Simple Summary
The large eggs of Chinese oak silkworm (Antheraea pernyi) have long been employed in China as an effective factitious host for mass-rearing Trichogramma parasitoids in agricultural and forestry pest management. Recent attention has turned to the Eri silkworm (Samia ricini) as a promising alternative host, owing to it possessing similarly large eggs with a broader geographical distribution. This study systematically assesses the suitability of six dominant Trichogramma species reared under five distinct treatments of S. ricini eggs. We identify the optimal egg treatment protocol to enhance Trichogramma productivity, thereby providing a scalable solution for industrial-scale parasitoid rearing. Our findings contribute to the optimization of sustainable biological control strategies against lepidopteran pests in agricultural and forestry ecosystems.
Abstract
Trichogramma wasps are highly effective biological control agents, offering an environmentally sustainable solution for pest management through their parasitism of insect eggs. This study evaluates the parasitism performance of six Trichogramma species—T. dendrolimi, T. chilonis, T. leucaniae, T. ostriniae, T. japonicum, and T. pretiosum—on five treatments of Eri silkworm (ES) eggs, a potential alternative to the large eggs of Antheraea pernyi for mass rearing. The ES egg treatments included the following: manually extracted, unfertilized, and washed eggs (MUW); naturally laid, unfertilized, and washed eggs (NUW); naturally laid, unfertilized, and unwashed eggs (NUUW); naturally laid, fertilized, and washed eggs (NFW); and naturally laid, fertilized, and unwashed eggs (NFUW). The results demonstrate that all Trichogramma species, except T. japonicum, successfully parasitized ES eggs across all treatments. Notably, washed eggs consistently supported higher parasitism and emergence rates compared to unwashed eggs, while unfertilized eggs outperformed fertilized eggs in these metrics. Among the treatments, unfertilized and washed eggs (MUW and NUW) exhibited the shortest pre-emergence time and the highest number of emerged adults, with no significant differences in female progeny ratios across most species. A striking exception was T. dendrolimi, which showed a significantly higher female offspring ratio in the MUW treatment. These findings highlight that MUW eggs of ES are a highly suitable alternative host for the mass production of Trichogramma wasps. This study provides critical insights for optimizing host egg treatments to enhance the efficiency of Trichogramma-based biological control programs.
1. Introduction
Biological control is a sustainable pest management strategy that leverages natural food chain dynamics and ecological interactions to suppress target pest populations [1]. This approach helps to minimize the use of chemical pesticides that are responsible for environmental pollution and side effects in non-target organisms and ecosystem services, and it helps to sustain biodiversity, making it an environmentally friendly alternative for pest control [2,3]. Trichogramma (Hymenoptera: Trichogrammatidae), a genus of egg parasitoids, are known to parasitize eggs of multiple different agricultural pests [4,5,6,7,8,9]. As highly effective natural enemies of agricultural and forestry pests, Trichogramma wasps play a pivotal role in integrated pest management [10]. Their deployment in biological control programs not only reduces reliance on chemical pesticides—ensuring pollution-free and residue-free green pest control—but also offers sustainable, long-term suppression of pest populations. This aligns with the growing global demand for safe, healthy, and eco-friendly agricultural products [11,12].
Globally, Trichogramma is the most extensively utilized parasitoid wasp for controlling lepidopteran pests and is amenable to large-scale factory production [13,14]. The alternative hosts employed for the mass rearing of Trichogramma are termed factitious hosts, which can be classified into two categories based on size: large eggs (e.g., Chinese oak silkworm (Antheraea pernyi Guérin-Méneville) and Eri silkworm (Samia ricini William Jones) eggs, which support the development of three or more wasps per egg; and small eggs (e.g., rice moth (Corcyra cephalonica Stainton) and angoumois grain moth (Sitotroga cerealella Olivier) eggs), which typically yield only one or two wasps per egg [15,16,17]. Ideal factitious hosts should exhibit cost-effectiveness, operational efficiency, high quality, and extended storage viability. However, current rearing technologies relying on A. pernyi (large eggs), as well as small eggs including S. cerealella and C. cephalonica, face notable limitations. Antheraea pernyi eggs are constrained by seasonal availability, geographical restrictions, and limited suitability for certain Trichogramma species, while small eggs entail high production costs, low yields, and labor-intensive processes [18,19]. Other methodologies, e.g., artificial host eggs, are not practicable yet [20,21,22]. These challenges pose significant barriers to the widespread adoption and scalability of Trichogramma-based biological control programs.
The Eri silkworm (Samia ricini) (ES) is among the most widely reared silk-producing insects globally, alongside key species such as the domestic silkworm (Bombyx mori), Antheraea pernyi [23,24], and other regionally high-potential species like Caligula japonica [25]. ES offers significant advantages for mass rearing, including low production costs, simple feeding requirements, rapid reproduction, high fecundity, and strong disease resistance, with an annual capacity of 6–7 generations in southern China [26,27]. As a factitious host for Trichogramma wasps, ES eggs demonstrate exceptional suitability, yielding 20–30 adult wasps per egg. Field trials have shown outstanding efficacy in sugarcane stem borer control, achieving parasitism rates of 65.65–94.18% [26]. Notably, Trichogramma chilonis exhibits significantly higher parasitism rates on ES eggs compared to A. pernyi eggs, confirming ES to be a superior alternative host for Trichogramma mass production in biological control programs [28].
Host egg characteristics are critical determinants of parasitoid reproductive efficiency, making the selection of optimal factitious hosts a fundamental consideration for successful biological control [29,30]. Several studies have found different suitability of parasitoid wasps for hosts. The characteristics of the host, including egg size, eggshell thickness, nutrient content of the host egg fluid, and age of the eggs, have been demonstrated to influence parasitism by Trichogramma [31,32].
Trichogramma species demonstrate distinct host egg preferences that are influenced by the nutritional composition and physiological state of the eggs [33]. Notably, studies have shown that T. dendrolimi preferentially parasitizes dissected, unfertilized A. pernyi eggs, making this combination particularly suitable for mass rearing programs [15]. Previous studies have shown that the host preference of Trichogramma can be influenced by the fertilization status of the host eggs. The study revealed that, in no-choice tests, T. dendrolimi parasitized significantly more unfertilized eggs than fertilized ones, whereas T. ostriniae parasitized significantly more fertilized eggs. In contrast, in choice tests, T. dendrolimi preferred unfertilized eggs, while T. ostriniae showed no clear preference [34]. Similar preferences have been observed in other egg parasitoids, including Anastatus gansuensis sp. Chen & Zang, Mesocomys trabalae Yao, Yang and Zhao, Anastatus fulloi Sheng & Wang, and Anastatus meilingensis Sheng, which exhibit greater host acceptance for unwashed, unfertilized A. pernyi eggs [35]. These findings suggest that the differential parasitism performance of Trichogramma species may be partly explained by their oviposition preferences, which are influenced by egg fertilization status. Furthermore, the results underscore the importance of host egg pre-treatment in mass rearing and field releases to improve biological control effectiveness.
The large host egg pretreatment methods significantly impact both parasitoid acceptance and offspring performance. Key treatment variables include the method of egg collection (dissected vs. naturally laid), fertilization status, and washing procedure [36]. Currently, A. pernyi eggs serve as the primary large-egg intermediate host in commercial production, typically obtained through the dissection of unfertilized female moths followed by water washing [15,35]. However, despite the growing interest in ES eggs as an alternative host, no comprehensive studies have evaluated how different ES egg treatments affect parasitism preferences or offspring performance in Trichogramma wasps.
The aim of the present study is to assess the adaptation of six dominant Trichogramma wasps, which are widely used in the field, to different treatments of ES eggs, in order to determine the optimal treatment of ES eggs for rearing Trichogramma.
2. Materials and Methods
2.1. Hosts
An Eri silkworm (ES, Samia ricini) colony was maintained at our research facility in Guiyang City, Guizhou Province, China. Larvae were reared under controlled laboratory conditions using fifth instar. Following cocoon formation, pupae were transferred to a moth emergence chamber maintained at 25 ± 3 °C and 80 ± 5% relative humidity under natural photoperiod conditions. Daily monitoring commenced 10–11 days post-cocooning to record adult emergence. Randomly selected newly emerged (<8 h) moths were used as experimental samples. We established five distinct ES egg treatment protocols: (1) Manually extracted unfertilized washed eggs (MUW): Eggs were dissected from unmated female abdomens, then washed and dried (washed with distilled water and then air-dried under the above controlled conditions). (2) Naturally laid unfertilized unwashed eggs (NUUW): Collected after 24 h oviposition by unmated females. (3) Naturally laid unfertilized washed eggs (NUW): NUUW eggs that were subsequently washed. (4) Naturally laid fertilized unwashed eggs (NFUW): Collected after 24 h oviposition from mated females. (5) Naturally laid fertilized washed eggs (NFW): NFUW eggs that were subsequently washed.
2.2. Parasitoids
Six Trichogramma species were obtained for this study: T. chilonis, T. dendrolimi, and T. japonicum were field-collected from Chilo suppressalis Walker egg masses in Changchun City, Jilin Province, China (43.89° N, 125.32° E); T. leucaniae and T. ostriniae were collected from Leguminivora glycinivorella Matsumura eggs in Heihe City, Heilongjiang Province, China (50.22° N, 127.53° E); and T. pretiosum was obtained from Nanjing Agricultural University, China, as a laboratory strain. All field-collected species were authenticated using morphological examination of male genital capsules and rDNA-ITS2 sequence analysis (GenBank accession numbers: T. chilonis: FR750277; T. dendrolimi: FR750279; T. leucaniae: HG518480; T. ostriniae: HE648326; T. japonicum: FN822757). All Trichogramma species were reared for up to 10 generations on C. cephalonica eggs. Rearing was conducted under controlled environmental conditions: temperature: 26 ± 1 °C; relative humidity: 75 ± 5%; photoperiod: 14L:10D (light–dark).
2.3. Parasitic Suitability of Differently Processed ES Eggs for Trichogramma Wasps
A non-toxic adhesive was used to attach five ES eggs to strips of paper card with 1 cm distance between the eggs, and then one egg card was transferred to glass tubes of 3.5 cm in diameter and 10 cm in length, and fifteen newly emerged Trichogramma adult females were introduced, all of which mated within 8 h of their emergence. After 24 h, the parasitoid wasps were removed. The parasitized eggs were placed in an incubator (MLR-351H, SANYO Electric Co., Ltd., Osaka, Japan) at a temperature of 25 ± 1 °C, relative humidity of 70 ± 5%, and light of 14L:10D to continue their development. After 6 days, the parasitism of the ES eggs of the different treatments was observed and recorded (gray egg were the parasitized eggs), and the parasitism rate was counted. After wasps had emerged, the emergence rate, female rate (number of females/total number of emerged wasps), number of emerged adult wasps/egg, and pre-emergence time (time elapsed from the start of parasitism to the emergence of the adult wasp) of Trichogramma wasps in each treatment group were recorded every day, and each treatment group was replicated 30 times.
2.4. Data Analysis
Linear models (LMs) were used to analyze the pre-emergence time and the number of emerged adults per egg, as residuals for these variables met the assumptions of normality. For parasitism rate, emergence rate, and female ratio, which are proportions, generalized linear models (GLMs) with a binomial distribution were applied. The explanatory variables included parasitoid species (PS), extraction (E), fertilization (F), washing (W), and their interactions (PS × E, PS × F, PS × W, PS × F × W).
Model significance was assessed using analysis of variance (ANOVA or deviance analysis, depending on the model type). Because several interactions were statistically significant, multiple comparisons were conducted using Tukey’s HSD test on the full dataset with the ‘multcomp’ package in R (version 4.1.1). All figures were produced using the ‘ggplot2’ package.
3. Results
3.1. Effect of Different ES Egg Treatments on Trichogramma Parasitism Rates
The parasitism rate was significantly influenced by the parasitoid species (PS), host egg treatments (E, F, W), and their interactions (Figure 1, Table 1). Notably, significant interactions were observed between PS and W, as well as between PS, F, and W.
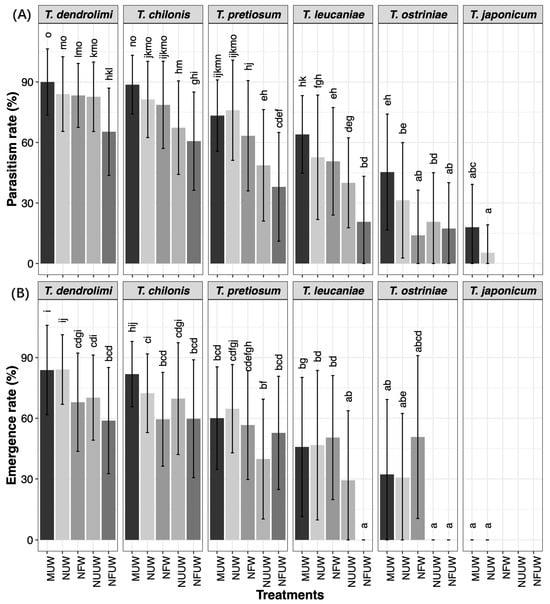
Figure 1.
Mean (±SE) of parasitism rate (A) and emergence rate (B) of Trichogramma of ES eggs in different treatments. Different letters on the bars indicate significant differences (p < 0.05, Tukey’s HSD test).

Table 1.
Linear models (LMs) and generalized linear models (GLMs) were used to assess the effects of parasitoid wasp species (PS), host egg treatments—extraction (E), fertilization (F), and washing (W)—and their interactions on several biological indexes of Trichogramma.
Parasitism variation by wasp species: T. dendrolimi parasitized MUW, NUW, and NFW eggs at significantly higher rates than NUUW and NFUW eggs. T. chilonis, T. leucaniae, T. ostriniae, and T. japonicum exhibited the highest parasitism rates in MUW eggs compared to other treatments. T. pretiosum showed significantly higher parasitism in MUW and NUW eggs than on other treatments.
Host egg-specific parasitism patterns: MUW eggs: T. dendrolimi (89.3%) and T. chilonis (86.7%) achieved the highest parasitism rates, while T. japonicum had the lowest. NUW eggs: The highest parasitism was observed for T. dendrolimi (84.0%), followed by T. chilonis (81.3%) and T. pretiosum (76.0%). T. leucaniae, T. ostriniae, and T. japonicum exhibited progressively lower rates. NFW eggs: T. dendrolimi (88%) and T. chilonis (78.6%) outperformed T. pretiosum (63.3%), T. leucaniae (50.7%), and T. ostriniae (12.7%). Notably, T. japonicum failed to parasitize NFW eggs (0%). NUUW eggs: T. dendrolimi and T. chilonis showed the highest parasitism (77.3%), significantly surpassing other species. T. japonicum again showed no parasitism. NFUW eggs: T. dendrolimi (63.3%) and T. chilonis (60.7%) led to parasitism, followed by T. pretiosum, T. leucaniae, and T. ostriniae.
3.2. Parasitic Suitability of Differently Treated ES Eggs for Trichogramma Wasps
The parasitic adaptation of Trichogramma wasps to various ES egg treatments was significantly affected by host egg treatment, wasp species, and their interactions (Table 1 and Table 2). The key parameters of parasitism success—emergence rate, number of emerged adults, female ratio, and pre-emergence time—showed distinct patterns across treatments and species.

Table 2.
Effects of ES egg treatments on emergence rate, number of emerged adults, female rate, and pre-emergence time in Trichogramma species. Values (mean ± SE) followed by different lower-case letters indicate significant differences among these parasitoid species on the host eggs (p < 0.05, Tukey’s HSD test). (T. dendrolim: TD; T. chilonis: TC; T. pretiosum: TP; T. leucaniae: TL; T. ostriniae: TO).
Suitability variation by wasp species: T. dendrolimi demonstrated optimal emergence rate on NUW eggs (84.2%; p < 0.05), and produced maximum adult yield in NUW treatment (30.7 adults; p = 0.021), highest female ratio from MUW eggs (88.7%; p = 0.048), and shortest pre-emergence time on MUW, NUW, and NFW eggs compared to NFUW (p < 0.05). T. chilonis achieved peak emergence rate on MUW eggs (81.8%; p < 0.001), maximum adult production in NUW treatment (29.9 adults; p < 0.05), consistently high female ratios (>80%) across all treatments (p = 0.08), and a stable pre-emergence time (10.75–11.0 days; p = 0.249). T. pretiosum demonstrated highest emergence rate on NUW eggs (64.7%; p < 0.05), maximum adult production in MUW (23.8) and NUW (24.4) treatments (p < 0.05), and significantly shorter pre-emergence time on MUW, NUW, NFW, and NUUW vs. NFW (p < 0.05) eggs. T. leucaniae failed to emerge from NFUW eggs (0% emergence), and demonstrated higher emergence rates on MUW, NUW, and NFW vs. NUUW (p < 0.05) eggs, consistent adult production (23.7–25.3) (p = 0.506) and female ratios (83.7–87.8%) (p = 0.191) across treatments, and shorter pre-emergence on MUW/NUW (14.3 days) vs. NFW/NUUW (p < 0.05) eggs. T. ostriniae demonstrated similar emergence rates across MUW, NUW, and NFW (p = 0.481) eggs, highest adult production in MUW (19.5) and NFW (16.9) (p < 0.05) eggs, significantly higher female ratio in MUW (79.6%) vs. NUW/NFW (p < 0.05) eggs, and shorter pre-emergence in MUW (14.4 days) vs. NUW (15.2) and NFW (15.3) (p < 0.05) eggs.
Host egg-specific suitability patterns: MUW eggs: T. dendrolimi and T. chilonis exhibited the highest emergence rates, followed by T. pretiosum and T. leucaniae, with the lowest rates observed in T. ostriniae (p < 0.05). T. chilonis produced significantly more adult wasps than other species (p < 0.05). T. pretiosum showed complete female production (thelytoky) (p < 0.05). T. dendrolimi and T. chilonis had shortest pre-emergence periods (p < 0.05). NUW eggs: T. dendrolimi exhibited a significantly higher emergence rate compared to other species (p < 0.05). T. dendrolimi and T. chilonis showed highest adult production (p < 0.05). The highest female ratio among T. pretiosum had highest female ratio, followed by T. chilonis, T. dendrolimi, and T. leucaniae, with the lowest in T. ostriniae (p < 0.001). T. dendrolimi and T. chilonis developed fastest, while T. ostriniae was slowest (p < 0.05). NFW eggs: T. dendrolimi exhibited the highest emergence rate (p < 0.05) and produced the most adults (p < 0.05). T. pretiosum had the highest female ratio, while T. ostriniae had the lowest (p < 0.05). T. dendrolimi and T. chilonis developed significantly faster than others (p < 0.05). NUUW eggs: T. dendrolimi and T. ostriniae had significantly higher emergence rates compared to T. pretiosum and T. leucaniae, while T. ostriniae failed to emerge (p < 0.05). T. dendrolimi and T. ostriniae also produced more adults than T. leucaniae, while T. pretiosum produced the fewest (p < 0.05). T. pretiosum showed the highest female ratio (p < 0.001). T. dendrolimi and T. ostriniae developed fastest (p < 0.05). NFUW eggs: There were no significant differences in emergence rates among T. dendrolimi, T. chilonis, and T. pretiosum, while T. leucaniae and T. ostriniae failed to emerge (p = 0.059). T. dendrolimi and T. ostriniae produced more adults than T. pretiosum (p < 0.05). T. pretiosum, T. dendrolimi, and T. ostriniae had the highest female proportion (p < 0.001). T. dendrolimi and T. chilonis developed significantly faster than T. pretiosum (p < 0.05).
4. Discussion
The parasitism efficiency of Trichogramma wasps is governed by both extrinsic (e.g., humidity, light, temperature) and intrinsic host factors (e.g., egg age, size, surface chemistry, and nutrient content) [37]. For mass rearing on factitious hosts, host egg acceptability and suitability are critical determinants of success [38,39]. Our study reveals that all tested Trichogramma species—except T. japonicum—successfully parasitized all egg treatments, with T. japonicum restricted to unfertilized washed eggs and failing to produce viable offspring. Notably, all species exhibited a pronounced preference for manually extracted, unfertilized washed eggs, aligning with prior reports that host fertilization status profoundly impacts parasitism success and offspring fitness [40,41]. While female ratios and pre-emergence times were similar between fertilized and unfertilized eggs, emergence rates were consistently higher in unfertilized eggs.
Insect egg surface secretions perform three critical biological functions: (1) lubrication during oviposition through the reproductive tract, (2) substrate adhesion for the egg’s attachment to plant surfaces, and (3) protective barrier formation against abiotic stressors (desiccation, precipitation) and biotic threats (microbial pathogens, predators) [42,43]. The surface distribution of these secretions governs their protective efficacy, influencing the wasp’s oviposition capability and, ultimately, parasitism outcomes [44]. This aligns with previous findings of light-yellow secretions coating ES eggs [45]. Our results demonstrate significantly higher parasitism and emergence rates in Trichogramma wasps on washed eggs versus unwashed eggs. This enhanced performance likely results from the elimination of surface secretions during washing, which presumably reduces the physical and chemical barriers to oviposition. Furthermore, consistent with previous reports [46,47], we confirm that host egg characteristics—particularly shell hardness and thickness—constitute critical limiting factors for parasitoid success, with thicker or harder eggshells substantially impeding parasitism efficiency and compromising mass rearing outcomes. Notably, in washed ES eggs, Trichogramma exhibited significantly greater parasitism rates in manually extracted eggs compared to naturally laid eggs. This preference may reflect (1) the reduced shell thickness in processed eggs, facilitating ovipositor penetration, and (2) the partial removal of outer shell layers during the extraction procedure. Together, these modifications appear to create more favorable conditions for parasitoid development.
The nutritional quality of host eggs fundamentally determines parasitoid wasp development success, serving as the exclusive nutrient source for larval growth [48]. During embryogenesis, fertilized eggs undergo dynamic biochemical changes—including depletion of sugars, polyols, and proteins—that alter their nutritional profile and consequently impact parasitoid development [49]. Our findings reveal that T. dendrolimi preferentially parasitizes unfertilized eggs, contrasting with reports of other Trichogramma species favoring fertilized dinoflagellate eggs [50]. This nutritional paradox warrants further investigation into the physiological and molecular differences between fertilized and unfertilized Equisetum eggs, particularly regarding why unfertilized eggs appear nutritionally inferior for parasitoid development.
Previous studies document T. ostriniae’s inability to develop in A. pernyi eggs and T. leucaniae’s poor performance on this host [51]. Our results demonstrate that both species show markedly improved adaptability to ES eggs, suggesting that multi-generational domestication could optimize rearing protocols. For T. japonicum, the physical constraints of A. pernyi eggs (surface structure, shell thickness) completely prevent parasitism. Chinese researchers have overcome similar limitations using multiparasitism technology, where T. dendrolimi and T. chilonis create oviposition holes, enabling T. ostriniae emergence [18]. Although T. japonicum can parasitize MUW and NUW ES eggs in our study, emergence failure suggests that similar co-parasitism strategies may be required.
The established that A. pernyi-based production infrastructure can be modified for ES eggs by adjusting processes to accommodate their distinct characteristics. Such adaptations would leverage existing systems while improving the cost-efficiency and scalability of biological control programs. Future development of T. japonicum products may depend on (1) multiparasitism techniques with compatible Trichogramma species and (2) the optimization of ES egg processing methods to enhance parasitoid emergence success.
Author Contributions
Conceptualization, Y.-H.Z., Q.-R.B. and L.-S.Z.; methodology, J.-Z.X., T.-H.L. and L.-S.Z.; validation, Q.-R.B. and L.-S.Z.; formal analysis, Y.-H.Z. and L.-S.Z.; investigation, J.-Z.X.; resources, Y.-H.Z., J.-F.M., H.-Y.Q. and L.-S.Z.; data curation, J.-Z.X. and T.-H.L.; writing—original draft preparation, J.-Z.X., W.M.W.W.K. and T.-H.L.; writing—review and editing, Y.-H.Z., Q.-R.B., L.S.M. and L.-S.Z.; visualization, W.M.W.W.K. and L.-S.Z.; supervision, Y.-H.Z., H.-Y.Q., Q.-R.B. and L.-S.Z.; project administration, Y.-H.Z. and L.-S.Z.; funding acquisition, Q.-R.B., J.-F.M. and L.-S.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key R&D Program of China (2023YFD1400600), the China Agriculture Research System of MOF and MARA (CARS-18-ZJ0101), the Central Government Guides Local Science and Technology Development Fund Projects [Qiankehezhongyindi (2023) 001, (2024) 007], and Qiankehe Platform Talent (BQW [2024]004, KXJZ [2024]042).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
Author Jian-Fei Mei was employed by the company Guizhou Zhuohao Agricultural Technology Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Heimpel, G.E.; Mills, N.J. Biological Control: Ecology and Applications; Cambridge University Press: New York, NY, USA, 2017; pp. 1–380. ISBN 9780521845144. [Google Scholar]
- Desneux, N.; Han, P.; Mansour, R.; Arnó, J.; Brévault, T.; Campos, M.R.; Chailleux, A.; Guedes, R.N.C.; Karimi, J.; Konan, K.A.J.; et al. Integrated pest management of Tuta absoluta: Practical implementations across different world regions. J. Pest Sci. 2022, 95, 17–39. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.-M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Desneux, N.; Wajnberg, E.; Wyckhuys, K.A.G.; Burgio, G.; Arpaia, S.; Narváez-Vasquez, C.A.; González-Cabrera, J.; Catalán Ruescas, D.; Tabone, E.; Frandon, J.; et al. Biological invasion of European tomato crops by Tuta absoluta: Ecology, geographic expansion and prospects for biological control. J. Pest Sci. 2010, 83, 197–215. [Google Scholar] [CrossRef]
- Tabone, E.; Bardon, C.; Desneux, N.; Wajnberg, E. Parasitism of different Trichogramma species and strains on Plutella xylostella L. on greenhouse cauliflower. J. Pest Sci. 2010, 83, 251–256. [Google Scholar] [CrossRef]
- Pizzol, J.; Pintureau, B.; Khoualdia, O.; Desneux, N. Temperature-dependent differences in biological traits between two strains of Trichogramma cacoeciae (Hymenoptera: Trichogrammatidae). J. Pest Sci. 2010, 83, 447–452. [Google Scholar] [CrossRef]
- Qu, Y.; Chen, X.; Monticelli, L.S.; Zhang, F.; Desneux, N.; Huijie, D.; Ramirez-Romero, R.; Wang, S. Parasitism performance of the parasitoid Trichogramma dendrolimi on the plum fruit moth Grapholitha funebrana. Entomol. Gen. 2020, 40, 385–395. [Google Scholar] [CrossRef]
- Wang, Y.; Iqbal, A.; Ahmed, K.S.; Zhou, Y.-Y.; Zhang, C. Reproductive Success of Trichogramma ostriniae over Trichogramma dendrolimi in Multi-Generational Rearing on Corn Borer Eggs. Insects 2025, 16, 297. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, T.; Chen, L.; Ren, J.; Ullah, F.; Yi, S.; Pan, Y.; Zhou, S.; Guo, W.; Fu, K.; et al. Trichogramma chilonis is a promising biocontrol agent against Tuta absoluta in China: Results from laboratory and greenhouse experiments. Entomol. Gen. 2024, 44, 357–365. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; He, K.-L.; Zhang, F.; Lu, X.; Babendreier, D. Mass rearing and release of Trichogramma for biological control of insect pests of corn in China. Biol. Control 2014, 68, 136–144. [Google Scholar] [CrossRef]
- Raven, C.; Nahrung, H.F. Trichogramma spp. as potential augmentative biocontrol agents of Poinciana looper, Pericyma cruegeri (Butler) (Lepidoptera: Noctuidae). Urban For. Urban Green. 2020, 50, 126656. [Google Scholar] [CrossRef]
- Lü, X.; Qiu, R.-R.; Han, S.-C.; Desneux, N.; Zang, L.-S.; Li, J. Reproductive and biological control performance of in vitro and in vivo reared Trichogramma dendrolimi on Spodoptera frugiperda. Entomol. Gen. 2025, 45, 175–183. [Google Scholar] [CrossRef]
- Van Bergeijk, K.E.; Bigler, F.; Kaashoek, N.K.; Pak, G.A. Changes in host acceptance and host suitability as an effect of rearing Trichogramma maidis on a factitious host. Entomol. Exp. Appl. 1989, 52, 229–238. [Google Scholar] [CrossRef]
- Bertin, A.; Pavinato, V.A.C.; Parra, J.R.P. Fitness-related changes in laboratory populations of the egg parasitoid Trichogramma galloi and the implications of rearing on factitious hosts. Biocontrol 2017, 62, 435–444. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, Z.-P.; Hou, Y.-Y.; Yang, X.; Wang, S.; Dai, H.-J.; Xu, Y.-Y.; Zang, L.-S. Manually-extracted unfertilized eggs of Chinese oak silkworm, Antheraea pernyi, enhance mass production of Trichogramma parasitoids. Entomol. Gen. 2020, 40, 397–406. [Google Scholar] [CrossRef]
- Cherif, A.; Mansour, R.; Grissa-Lebdi, K. The egg parasitoids Trichogramma: From laboratory mass rearing to biological control of lepidopteran pests. Biocontrol Sci. Technol. 2021, 31, 661–693. [Google Scholar] [CrossRef]
- Ghosh, E.; Ballal, C.R. Effect of age dependent cold storage of factitious host Corcyra cephalonica (Stainton) (Lepidoptera: Pyralidae) for their continuous production and Trichogramma chilonis (Ishii) (Hymenoptera: Trichogrammatidae) rearing. J. Asia-Pac. Entomol. 2017, 20, 928–934. [Google Scholar] [CrossRef]
- Li, T.-H.; Tian, C.-Y.; Zang, L.-S.; Hou, Y.-Y.; Ruan, C.-C.; Yang, X.; Monticelli, L.; Desneux, N. Multiparasitism with Trichogramma dendrolimi on egg of Chinese oak silkworm, Antheraea pernyi, enhances emergence of Trichogramma ostriniae. J. Pest Sci. 2019, 92, 707–713. [Google Scholar] [CrossRef]
- Gowda G., B.; Pandi G., G.P.; Ullah, F.; Patil, N.B.; Sahu, M.; Adak, T.; Pokhare, S.; Yadav, M.K.; Mahendiran, A.; Mittapelly, P.; et al. Performance of Trichogramma japonicum under field conditions as a function of the factitious host species used for mass rearing. PLoS ONE 2021, 16, e0256246. [Google Scholar]
- Cônsoli, F.L.; Parra, J.R.P. Development of an artificial host egg for in vitro egg laying of Trichogramma galloi and T. pretiosum using plastic membranes. Entomol. Exp. Appl. 1999, 91, 327–336. [Google Scholar] [CrossRef]
- Wang, H.; Yu, Z.; Ren, X.; Li, Y.; Yan, Z. Extracting Venom from the Parasitoid Wasp Trichogramma dendrolimi Using an Artificial Host. J. Vis. Exp. 2023, 200, 66032. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Jiang, Z.; Yang, Z.; Jiang, W.; Wang, X.; Lu, Y.; Lin, H.; Ruan, C.; Peng, Z.; Zhang, J.; et al. Insights into the composition and evolution of venom proteins collected from artificial host eggs in Trichogramma dendrolimi. Entomol. Gen. 2024, 44, 1557–1568. [Google Scholar] [CrossRef]
- Gogoi, K.; Luikham, R.; Shabnam, A.A.; Vijayakumari, K.M. Comparative rearing performance of borduar and titabar ecoraces of eri silkworm (Samia ricini) in different crops. Plant Arch. 2022, 22, 50–55. [Google Scholar] [CrossRef]
- Parbin, H.; Ahmed, A.; Chutia, B.C.; Nath, C.; Saikia, B. Evaluation of economic characters of C2 breed of eri silkworm (Samia ricini, Donovan) reared on castor (Ricinus communis) and kesseru (Heteropanax fragrans Roxb.) food plant. UPJOZ 2022, 43, 78–83. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.-M.; Wang, S.; Ye, X.-H.; Chen, M.-Y.; Zang, L.-S. A chromosome-level genome assembly of Caligula japonica as a resource for evolutionary studies in Lepidoptera. Entomol. Gen. 2023, 43, 1183–1192. [Google Scholar] [CrossRef]
- Liu, Z.-C.; Wang, G.-R.; Wu, J.-Q.; Sun, S.-R.; Wng, Z.-Y.; Chen, D.-J.; Zeng, J.-C.; Liang, Y.-F.; Tan, L.-C. Rearing the factitious host of Trichogrammatid wasp (Eri silkworm) on artificial diets. Acta Entomol. Sin. 1983, 2, 165–171. [Google Scholar]
- Zanetti, F.; Chieco, C.; Alexopoulou, E.; Vecchi, A.; Bertazza, G.; Monti, A. Comparison of new castor (Ricinus communis L.) genotypes in the mediterranean area and possible valorization of residual biomass for insect rearing. Ind. Crop Prod. 2017, 107, 581–587. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Xue, J.-Z.; Tariq, T.; Li, T.-H.; Qian, H.-Y.; Cui, W.-H.; Tian, H.; Monticelli, L.S.; Desneux, N.; Zang, L.-S. Parasitism and suitability of Trichogramma chilonis on large eggs of two factitious hosts: Samia cynthia ricini and Antheraea pernyi. Insects 2023, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Siam, A.; Zohdy, N.Z.M.; ELHafez, A.M.A.; Moursy, L.E.; Sherif, H.A.E.L. Effect of different cold storage periods of rearing host eggs on the performance of the parasitoid Trichogramma evanescens (Westwood) (Hymenoptera: Trichogrammatidae). Egypt. J. Biol. Pest Control. 2019, 29, 34. [Google Scholar] [CrossRef]
- Keinan, Y.; Keasar, T. Evidence for trans-generational effects on egg maturation schedules in a syn-ovigenic parasitoid. J. Insect Physiol. 2019, 117, 103910. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Zhang, Y.-F.; Song, Q.-T.; Zhang, F.; Li, Y.-X. The suitability of Ostrinia furnacalis (Lepidoptera: Crambidae) eggs for Trichogramma dendrolimi (Hymenoptera: Trichogrammatidae) can be changed by T. ostriniae. Appl. Entomol. Zool. 2014, 49, 265–272. [Google Scholar] [CrossRef]
- Pehlivan, S. Role of host diet on the fitness of the egg parasitoid species, Trichogramma evanescens Westwood (Hymenoptera: Trichogrammatidae). Egypt. J. Biol. Pest Control. 2021, 31, 10. [Google Scholar] [CrossRef]
- Jiang, Z.-X.; Zhou, S.-W.; Sun, Y.; Zou, K.; Li, T.-A.; Zhang, J.-L.; Chen, G.-H.; Zhang, X.-M. Assessment of the suitability of three native Trichogramma species for biological control of Tuta absoluta in China. Entomol. Gen. 2024, 44, 367–375. [Google Scholar] [CrossRef]
- Li, X.-Y.; Lei, Q.; Hua, H.-Q.; Song, H.-F.; Wang, S.; Ramirez-Romero, R.; Dai, H.; Li, J.; Li, Y.-X. Impact of host suitability on oviposition preference toward fertilized and unfertilized host eggs in two Trichogramma parasitoid species. Entomol. Gen. 2019, 39, 313–323. [Google Scholar] [CrossRef]
- Chen, Y.; Iqbal, A.; Lv, R.; Wang, X.; Desneux, N.; Zang, L. Chinese oak silkworm Antherae pernyi egg, a suitable factitious host for rearing eupelmid egg parasitoids. Pest Manag. Sci. 2022, 78, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Xavier, G.M.; Gonzaga, M.O.; de Castro, V.C.; Silva, W.D.; Valentim, A.M.; Rios Moura, R. Effects of host size on progeny sex and survivorship of Hymenoepimecis pinheirensis. Behav. Ecol. 2024, 35, arae068. [Google Scholar] [CrossRef]
- Ayvaz, A.; Karasu, E.; Karabörklü, S.; Tunçbilek, A.Ş. Effects of cold storage, rearing temperature, parasitoid age and irradiation on the performance of Trichogramma evanescens Westwood (Hymenoptera: Trichogrammatidae). J. Stored Prod. Res. 2008, 44, 232–240. [Google Scholar] [CrossRef]
- Cebolla, R.; Bru, P.; Urbaneja, A.; Tena, A. Does host quality dictate the outcome of interference competition between sympatric parasitoids? Effects on their coexistence. Anim. Behav. 2017, 127, 75–81. [Google Scholar] [CrossRef]
- Muchemi, S.K.; Zebitz, C.P.W.; Borgemeister, C.; Akutse, K.S.; Foba, C.N.; Ekesi, S.; Fiaboe, K.K.M. Acceptability and suitability of three Liriomyza leafminer species as host for the endoparasitoid Chrysocharis flacilla (Hymenoptera: Eulophidae). J. Econ. Entomol. 2018, 111, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Du, W.-M.; Xu, J.; Hou, Y.-Y.; Lin, Y.; Zang, L.-S.; Yang, X.; Zhang, J.-J.; Ruan, C.-C.; Desneux, N. Trichogramma parasitoids can distinguish between fertilized and unfertilized host eggs. J. Pest Sci. 2018, 91, 771–780. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Zhan, H.-X.; Zhang, F.; Babendreier, D.; Zhong, Y.-Z.; Lou, Q.-Z.; Zhong, Y.; Zhang, J.-P. Development and fecundity of Trissolcus japonicus on fertilized and unfertilized eggs of the brown marmorated stink bug, Halyomorpha halys. J. Pest Sci. 2018, 91, 1335–1343. [Google Scholar] [CrossRef]
- Marchini, D.; Marri, L.; Rosetto, M.; Manetti, A.G.O.; Dallai, R. Presence of antibacterial peptides on the laid egg chorion of the medfly Ceratitis capitata. Biochem. Biophys. Res. Commun. 1997, 240, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, C.G.C.; Rezende, G.L.; Lamers, G.E.M.; Van Der Zee, M. The extraembryonic serosa protects the insect egg against desiccation. Proc. R. Soc. B Biol. Sci. 2013, 280, 20131082. [Google Scholar] [CrossRef] [PubMed]
- Gaudreau, M.; Abram, P.K.; Brodeur, J. Host egg pigmentation protects developing parasitoids from ultraviolet radiation. Oikos 2017, 126, 1419–1427. [Google Scholar] [CrossRef]
- Lobdell, C.E.; Yong, T.; Hoffmann, M.P. Host color preferences and short-range searching behavior of the egg parasitoid Trichogramma ostriniae. Entomol. Exp. Appl. 2005, 116, 127–134. [Google Scholar] [CrossRef]
- Hassan, S.A.; Liscsinszky, H.; Zhang, G. The Oak-silkworm Egg Antheraea pernyi (Lepidoptera: Anthelidae) as a Mass Rearing Host for Parasitoids of the Genus Trichogramma (Hymenoptera: Trichogrammatidae). Biocontrol Sci. Technol. 2004, 14, 269–279. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.-C.; Du, W.-M.; Zang, L.-S.; Ruan, C.-C.; Zhang, J.-J.; Zou, Z.; Monticelli, L.S.; Harwood, J.D.; Desneux, N. Multi-parasitism: A promising approach to simultaneously produce Trichogramma chilonis and T. dendrolimi on eggs of Antheraea pernyi. Entomol. Gen. 2021, 41, 627–636. [Google Scholar] [CrossRef]
- Harvey, J.A.; Malcicka, M. Nutritional integration between insect hosts and koinobiont parasitoids in an evolutionary framework. Entomol. Exp. Appl. 2016, 159, 181–188. [Google Scholar] [CrossRef]
- Krugner, R. Suitability of non-fertilized eggs of Homalodisca vitripennis for the egg parasitoid Gonatocerus morrilli. Biocontrol 2014, 59, 167–174. [Google Scholar] [CrossRef]
- Yang, X.; Qu, Y.-L.; Wu, Z.-Y.; Lin, Y.; Ruan, C.-C.; Desneux, N.; Zang, L.-S. Parasitism and suitability of fertilized and nonfertilized eggs of the rice striped stem borer, Chilo suppressalis (Lepidoptera: Crambidae), for Trichogramma parasitoids. J. Econ. Entomol. 2016, 109, 1524–1528. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wen, X.-Y.; Hou, Y.-Y.; Desneux, N.; Ali, A.; Zang, L.-S. Suitability of Chinese oak silkworm eggs for the multigenerational rearing of the parasitoid Trichogramma leucaniae. PLoS ONE 2020, 15, e0231098. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).