Effects of Yeast on the Growth and Development of Drosophila melanogaster and Pardosa pseudoannulata (Araneae: Lycsidae) Through the Food Chain
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Rearing
2.2. Generation of Fruit Flies with Different Nutrients
2.3. Juvenile Survival and Growth
2.4. Total Fat, Protein, and Glucose Were Measured
2.5. Statistics
3. Results
3.1. Growth and Development of Drosophila melanogaster
3.2. Survival and Growth of Spiderlings
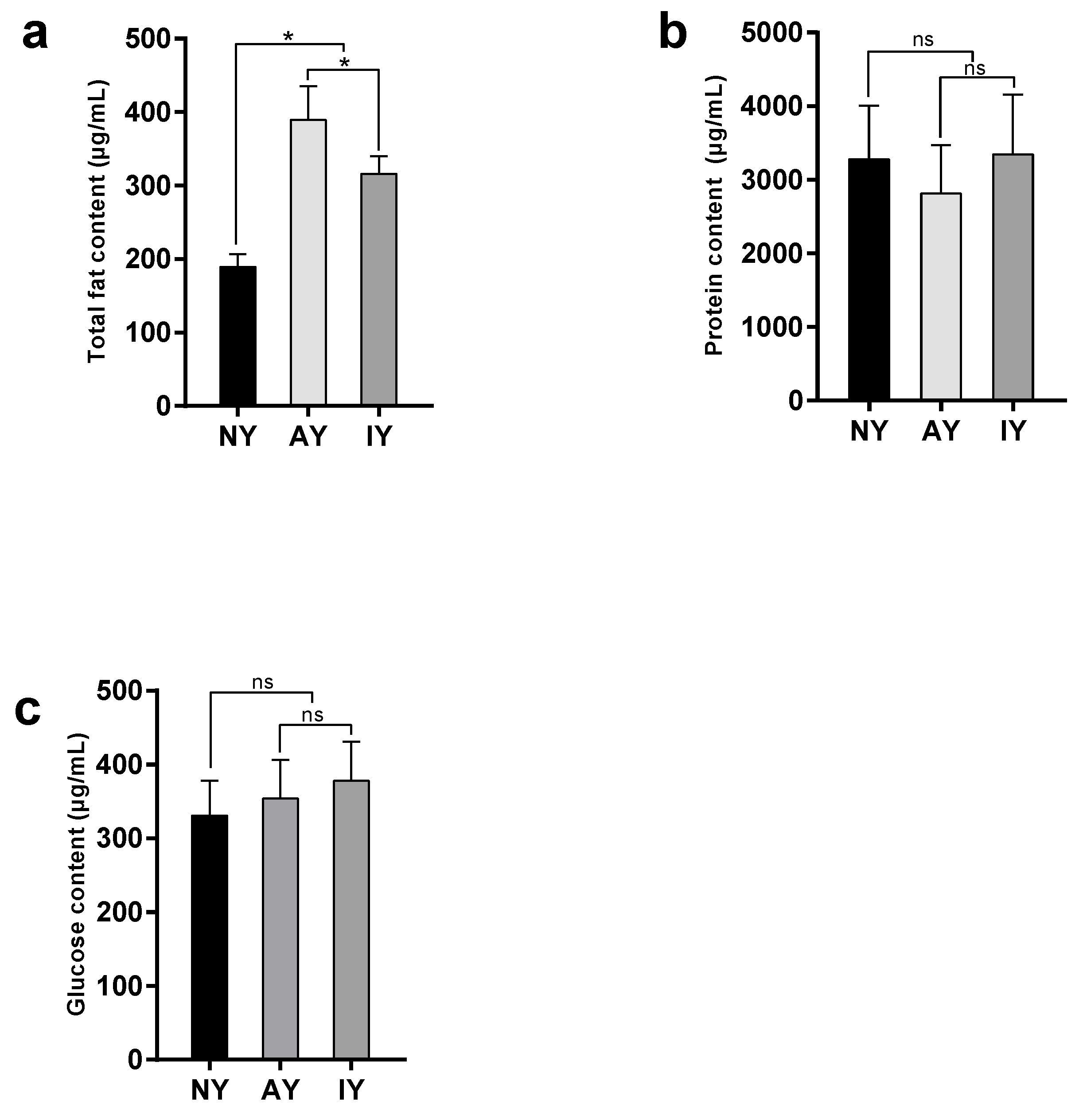
3.3. The Content of Nutrients in D. melanogaster and P. pseudoannulata
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chatterjee, S.; Isaia, M.; Venturino, E. Spiders as biological controllers in the agroecosystem. J. Theor. Biol. 2009, 258, 352–362. [Google Scholar] [CrossRef]
- Samu, F.; Sunderland, K.D.; Szinetar, C. Scale-dependent dispersal and distribution patterns of spiders in agricultural systems: A review. J. Arachnol. 1999, 27, 325–332. [Google Scholar] [CrossRef]
- Yang, H.; Peng, Y.; Tian, J.; Wang, J.; Wei, B.; Xie, C.; Wang, Z. Rice field spiders in China: A review of the literature. J. Econ. Entomol. 2018, 111, 53–64. [Google Scholar] [CrossRef]
- Kaspari, M. The invisible hand of the periodic table: How micronutrients shape ecology. Annual Review of Ecology. Evol. Syst. 2021, 52, 199–219. [Google Scholar] [CrossRef]
- Sobczyk, Ł.; Filipiak, M.; Czarnoleski, M. Sexual dimorphism in the multielemental stoichiometric phenotypes and stoichiometric niches of spiders. Insects 2020, 11, 484. [Google Scholar] [CrossRef] [PubMed]
- Trubl, P.; Johnson, J.C. Ecological stoichiometry of the black widow spider and its prey from desert, urban and laboratory populations. J. Arid Environ. 2019, 163, 18–25. [Google Scholar] [CrossRef]
- Wilder, S.M.; Schneider, J.M. Micronutrient consumption by female Argiope bruennichi affects offspring survival. J. Insect Physiol. 2017, 100, 128–132. [Google Scholar] [CrossRef]
- Ludwig, L.; Barbour, M.A.; Guevara, J.; Avilés, L.; González, A.L. Caught in the web: Spider web architecture affects prey specialization and spider–prey stoichiometric relationships. Ecol. Evol. 2018, 8, 6449–6462. [Google Scholar] [CrossRef]
- Wilder, S.M. Spider Nutrition: An Integrative Perspective; Advances in Insect Physiology; Academic Press: Cambridge, MA, USA, 2011; Volume 40, pp. 87–136. [Google Scholar] [CrossRef]
- Michalko, R.; Pekár, S.; Entling, M.H. An updated perspective on spiders as generalist predators in biological control. Ecologia 2019, 189, 21–36. [Google Scholar] [CrossRef]
- Herzog, C.; Reeves, J.T.; Ipek, Y.; Jilling, A.; Hawlena, D.; Wilder, S.M. Multi-elemental consumer-driven nutrient cycling when predators feed on different prey. Oecologia 2023, 202, 1–14. [Google Scholar] [CrossRef]
- Jensen, K.; Mayntz, D.; Toft, S.; Raubenheimer, D.; Simpson, S.J. Prey nutrient composition has different effects on Pardosa wolf spiders with dissimilar life histories. Oecologia 2010, 165, 577–583. [Google Scholar] [CrossRef]
- Wilson, J.K.; Ruiz, L.; Davidowitz, G. Dietary protein and carbohydrates affect immune function and performance in a specialist herbivore insect (Manduca sexta). Physiol. Biochem. Zool. 2019, 92, 58–70. [Google Scholar] [CrossRef]
- Vargas, M.A.; Luo, N.; Yamaguchi, A.; Kapahi, P. A role for S6 kinase and serotonin in postmating dietary switch and balance of nutrients in D. melanogaster. Curr. Biol. 2010, 20, 1006–1011. [Google Scholar] [CrossRef]
- Raubenheimer, D.; Simpson, S.J.; Mayntz, D. Nutrition, ecology and nutritional ecology: Toward an integrated framework. Funct. Ecol. 2009, 23, 4–16. [Google Scholar] [CrossRef]
- Wasko, D.K.; Sasa, M. Food resources influence spatial ecology, habitat selection, and foraging behavior in an ambush-hunting snake (Viperidae: Bothrops asper): An experimental study. Zoology 2012, 115, 179–187. [Google Scholar] [CrossRef]
- Glaudas, X.; Alexander, G.J. Food supplementation affects the foraging ecology of a low-energy, ambush-foraging snake. Behav. Ecol. Sociobiol. 2017, 71, 5. [Google Scholar] [CrossRef]
- Zhao, J.Z. List of spiders in Chinese cotton fields (I). Acta Arachnol. Sin. 1992, 1, 23–30. [Google Scholar]
- Raubenheimer, D.; Simpson, S.J. Nutritional ecology and foraging theory. Curr. Opin. Insect Sci. 2018, 27, 38–45. [Google Scholar] [CrossRef]
- Settle, W.H.; Ariawan, H.; Astuti, E.T.; Cahyana, W.; Hakim, A.L.; Hindayana, D.; Lestari, A.S. Managing tropical rice pests through conservation of generalist natural enemies and alternative prey. Ecology 1996, 77, 1975–1988. [Google Scholar] [CrossRef]
- Barry, K.L.; Wilder, S.M. Macronutrient intake affects reproduction of a predatory insect. Oikos 2012, 122, 1058–1064. [Google Scholar] [CrossRef]
- Keswani, S.V. Spiders of agro-ecosystems: A review. Int. J. Multdiciplinary Res. 2023, 23, 1–18. [Google Scholar]
- Toft, S. Nutritional aspects of spider feeding. In Spider Ecophysiology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 373–384. [Google Scholar] [CrossRef]
- Hou, F.; Ni, Z.H.; Zou, M.T.; Zhu, R.; Yi, T.C.; Guo, J.J.; Jin, D.C. The effects of alternative foods on life history and cannibalism of Amblyseius herbicolus (Acari: Phytoseiidae). Insects 2022, 13, 1036. [Google Scholar] [CrossRef]
- Hawley, J.; Simpson, S.J.; Wilder, S.M. Effects of prey macronutrient content on body composition and nutrient intake in a web-building spider. PLoS ONE 2014, 9, e99165. [Google Scholar] [CrossRef]
- Feng, Q.; Wen, L.; Ma, J.; Yu, L.; Li, C.; Jiao, X. The effects of prey lipid on female mating and reproduction of a wolf spider. Curr. Zool. 2022, 68, 726–733. [Google Scholar] [CrossRef]
- Kowarik, C.; Martin-Creuzburg, D.; Robinson, C.T. Cross-ecosystem linkages: Transfer of polyunsaturated fatty acids from streams to riparian spiders via emergent insects. Front. Ecol. Evol. 2021, 9, 707570. [Google Scholar] [CrossRef]
- Mayntz, D.; Toft, S. Nutrient composition of the prey’s diet affects growth and survivorship of a generalist predator. Oecologia 2001, 127, 207–213. [Google Scholar] [CrossRef]
- Wen, L.; Jiao, X.; Liu, F.; Zhang, S.; Li, D. High-lipid prey reduce juvenile survivorship and delay egg laying in a small linyphiid spider Hylyphantes graminicola. J. Exp. Biol. 2020, 223, jeb237255. [Google Scholar] [CrossRef]
- Günther, C.S.; Goddard, M.R. Do yeasts and Drosophila interact just by chance? Fungal Ecol. 2018, 38, 37–43. [Google Scholar] [CrossRef]
- Wen, L.; Zhang, Z.; Zhang, S.; Liu, F.; Jiao, X.; Li, D. Influence of maternal diet on offspring survivorship, growth, and reproduction in a sheetweb spider. Biol. Open 2020, 9, bio056846. [Google Scholar] [CrossRef]
- Xiu, B.; Wu, Q. Effects of yeast powder on fecundity and growth of Drosophila melanogaster. J. Tongji Univ. Med. Ed. 2002, 23, 204–206. [Google Scholar] [CrossRef]
- Wang, Z.L.; Wang, X.P.; Li, C.R.; Xia, Z.Z.; Li, S.X. Effect of dietary protein and carbohydrates on survival and growth in larvae of the Henosepilachna vigintioctopunctata (F.) Coleoptera: Coccinellidae. J. Insect Sci. 2018, 18, 3. [Google Scholar] [CrossRef]
- Cammack, J.A.; Tomberlin, J.K. The impact of diet protein and carbohydrate on select life-history traits of the black soldier fly Hermetia illucens (L.) (Diptera: Stratiomyidae). Insects 2017, 8, 56. [Google Scholar] [CrossRef]
- Miranda, C.D.; Cammack, J.A.; Tomberlin, J.K. Life-history traits of the black soldier fly, Hermetia illucens (L.) (Diptera: Stratiomyidae), reared on three manure types. Animals 2019, 9, 281. [Google Scholar] [CrossRef]
- Jang, T.; Lee, K.P. Comparing the impacts of macronutrients on life-history traits in larval and adult Drosophila melanogaster: The use of nutritional geometry and chemically defined diets. J. Exp. Biol. 2018, 221, jeb181115. [Google Scholar] [CrossRef]
- Mayntz, D.; Toft, S.; Vollrath, F. Effects of prey quality and availability on the life history of a trap-building predator. Oikos 2003, 101, 631–638. [Google Scholar] [CrossRef]
- Toft, S.; Li, D.; Mayntz, D. A specialized araneophagic predator’s short-term nutrient utilization depends on the macronutrient content of prey rather than on prey taxonomic affiliation. Physiol. Entomol. 2010, 35, 317–327. [Google Scholar] [CrossRef]
- Jensen, K.; Mayntz, D.; Wang, T.; Simpson, S.J.; Overgaard, J. Metabolic consequences of feeding and fasting on nutritionally different diets in the wolf spider Pardosa prativaga. J. Insect Physiol. 2010, 56, 1095–1100. [Google Scholar] [CrossRef]
- Wiggins, W.D.; Wilder, S.M. Mismatch between dietary requirements for lipid by a predator and availability of lipid in prey. Oikos 2018, 127, 1024–1032. [Google Scholar] [CrossRef]
- Grangeteau, C.; Yahou, F.; Everaerts, C.; Dupont, S.; Farine, J.-P.; Beney, L.; Ferveur, J.-F. Yeast quality in juvenile diet affects Drosophila melanogaster adult life traits. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Kleinbaum, D.G.; Klein, M. Kaplan-Meier Survival curves and the Log-Rank Test. In Survival Analysis: A Self-Learning Text; Springer: New York, NY, USA, 2011; pp. 55–96. [Google Scholar] [CrossRef]
- Anderson, J.F. Responses to starvation in the spiders Lycosa lenta Hentz and Filistata hibernalis (Hentz). Ecology 1974, 55, 576–585. [Google Scholar] [CrossRef]
- Murgier, J.; Everaerts, C.; Farine, J.P.; Ferveur, J.F. Live yeast in juvenile diet induces species-specific effects on Drosophila adult behaviour and fitness. Sci. Rep. 2019, 9, 8873. [Google Scholar] [CrossRef]
- Hu, D.; Li, W.; Wang, J.; Peng, Y.Q.; Yun, Y.L.; Peng, Y. Interaction of high temperature stress and Wolbachia Infection on the Biological Characteristic of Drosophila melanogaster. Insects 2023, 14, 558. [Google Scholar] [CrossRef]
- Anagnostou, C.; Dorsch, M.; Rohlfs, M. Influence of dietary yeasts on Drosophila melanogaster life-history traits. Entomol. Exp. Appl. 2010, 136, 1–11. [Google Scholar] [CrossRef]
- Jiménez-Padilla, Y.; Adewusi, B.; Lachance, M.A.; Sinclair, B.J. Live yeasts accelerate Drosophila melanogaster larval development. J. Exp. Biol. 2024, 227, jeb247932. [Google Scholar] [CrossRef]
- Wang, N.; Xu, Y.Z.; Guo, X.Z.; Wang, B.; He, Q.Y. Effects of yeast culture addition on development and oviposition of Drosophila melanogaster. Anim. Husb. Feed Sci. 2018, 39, 16–18. [Google Scholar] [CrossRef]
- Mohapatra, A.K.; Pandey, P. Fecundity of inbred fruit fly Drosophila melanogaster on different solid culture media: An analysis. J. Appl. Nat. Sci. 2018, 10, 1109–1114. [Google Scholar] [CrossRef]
- Becher, P.G.; Flick, G.; Rozpędowska, E.; Schmidt, A.; Hagman, A.; Lebreton, S.; Bengtsson, M. Yeast, not fruit volatiles mediate Drosophila melanogaster attraction, oviposition and development. Funct. Ecol. 2012, 26, 822–828. [Google Scholar] [CrossRef]
- Pimentel, D.; Acquay, H.; Biltonen, M.; Rice, P.; Silva, M.; Nelson, J.; D’amore, M. Environmental and economic costs of pesticide use. BioScience 1992, 42, 750–760. [Google Scholar] [CrossRef]
- Mendelsohn, A.R.; Larrick, J.W. Dietary restriction: Critical co-factors to separate health span from life span benefits. Rejuvenation Res. 2012, 15, 523–529. [Google Scholar] [CrossRef]
- Rantisi, J.G. Transgenerational Inheritance of Starvation Effects on Lifespan and Reproduction in Caenorhabditis Elegans. Ph.D. Thesis, University of Oregon, Eugene, OR, USA, 2018. [Google Scholar]
- Joy, T.K.; Arik, A.J.; Corby-Harris, V.; Johnson, A.A.; Riehle, M.A. The impact of larval and adult dietary restriction on lifespan, reproduction and growth in the mosquito Aedes aegypti. Exp. Gerontol. 2010, 45, 685–690. [Google Scholar] [CrossRef]
- Le Bourg, É. Predicting whether dietary restriction would increase longevity in species not tested so far. Ageing Res. Rev. 2010, 9, 289–297. [Google Scholar] [CrossRef]
- Toft, S.; Wise, D.H. Growth, development, and survival of a generalist predator fed single-and mixed-species diets of different quality. Oecologia 1999, 119, 191–197. [Google Scholar] [CrossRef]
- Wen, L.; Wang, L.; Wang, Z.; Zhang, H.; Hu, L.; Peng, B.; Li, C. The Critical Role of Arachidonic Acid on Molting in Spiders. Curr. Zool. 2024, 71, 373–380. [Google Scholar] [CrossRef]
- Miyashita, K. Growth and development of Lycosa T-insignita Boes. et Str. (Araneae: Lycosidae) under different feeding conditions. Appl. Entomol. Zool. 1968, 3, 81–88. [Google Scholar] [CrossRef]
- Kleinteich, A.; Wilder, S.M.; Schneider, J.M. Contributions of juvenile and adult diet to the lifetime reproductive success and lifespan of a spider. Oikos 2015, 124, 130–138. [Google Scholar] [CrossRef]
- Toft, S. Value of the aphid Rhopalosiphum padi as food for cereal spiders. J. Appl. Ecol. 1995, 32, 552. [Google Scholar] [CrossRef]
- Lefcheck, J.S.; Whalen, M.A.; Davenport, T.M.; Stone, J.P.; Duffy, J.E. Physiological effects of diet mixing on consumer fitness: A meta-analysis. Ecology 2013, 94, 565–572. [Google Scholar] [CrossRef]
- Jensen, K.; Mayntz, D.; Toft, S.; Clissold, F.J.; Hunt, J.; Raubenheimer, D.; Simpson, S.J. Optimal foraging for specific nutrients in predatory beetles. Proc. R. Soc. B Biol. Sci. 2012, 279, 2212–2218. [Google Scholar] [CrossRef]
- Jakob, E.M.; Marshall, S.D.; Uetz, G.W. Estimating fitness: A comparison of body condition indices. Oikos 1996, 2, 61–67. [Google Scholar] [CrossRef]
- Matsuda, I.; Clauss, M.; Tuuga, A.; Sugau, J.; Hanya, G.; Yumoto, T.; Hummel, J. Factors affecting leaf selection by foregut-fermenting proboscis monkeys: New insight from in vitro digestibility and toughness of leaves. Sci. Rep. 2017, 7, 42774. [Google Scholar] [CrossRef]
- Li, Z.; Wei, Y.; Rogers, E. Food choice of white-headed langurs in Fusui, China. Int. J. Primatol. 2003, 24, 1189–1205. [Google Scholar] [CrossRef]
- Wilder, S.M.; Mayntz, D.; Toft, S.; Rypstra, A.L.; Pilati, A.; Vanni, M.J. Intraspecific variation in prey quality: A comparison of nutrient presence in prey and nutrient extraction by predators. Oikos 2010, 119, 350–358. [Google Scholar] [CrossRef]
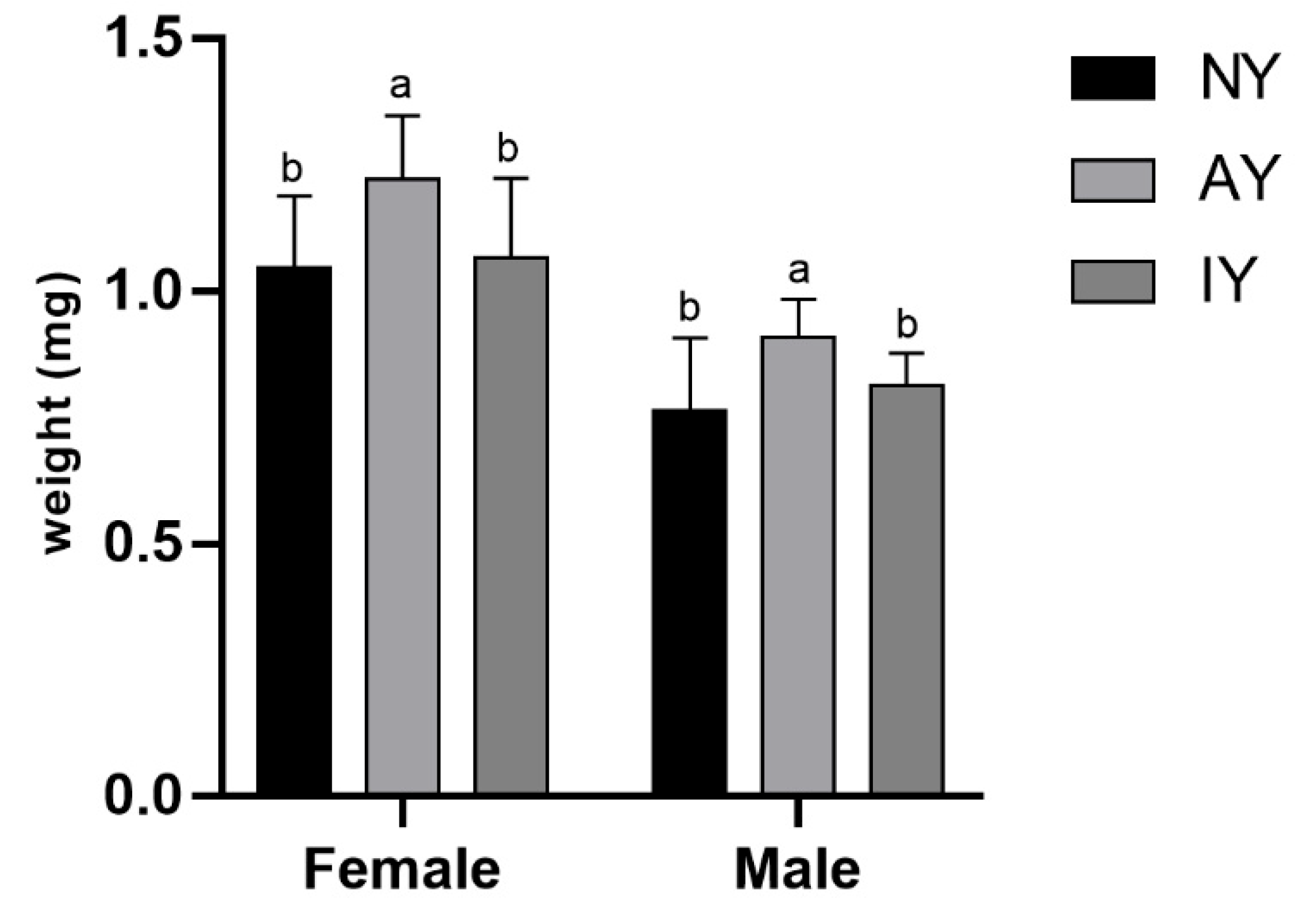
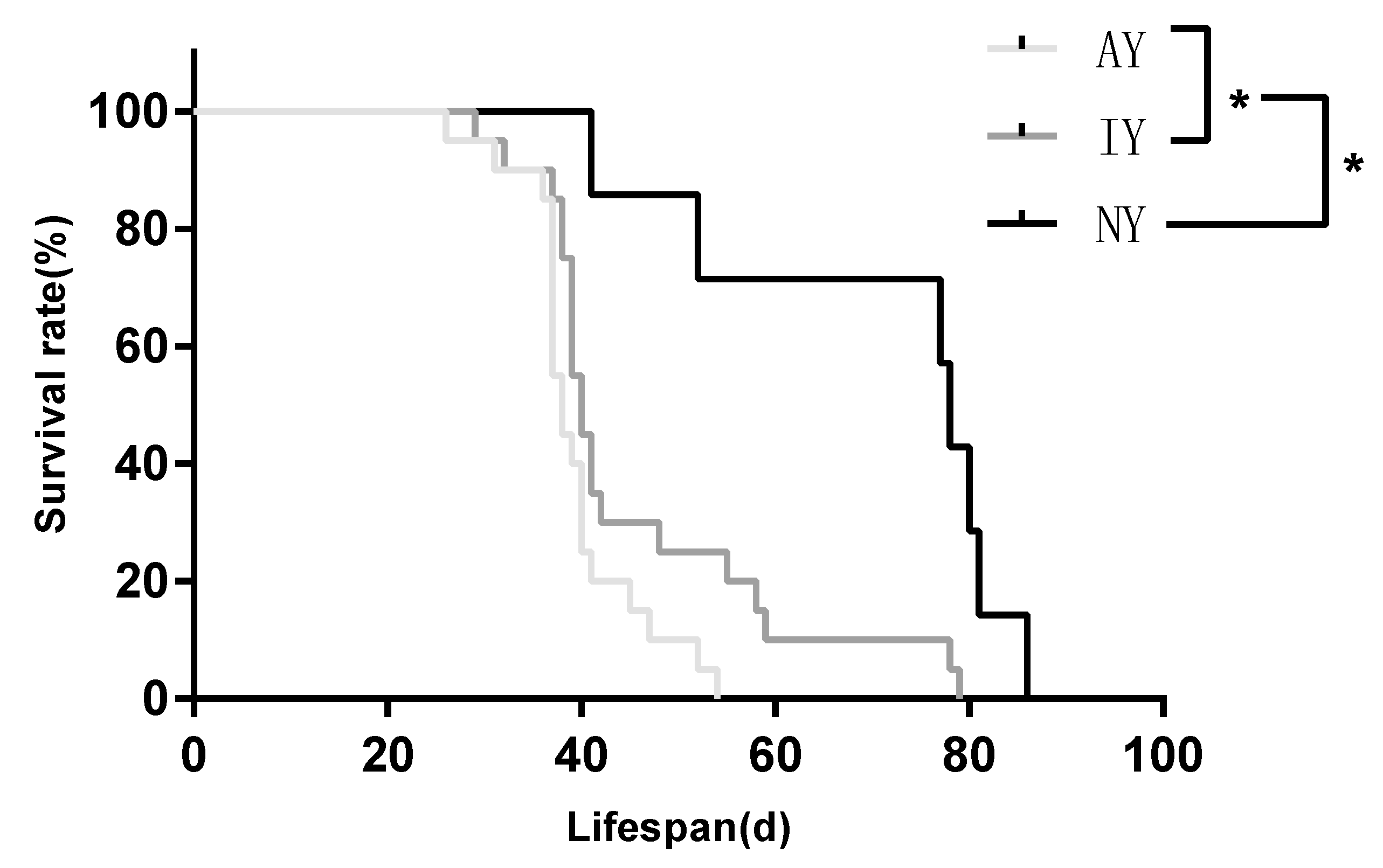
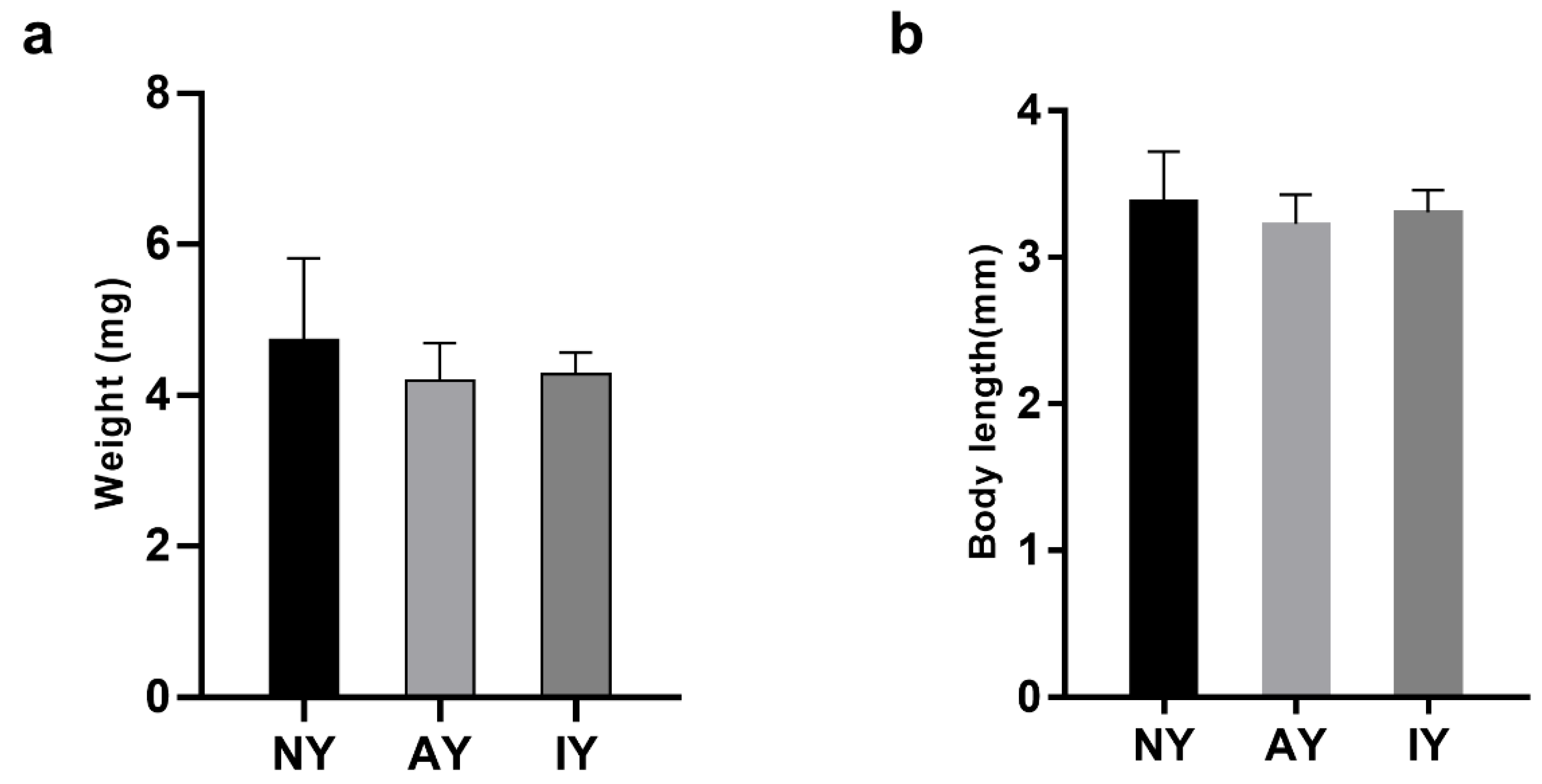
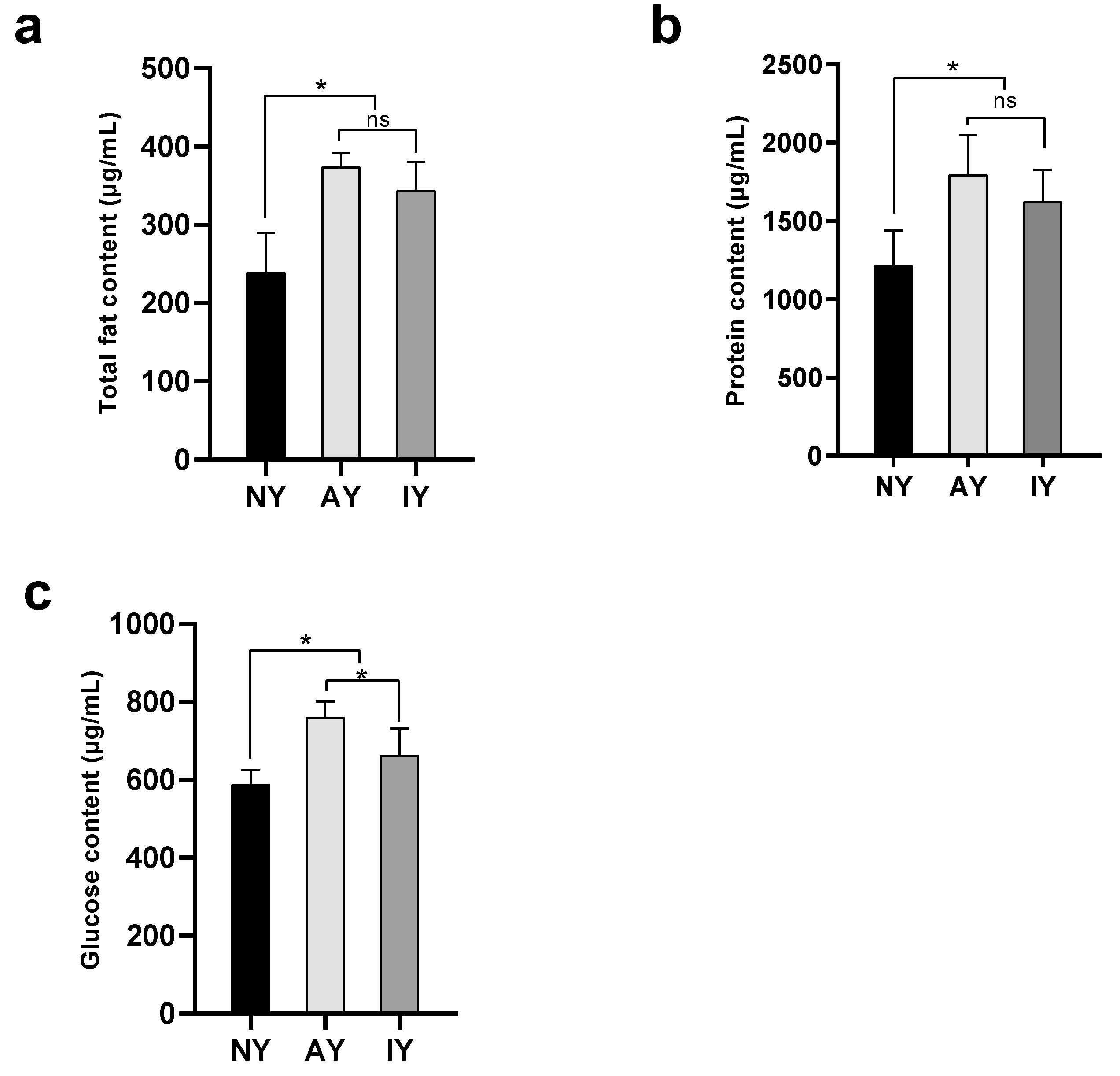
| Treatment Group | |||
|---|---|---|---|
| Biological Properties | NY | AY | IY |
| Larval duration (d) | 7.4 ± 0.55 a | 5.0 ± 0.71 b | 5.8 ± 0.84 b |
| Pupal duration (d) | 6.8 ± 0.84 a | 3.2 ± 0.84 c | 4.8 ± 0.84 b |
| Developmental duration (d) | 14.2 ± 1.10 a | 8.2 ± 0.84 c | 10.6 ± 0.89 b |
| The number of pupations | 62.8 ± 3.70 c | 118.4 ± 5.86 a | 99.4 ± 1.67 b |
| The number of eclosions | 49.6 ± 3.29 c | 100.4 ± 6.84 a | 83.40 ± 2.41 b |
| Eclosion rate (%) | 79.03 ± 0.04 b | 84.74 ± 0.02 a | 83.89 ± 0.01 a |
| Treatment Group | |||
|---|---|---|---|
| Instar | NY | AY | IY |
| 2nd instar | 14.31 ± 2.78 a | 11.15 ± 1.50 b | 14.08 ± 3.50 a |
| 3rd instar | 7.69 ± 0.95 a | 8.61 ± 1.04 a | 8.31 ± 1.44 a |
| 4th instar | 7.69 ± 0.75 a | 7.38 ± 0.51 a | 7.77 ± 0.83 a |
| 5th instar | 11.08 ± 2.02 a | 10.62 ± 1.04 a | 9.92 ± 1.19 a |
| 6th instar | 13.54 ± 3.23 | — | — |
| 7th instar | 26.62 ± 3.66 | — | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Liu, R.; Li, W.; Zhao, Y.; Peng, Y. Effects of Yeast on the Growth and Development of Drosophila melanogaster and Pardosa pseudoannulata (Araneae: Lycsidae) Through the Food Chain. Insects 2025, 16, 795. https://doi.org/10.3390/insects16080795
Peng Y, Liu R, Li W, Zhao Y, Peng Y. Effects of Yeast on the Growth and Development of Drosophila melanogaster and Pardosa pseudoannulata (Araneae: Lycsidae) Through the Food Chain. Insects. 2025; 16(8):795. https://doi.org/10.3390/insects16080795
Chicago/Turabian StylePeng, Yaqi, Rui Liu, Wei Li, Yao Zhao, and Yu Peng. 2025. "Effects of Yeast on the Growth and Development of Drosophila melanogaster and Pardosa pseudoannulata (Araneae: Lycsidae) Through the Food Chain" Insects 16, no. 8: 795. https://doi.org/10.3390/insects16080795
APA StylePeng, Y., Liu, R., Li, W., Zhao, Y., & Peng, Y. (2025). Effects of Yeast on the Growth and Development of Drosophila melanogaster and Pardosa pseudoannulata (Araneae: Lycsidae) Through the Food Chain. Insects, 16(8), 795. https://doi.org/10.3390/insects16080795






