Lack of Genetic Differentiation of Five Triatomine Species Belonging to the Triatoma rubrovaria Subcomplex (Hemiptera, Reduviidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
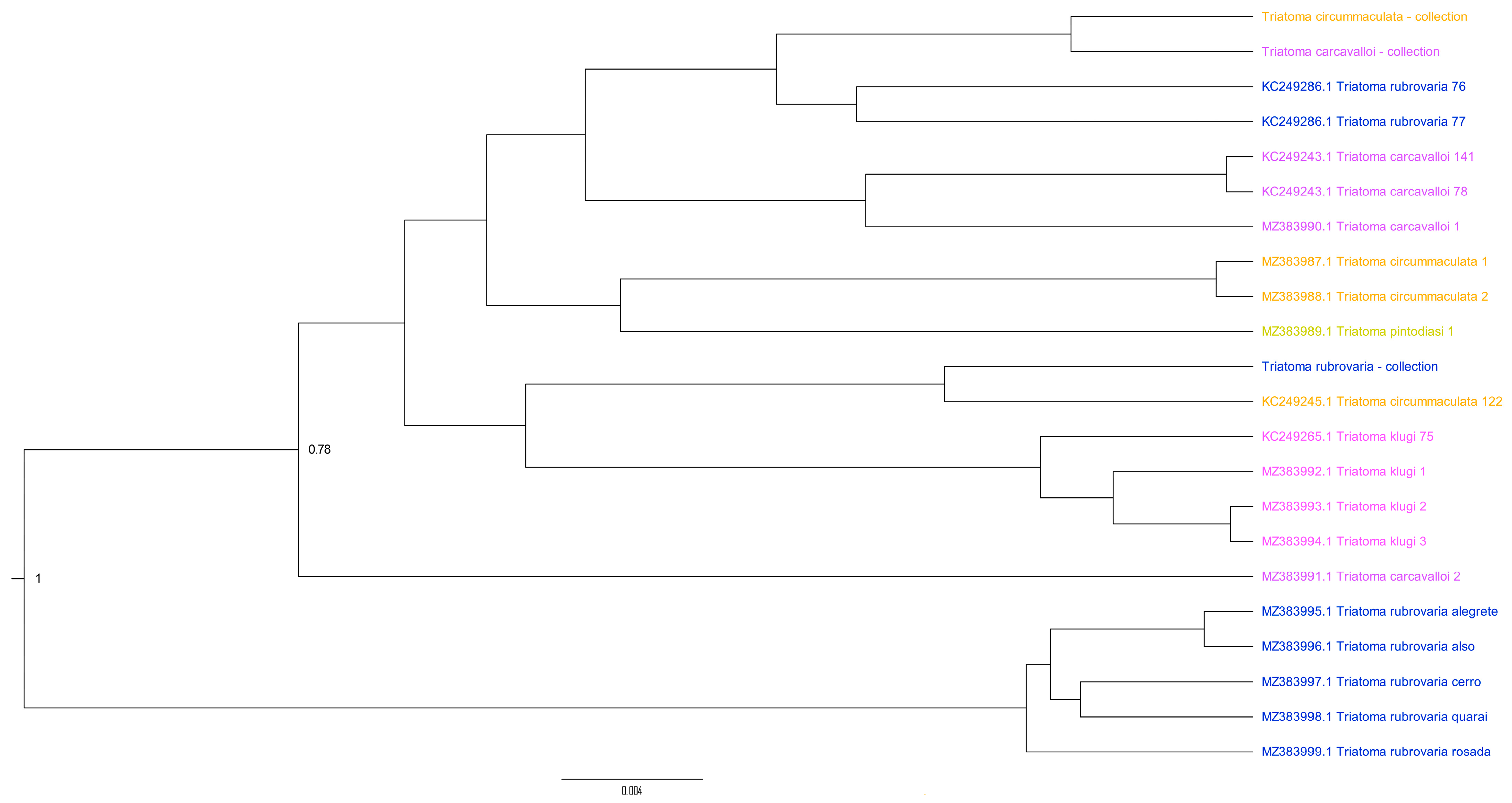
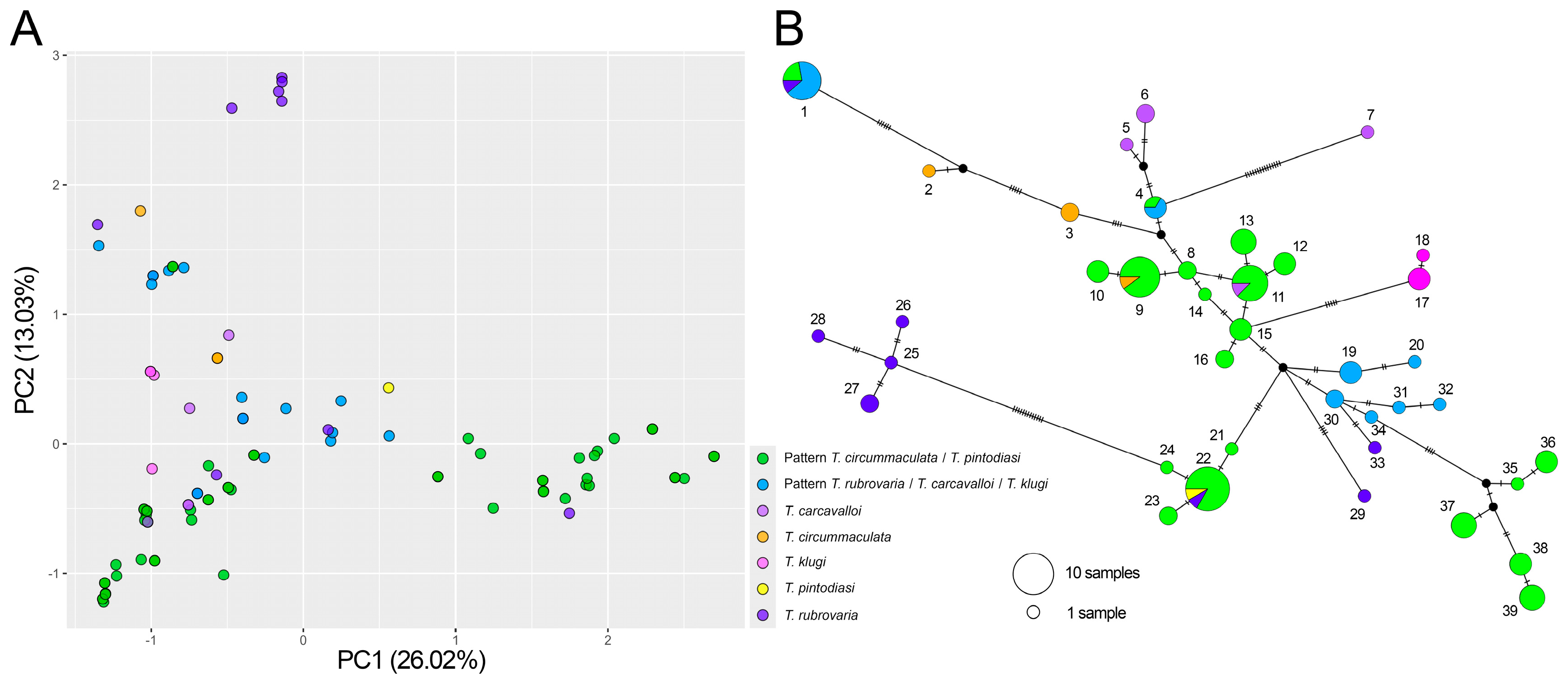
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| mtCytb | Mitochondrial Cytochrome b |
| PP | Posterior Probability |
| PCA | Principal Component Analysis |
| hBAPS | Hierarchical Bayesian Analysis of Population Structure |
| ESS | Effective Sample Size |
| K2P | Kimura 2-Parameter |
| MCMC | Markov Chain Monte Carlo |
Appendix A
| Collection Site | Sample ID | Life Stage | Morphogroup/Species | Ecotype | Haplotype | GenBank Accession Number | Reference |
|---|---|---|---|---|---|---|---|
| Caçapava do Sul | 62 | Nymph (N5) | T. circummaculata/T. pintodiasi | Sylvatic | 22 | PV184590 | This study |
| Caçapava do Sul | 63 | Nymph (N5) | T. circummaculata/T. pintodiasi | Sylvatic | 22 | PV184591 | This study |
| Caçapava do Sul | 67 | Nymph (N2) | T. circummaculata/T. pintodiasi | Sylvatic | 22 | PV184592 | This study |
| Caçapava do Sul | 70 | Nymph (N3) | T. rubrovaria/T. carcavalloi/T. klugi | Sylvatic | 32 | PV184593 | This study |
| Caçapava do Sul | 71 | Nymph (N2) | T. circummaculata/T. pintodiasi | Sylvatic | 22 | PV184594 | This study |
| Caçapava do Sul | 77 | Nymph (N3) | T. circummaculata/T. pintodiasi | Sylvatic | 1 | PV184595 | This study |
| Caçapava do Sul | 476 | Nymph (N2) | T. circummaculata/T. pintodiasi | Sylvatic | 24 | PV184596 | This study |
| Caçapava do Sul | 481 | Nymph (N1) | T. circummaculata/T. pintodiasi | Sylvatic | 15 | PV184597 | This study |
| Caçapava do Sul | 485 | Nymph (N5) | T. circummaculata/T. pintodiasi | Sylvatic | 22 | PV184598 | This study |
| Caçapava do Sul | 491 | Nymph (N5) | T. circummaculata/T. pintodiasi | Sylvatic | 22 | PV184599 | This study |
| Caçapava do Sul | 493 | Nymph (N5) | T. circummaculata/T. pintodiasi | Sylvatic | 22 | PV184600 | This study |
| Cachoeira do Sul | 87 | Nymph (N2) | T. circummaculata/T. pintodiasi | Sylvatic | 9 | PV184601 | This study |
| Cachoeira do Sul | 90 | Nymph (N3) | T. circummaculata/T. pintodiasi | Sylvatic | 10 | PV184602 | This study |
| Cachoeira do Sul | 92 | Nymph (N3) | T. circummaculata/T. pintodiasi | Sylvatic | 9 | PV184603 | This study |
| Cachoeira do Sul | 93 | Nymph (N2) | T. circummaculata/T. pintodiasi | Sylvatic | 10 | PV184604 | This study |
| Cachoeira do Sul | 278 | Nymph (N5) | T. circummaculata/T. pintodiasi | Sylvatic | 9 | PV184605 | This study |
| Cachoeira do Sul | 280 | Nymph (N5) | T. circummaculata/T. pintodiasi | Sylvatic | 10 | PV184606 | This study |
| Cachoeira do Sul | 281 | Nymph (N5) | T. circummaculata/T. pintodiasi | Sylvatic | 9 | PV184607 | This study |
| Cachoeira do Sul | 283 | Nymph (N3) | T. circummaculata/T. pintodiasi | Sylvatic | 9 | PV184608 | This study |
| Cachoeira do Sul | 284 | Nymph (N5) | T. circummaculata/T. pintodiasi | Sylvatic | 9 | PV184609 | This study |
| Cachoeira do Sul | 285 | Nymph (N5) | T. circummaculata/T. pintodiasi | Sylvatic | 9 | PV184610 | This study |
| Cachoeira do Sul | 286 | Nymph (N2) | T. circummaculata/T. pintodiasi | Sylvatic | 14 | PV184611 | This study |
| Canguçu | 109 | Nymph (N2) | T. circummaculata/T. pintodiasi | Sylvatic | 37 | PV184612 | This study |
| Canguçu | 110 | Nymph (N2) | T. circummaculata/T. pintodiasi | Sylvatic | 37 | PV184613 | This study |
| Canguçu | 111 | Nymph (N2) | T. circummaculata/T. pintodiasi | Sylvatic | 38 | PV184614 | This study |
| Canguçu | 112 | Nymph (N2) | T. circummaculata/T. pintodiasi | Sylvatic | 37 | PV184615 | This study |
| Canguçu | 113 | Nymph (N2) | T. circummaculata/T. pintodiasi | Sylvatic | 39 | PV184616 | This study |
| Canguçu | 114 | Nymph (N2) | T. circummaculata/T. pintodiasi | Sylvatic | 37 | PV184617 | This study |
| Canguçu | 115 | Nymph (N2) | T. circummaculata/T. pintodiasi | Sylvatic | 38 | PV184618 | This study |
| Canguçu | 116 | Nymph (N1) | T. circummaculata/T. pintodiasi | Sylvatic | 39 | PV184619 | This study |
| Canguçu | 117 | Nymph (N1) | T. circummaculata/T. pintodiasi | Sylvatic | 38 | PV184620 | This study |
| Canguçu | 118 | Nymph (N2) | T. circummaculata/T. pintodiasi | Sylvatic | 39 | PV184621 | This study |
| Canguçu | 119 | Nymph (N2) | T. circummaculata/T. pintodiasi | Sylvatic | 39 | PV184622 | This study |
| Encruzilhada do Sul | 99 | Nymph (N4) | T. circummaculata/T. pintodiasi | Sylvatic | 11 | PV184623 | This study |
| Encruzilhada do Sul | 101 | Nymph (N4) | T. circummaculata/T. pintodiasi | Sylvatic | 4 | PV184624 | This study |
| Encruzilhada do Sul | 103 | Nymph (N2) | T. circummaculata/T. pintodiasi | Sylvatic | 11 | PV184625 | This study |
| Encruzilhada do Sul | 104 | Nymph (N1) | T. circummaculata/T. pintodiasi | Sylvatic | 11 | PV184626 | This study |
| Encruzilhada do Sul | 105 | Nymph (N2) | T. circummaculata/T. pintodiasi | Sylvatic | 11 | PV184627 | This study |
| Encruzilhada do Sul | 106 | Nymph (N2) | T. circummaculata/T. pintodiasi | Sylvatic | 11 | PV184628 | This study |
| Encruzilhada do Sul | 107 | Nymph (N3) | T. circummaculata/T. pintodiasi | Sylvatic | 11 | PV184629 | This study |
| Encruzilhada do Sul | 108 | Nymph (N3) | T. circummaculata/T. pintodiasi | Sylvatic | 11 | PV184630 | This study |
| Lavras do Sul | 29 | Nymph (N4) | T. circummaculata/T. pintodiasi | Sylvatic | 22 | PV184631 | This study |
| Lavras do Sul | 35 | Nymph (N4) | T. rubrovaria/T. carcavalloi/T. klugi | Sylvatic | 1 | PV184632 | This study |
| Lavras do Sul | 37 | Nymph (N4) | T. circummaculata/T. pintodiasi | Sylvatic | 30 | PV184633 | This study |
| Lavras do Sul | 38 | Nymph (N4) | T. circummaculata/T. pintodiasi | Sylvatic | 19 | PV184634 | This study |
| Lavras do Sul | 39 | Nymph (N5) | T. circummaculata/T. pintodiasi | Sylvatic | 22 | PV184635 | This study |
| Lavras do Sul | 42 | Nymph (N5) | T. circummaculata/T. pintodiasi | Sylvatic | 21 | PV184636 | This study |
| Lavras do Sul | 45 | Nymph (N4) | T. rubrovaria/T. carcavalloi/T. klugi | Sylvatic | 30 | PV184637 | This study |
| Lavras do Sul | 52 | Nymph (N4) | T. rubrovaria/T. carcavalloi/T. klugi | Sylvatic | 19 | PV184638 | This study |
| Lavras do Sul | 54 | Nymph (N4) | T. rubrovaria/T. carcavalloi/T. klugi | Sylvatic | 31 | PV184639 | This study |
| Lavras do Sul | 81 | Nymph (N2) | T. rubrovaria/T. carcavalloi/T. klugi | Sylvatic | 1 | PV184640 | This study |
| Lavras do Sul | 82 | Nymph (N2) | T. rubrovaria/T. carcavalloi/T. klugi | Sylvatic | 1 | PV184641 | This study |
| Lavras do Sul | 83 | Nymph (N5) | T. rubrovaria/T. carcavalloi/T. klugi | Sylvatic | 19 | PV184642 | This study |
| Lavras do Sul | 84 | Nymph (N2) | T. rubrovaria/T. carcavalloi/T. klugi | Sylvatic | 1 | PV184643 | This study |
| Lavras do Sul | 89 | Nymph (N2) | T. circummaculata/T. pintodiasi | Sylvatic | 9 | PV184644 | This study |
| Lavras do Sul | 94 | Nymph (N3) | T. circummaculata/T. pintodiasi | Sylvatic | 8 | PV184645 | This study |
| Lavras do Sul | 95 | Nymph (N2) | T. circummaculata/T. pintodiasi | Sylvatic | 9 | PV184646 | This study |
| Lavras do Sul | 97 | Nymph (N1) | T. circummaculata/T. pintodiasi | Sylvatic | 8 | PV184647 | This study |
| Lavras do Sul | 231 | Nymph (N5) | T. rubrovaria/T. carcavalloi/T. klugi | Sylvatic | 1 | PV184648 | This study |
| Lavras do Sul | 236 | Nymph (N5) | T. rubrovaria/T. carcavalloi/T. klugi | Sylvatic | 1 | PV184649 | This study |
| Lavras do Sul | 253 | Nymph (N4) | T. rubrovaria/T. carcavalloi/T. klugi | Sylvatic | 34 | PV184650 | This study |
| Lavras do Sul | 258 | Nymph (N5) | T. circummaculata/T. pintodiasi | Sylvatic | 35 | PV184651 | This study |
| Lavras do Sul | 259 | Nymph (N3) | T. circummaculata/T. pintodiasi | Sylvatic | 36 | PV184652 | This study |
| Lavras do Sul | 261 | Nymph (N4) | T. circummaculata/T. pintodiasi | Sylvatic | 22 | PV184653 | This study |
| Lavras do Sul | 265 | Nymph (N5) | T. circummaculata/T. pintodiasi | Sylvatic | 36 | PV184654 | This study |
| Lavras do Sul | 276 | Nymph (N1) | T. circummaculata/T. pintodiasi | Sylvatic | 36 | PV184655 | This study |
| Lavras do Sul | 277 | Nymph (N2) | T. rubrovaria/T. carcavalloi/T. klugi | Sylvatic | 20 | PV184656 | This study |
| Lavras do Sul | 279 | Adult (♀) | Triatoma rubrovaria | Sylvatic | 22 | PV184657 | This study |
| Lavras do Sul | 298 | Nymph (N1) | T. rubrovaria/T. carcavalloi/T. klugi | Sylvatic | 1 | PV184658 | This study |
| Lavras do Sul | 424 | Nymph (N5) | T. circummaculata/T. pintodiasi | Sylvatic | 15 | PV184659 | This study |
| Lavras do Sul | 426 | Nymph (N2) | T. circummaculata/T. pintodiasi | Sylvatic | 23 | PV184660 | This study |
| Lavras do Sul | 432 | Nymph (N2) | T. circummaculata/T. pintodiasi | Sylvatic | 15 | PV184661 | This study |
| Lavras do Sul | 460 | Nymph (N2) | T. circummaculata/T. pintodiasi | Sylvatic | 13 | PV184662 | This study |
| Lavras do Sul | 463 | Nymph (N2) | T. circummaculata/T. pintodiasi | Sylvatic | 15 | PV184663 | This study |
| São Jerônimo | 7 | Nymph (N3) | T. circummaculata/T. pintodiasi | Sylvatic | 13 | PV184664 | This study |
| São Jerônimo | 8 | Nymph (N2) | T. circummaculata/T. pintodiasi | Sylvatic | 16 | PV184665 | This study |
| São Jerônimo | 11 | Nymph (N1) | T. circummaculata/T. pintodiasi | Sylvatic | 16 | PV184666 | This study |
| São Jerônimo | 13 | Nymph (N2) | T. rubrovaria/T. carcavalloi/T. klugi | Sylvatic | 4 | PV184667 | This study |
| São Jerônimo | 18 | Nymph (N1) | T. circummaculata/T. pintodiasi | Sylvatic | 12 | PV184668 | This study |
| São Jerônimo | 21 | Nymph (N5) | T. circummaculata/T. pintodiasi | Sylvatic | 13 | PV184669 | This study |
| São Jerônimo | 22 | Nymph (N3) | T. rubrovaria/T. carcavalloi/T. klugi | Sylvatic | 4 | PV184670 | This study |
| São Jerônimo | 23 | Nymph (N1) | T. circummaculata/T. pintodiasi | Sylvatic | 12 | PV184671 | This study |
| São Jerônimo | 25 | Nymph (N2) | T. circummaculata/T. pintodiasi | Sylvatic | 13 | PV184672 | This study |
| São Jerônimo | 461 | Nymph (N2) | T. circummaculata/T. pintodiasi | Sylvatic | 12 | PV184673 | This study |
| Quaraí, RS, Brazil | - | Adult | Triatoma rubrovaria | Colony | 1 | PV184674 | This study |
| São Francisco de Assis, RS, Brazil | - | Adult | Triatoma carcavalloi | Colony | 11 | PV184675 | This study |
| Caçapava do Sul, Brazil | - | Adult | Triatoma circummaculata | Colony | 9 | PV184676 | This study |
| Caçapava do Sul, RS, Brazil | T.rubrovaria76 | Adult | Triatoma rubrovaria | - | 33 | KC249286.1 | Justi et al. [21] |
| Quevedos, RS, Brazil | T.rubrovaria77 | Adult | Triatoma rubrovaria | - | 29 | KC249287.1 | Justi et al. [21] |
| Quaraí, RS, Brazil | rosada | Adult | Triatoma rubrovaria | - | 25 | MZ383999.1 | da Silva et al. [17] |
| Quaraí, RS, Brazil | quarai | Adult | Triatoma rubrovaria | - | 26 | MZ383998.1 | da Silva et al. [17] |
| Quaraí, RS, Brazil | cerro | Adult | Triatoma rubrovaria | - | 28 | MZ383997.1 | da Silva et al. [17] |
| Alegrete, RS, Brazil | salso | Adult | Triatoma rubrovaria | - | 27 | MZ383996.1 | da Silva et al. [17] |
| Alegrete, RS, Brazil | alegrete | Adult | Triatoma rubrovaria | - | 27 | MZ383995.1 | da Silva et al. [17] |
| São Francisco de Assis, RS, Brazil | T.klugi1 | Adult | Triatoma klugi | - | 17 | MZ383992.1 | da Silva et al. [17] |
| São Francisco de Assis, RS, Brazil | T.klugi2 | Adult | Triatoma klugi | - | 17 | MZ383993.1 | da Silva et al. [17] |
| São Francisco de Assis, RS, Brazil | T.klugi3 | Adult | Triatoma klugi | - | 17 | MZ383994.1 | da Silva et al. [17] |
| Nova Petrópolis, RS, Brazil | T.klugi75 | Adult | Triatoma klugi | - | 18 | KC249265.1 | Justi et al. [21] |
| Caçapava do Sul, RS, Brazil | T.carcavalloi1 | Adult | Triatoma carcavalloi | - | 5 | MZ383990.1 | da Silva et al. [17] |
| Lavras, RS, Brazil | T.carcavalloi2 | Adult | Triatoma carcavalloi | - | 7 | MZ383991.1 | da Silva et al. [17] |
| São Gerônimo, RS, Brazil | T.carcavalloi78 | Adult | Triatoma carcavalloi | - | 6 | KC249244.1 | Justi et al. [21] |
| - | T.carcavalloi141 | Adult | Triatoma carcavalloi | - | 6 | KC249243.1 | Justi et al. [21] |
| Piratini, RS, Brazil | T.circummaculata122 | Adult | Triatoma circummaculata | - | 2 | KC249245.1 | Justi et al. [21] |
| Lavras, RS, Brazil | T.circummaculata1 | Adult | Triatoma circummaculata | - | 3 | MZ383987.1 | da Silva et al. [17] |
| Caçapava do Sul, RS, Brazil | T.circummaculata2 | Adult | Triatoma circummaculata | - | 3 | MZ383988.1 | da Silva et al. [17] |
| Caçapava do Sul, RS, Brazil | T.pintodiasi1 | Adult | Triatoma pintodiasi | - | 22 | MZ383989.1 | da Silva et al. [17] |
References
- Chagas, C. Nova tripanozomiaze humana: Estudos sobre a morfolojia e o ciclo evolutivo do Schizotrypanum cruzi n. gen., n. sp., ajente etiolojico de nova entidade morbida do homem. Mem. Inst. Oswaldo Cruz. 1909, 1, 159–218. [Google Scholar] [CrossRef]
- World Health Organization. Chagas Disease (American Trypanosomiasis). 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis) (accessed on 10 January 2025).
- PAHO (Pan-American Health Organization). 2024. Available online: https://www.paho.org/en/news/16-10-2023-paho-pushes-elimination-more-30-communicable-diseases-americas-2030 (accessed on 10 January 2025).
- de Arias, A.R.; Monroy, C.; Guhl, F.; Sosa-Estani, S.; Santos, W.S.; Abad-Franch, F. Chagas disease control-surveillance in the Americas: The multinational initiatives and the practical impossibility of interrupting vector-borne Trypanosoma cruzi transmission. Mem. Inst. Oswaldo Cruz. 2022, 117, e210130. [Google Scholar] [CrossRef]
- Dias, J.C.P. Southern Cone Initiative for the elimination of domestic populations of Triatoma infestans and the interruption of transfusion Chagas disease: Historical aspects, present situation, and perspectives. Mem. Inst. Oswaldo Cruz. 2007, 102 (Suppl. S1), 11–18. [Google Scholar] [CrossRef] [PubMed]
- Bedin, C.; Wilhelms, T.; Villela, M.M.; Silva, G.C.C.; Riffel, A.P.K.; Sackis, P.; Mello, F. Residual foci of Triatoma infestans infestation: Surveillance and control in Rio Grande do Sul, Brazil, 2001–2018. Rev. Soc. Bras. Med. Trop. 2021, 54, e0530-2020. [Google Scholar] [CrossRef]
- Almeida, C.E.; Vinhaes, M.C.; Almeida, J.R.; de Silveira, A.C.; Costa, J. Monitoring the domiciliary and peridomiciliary invasion process of Triatoma rubrovaria in the State of Rio Grande do Sul, Brazil. Mem. Inst. Oswaldo Cruz. 2000, 95, 761–768. [Google Scholar] [CrossRef]
- Souza, C.B.; de Santos, G.B.; dos Bedin, C.; Bergamin, M.; de Mello, F.; Villela, M.M. Triatoma rubrovaria (Hemiptera, Reduviidae, Triatominae) in the Pampa biome, Brazil: A retrospective study of its occurrence and abundance. Rev. Soc. Bras. Med. Trop. 2023, 65, e38. [Google Scholar] [CrossRef]
- Verly, T.; Costa, S.; Lima, N.; Mallet, J.; Odêncio, F.; Pereira, M.; Moreira, C.J.; Britto, C.; Pavan, M.G. Vector competence and feeding-excretion behavior of Triatoma rubrovaria (Blanchard, 1843) (Hemiptera: Reduviidae) infected with Trypanosoma cruzi TcVI. PLoS Neglected Tropical Diseases. 2020, 14, e0008712. [Google Scholar] [CrossRef] [PubMed]
- Salvatella, R.; Calegari, L.; Puime, A.; Basmadjian, Y.; Rosa, R.; Guerrero, J.; Martinez, M.; Mendaro, G.; Briano, D.; Montero, C.; et al. Perfil alimentario de Triatoma rubrovaria (Blanchard, 1843) (Hemiptera, Triatominae) en ámbitos peridomiciliarios, de una localidad rural de Uruguay. Rev. Soc. Bras. Med. Trop. São Paulo 1994, 36, 311–320. [Google Scholar] [CrossRef]
- Almeida, C.E.; Duarte, R.; Nascimento, R.G.d.; Pacheco, R.S.; Costa, J. Triatoma rubrovaria (Blanchard, 1843) (Hemiptera, Reduviidae, Triatominae) II: Trophic resources and ecological observations of five populations collected in the State of Rio Grande do Sul, Brazil. Mem. Inst. Oswaldo Cruz. 2002, 97, 1127–1131. [Google Scholar] [CrossRef][Green Version]
- Schofield, C.J.; Galvão, C. Classification, evolution, and species groups within the Triatominae. Acta Tropica. 2009, 110, 88–100. [Google Scholar] [CrossRef]
- Pita, S.; Lorite, P.; Nattero, J.; Galvão, C.; Alevi, K.C.C.; Teves, S.C.; Azeredo-Oliveira, M.T.V.; Panzera, F. New arrangements on several species subcomplexes of Triatoma genus based on the chromosomal position of ribosomal genes (Hemiptera-Triatominae). Infect. Genet. Evol. 2016, 43, 225–231. [Google Scholar] [CrossRef]
- Lent, H.; Wygodzinsky, P. Revision of the Triatominae (Hemiptera, Reduviidae), and their significance as vectors of Chagas’ disease. Bull. Am. Mus. Nat. Hist. 1979, 163, 125–520. [Google Scholar]
- Jurberj, J.; Carcavallo, U.R.; Lent, H. Panstrongylus sherlocki sp.n. do estado da Bahia, Brasil (Hemiptera, Reduviidae, Triatominae). Entomol. Vectores. 2001, 8, 261–274. [Google Scholar]
- Jurberg, J.; Cunha, V.; Cailleaux, S.; Raigorodschi, R.; Lima, M.S.; Rocha, D.d.S.; Moreira, F.F.F. Triatoma pintodiasi sp. nov. do subcomplexo T. rubrovaria (Hemiptera, Reduviidae, Triatominae). Rev. Pan-Amaz. Saúde 2013, 4, 43–56. [Google Scholar] [CrossRef][Green Version]
- da Silva, L.A.; Belintani, T.; de Paiva, V.F.; Nascimento, J.D.; Rimoldi, A.; Gardim, S.; Rocha, C.S.; de Mello, F.; Obara, M.T.; de Oliveira, J.; et al. Integrative taxonomy and phylogenetic systematics of the Triatoma rubrovaria subcomplex (Hemiptera, Triatominae). Acta Trop. 2023, 237, 106699. [Google Scholar] [CrossRef] [PubMed]
- Pavan, M.G.; Lazoski, C.; Monteiro, F.A. Speciation Processes in Triatominae. In Triatominae—The Biology of Chagas Disease Vectors. Entomology in Focus; Guarneri, A., Lorenzo, M., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Jurberg, J.; Barbosa, H.S.; Galvão, C.; Rocha, D.d.S.; Silva, M.B.A. Descrição de ovos e ninfas de Triatoma klugi (Hemiptera, Reduviidae, Triatominae). Iheringia Ser. Zool. 2010, 100, 43–54. [Google Scholar] [CrossRef][Green Version]
- Monteiro, F.A.; Barrett, T.V.; Fitzpatrick, S.; Cordon-Rosales, C.; Feliciangeli, D.; Beard, C.B. Molecular phylogeography of the Amazonian Chagas disease vectors Rhodnius prolixus and R. robustus. Mol. Ecol. 2003, 12, 997–1006. [Google Scholar] [CrossRef]
- Justi, S.A.; Russo, C.A.M.; Mallet, J.R.d.S.; Obara, M.T.; Galvão, C. Molecular phylogeny of Triatomini (Hemiptera: Reduviidae: Triatominae). Parasites Vectors. 2014, 7, 149. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Bouckaert, R.; Vaughan, T.G.; Barido-Sottani, J.; Duchêne, S.; Fourment, M.; Gavryushkina, A.; Heled, J.; Jones, G.; Kühnert, D.; de Maio, N.; et al. BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2019, 15, e1006650. [Google Scholar] [CrossRef]
- Bouckaert, R.R.; Drummond, A.J. bModelTest: Bayesian phylogenetic site model averaging and model comparison. BMC Evol. Biol. 2017, 17, 42. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 15 February 2024).
- Jombart, T. adegenet: A R package for the multivariate analysis of genetic markers. Bioinformatics 2008, 24, 1403–1405. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2 Elegant Graphics for Data Analysis, 2016th ed.; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Cheng, L.; Thomas, R.C.; Jukka, S.; David, M.A.; Jukka, C. Hierarchical and Spatially Explicit Clustering of DNA Sequences with BAPS Software. Mol. Biol. Evol. 2013, 30, 1224–1228. [Google Scholar] [CrossRef]
- Revell, L.J. Phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 2011, 3, 217–223. [Google Scholar] [CrossRef]
- Kassambara, A. ggpubr R Package: ggplot2-Based Publication Ready Plots. 2023. Available online: https://www.sthda.com/english/articles/24-ggpubr-publication-ready-plots/ (accessed on 22 February 2024).
- Wilke, C. Introduction to ggridges. 2024. Available online: https://wilkelab.org/ggridges/articles/introduction.html/ (accessed on 22 February 2024).
- Abad-Franch, F.; Pavan, M.G.; Jaramillo-O, N.; Palomeque, F.S.; Dale, C.; Chaverra, D.; Monteiro, F.A. Rhodnius barretti, a new species of Triatominae (Hemiptera: Reduviidae) from Western Amazonia. Mem. Inst. Oswaldo Cruz. 2013, 108 (Suppl. S1), 92–99. [Google Scholar] [CrossRef] [PubMed]
- Abad-Franch, F.; Monteiro, F.A.; Pavan, M.G.; Patterson, J.S.; Bargues, M.D.; Zuriaga, M.Á.; Aguilar, M.; Beard, C.B.; Mas-Coma, S.; Miles, M.A. Under pressure: Phenotypic divergence and convergence associated with microhabitat adaptations in Triatominae. Parasites Vectors 2021, 14, 195. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, F.A.; Donnelly, M.J.; Beard, C.B.; Costa, J. Nested clade and phylogeographic analyses of the Chagas disease vector Triatoma brasiliensis in Northeast Brazil. Mol. Phylogenet. Evol. 2004, 32, 46–56. [Google Scholar] [CrossRef]
- Monteiro, F.A.; Peretolchina, T.; Lazoski, C.; Harris, K.; Dotson, E.M.; Abad-Franch, F.; Tamayo, E.; Pennington, P.M.; Monroy, C.; Cordon-Rosales, C.; et al. Phylogeographic pattern and extensive mitochondrial DNA divergence disclose a species complex within the Chagas disease vector Triatoma dimidiata. PLoS ONE 2013, 8, e70974. [Google Scholar] [CrossRef]
- Pita, S.; Gómez-Palacio, A.; Lorite, P.; Dujardin, J.P.; Chavez, T.; Villacís, A.G.; Galvão, C.; Panzera, Y.; Calleros, L.; Pereyra-Mello, S.; et al. Multidisciplinary approach detects speciation within the kissing bug Panstrongylus rufotuberculatus populations (Hemiptera, Heteroptera, Reduviidae). Mem. Inst. Oswaldo Cruz. 2021, 116, e210259. [Google Scholar] [CrossRef]
- Schmid, M.; Guillaume, F. The role of phenotypic plasticity on population differentiation. Heredity 2017, 119, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Crispo, E. Modifying effects of phenotypic plasticity on interactions among natural selection, adaptation and gene flow. J. Evol. Biol. 2008, 21, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Pavan, M.G.; Rivas, G.B.S.; Dias, F.B.S.; Gurgel-Gonçalves, R. Looks can be deceiving: Cryptic species and phenotypic variation in Rhodnius spp., Chagas disease vectors. In Evolutionary Biology: Biodiversification from Genotype to Phenotype; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 345–372. [Google Scholar] [CrossRef]
- González, C.; Ornelas, J.F.; Gutiérrez-Rodríguez, C. Selection and geographic isolation influence hummingbird speciation: Genetic, acoustic and morphological divergence in the wedge-tailed sabrewing (Campylopterus curvipennis). BMC Evol. Biol. 2011, 11, 38. [Google Scholar] [CrossRef]
- Kautt, A.F.; Kratochwil, C.F.; Nater, A.; Machado-Schiaffino, G.; Olave, M.; Henning, F.; Torres-Dowdall, J.; Härer, A.; Hulsey, C.D.; Franchini, P.; et al. Contrasting signatures of genomic divergence during sympatric speciation. Nature 2020, 588, 106–111. [Google Scholar] [CrossRef]
- Nosil, P.; Feder, J.L.; Flaxman, S.M.; Gompert, Z. Tipping points in the dynamics of speciation. Nat. Ecol. Evol. 2017, 1, 0001. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caetano, A.R.; Mosmann, L.B.; Verly, T.; Costa, S.; Oliveira, J.; Britto, C.; Pavan, M.G. Lack of Genetic Differentiation of Five Triatomine Species Belonging to the Triatoma rubrovaria Subcomplex (Hemiptera, Reduviidae). Insects 2025, 16, 822. https://doi.org/10.3390/insects16080822
Caetano AR, Mosmann LB, Verly T, Costa S, Oliveira J, Britto C, Pavan MG. Lack of Genetic Differentiation of Five Triatomine Species Belonging to the Triatoma rubrovaria Subcomplex (Hemiptera, Reduviidae). Insects. 2025; 16(8):822. https://doi.org/10.3390/insects16080822
Chicago/Turabian StyleCaetano, Amanda R., Lucas B. Mosmann, Thaiane Verly, Stephanie Costa, Jader Oliveira, Constança Britto, and Márcio G. Pavan. 2025. "Lack of Genetic Differentiation of Five Triatomine Species Belonging to the Triatoma rubrovaria Subcomplex (Hemiptera, Reduviidae)" Insects 16, no. 8: 822. https://doi.org/10.3390/insects16080822
APA StyleCaetano, A. R., Mosmann, L. B., Verly, T., Costa, S., Oliveira, J., Britto, C., & Pavan, M. G. (2025). Lack of Genetic Differentiation of Five Triatomine Species Belonging to the Triatoma rubrovaria Subcomplex (Hemiptera, Reduviidae). Insects, 16(8), 822. https://doi.org/10.3390/insects16080822







