1/3 emarginate…….........................................................................................................................................................................................H. mendesi Silveira, Lima, and McHugh, 2022.