Determination of Rice Accession Status Using Infochemical and Visual Cues Emitted to Sustainably Control Diopsis apicalis Dalman
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Field and Leaf Sampling
2.2. Insects Used in the Study
2.3. Y-Tube Olfactometer Experiments
2.4. Preparation of Odor Sources Used for the Y-Tube Olfactometer
2.5. Screened Cage Free Choice Experiments
2.6. Statistical Analyses
3. Results
3.1. Behavior of SEFs in Response to Rice Variety Leaf Cues Paired with Clean Air
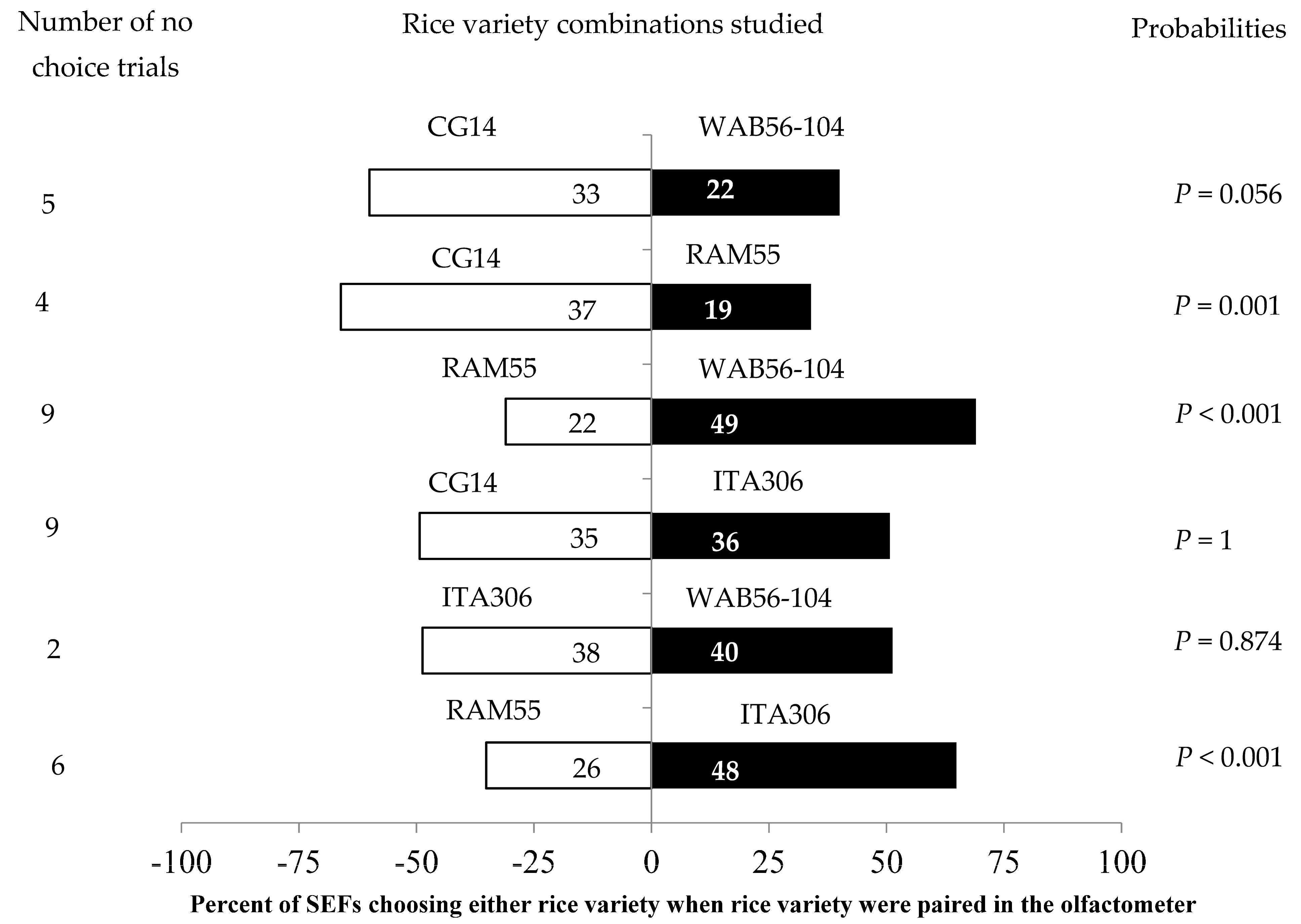
3.2. SEF Response to Rice Leaves from Two Varieties Paired with One Another
3.3. SEF Time Spent Before Reaching the Hosts in the Y-Tube Olfactometer
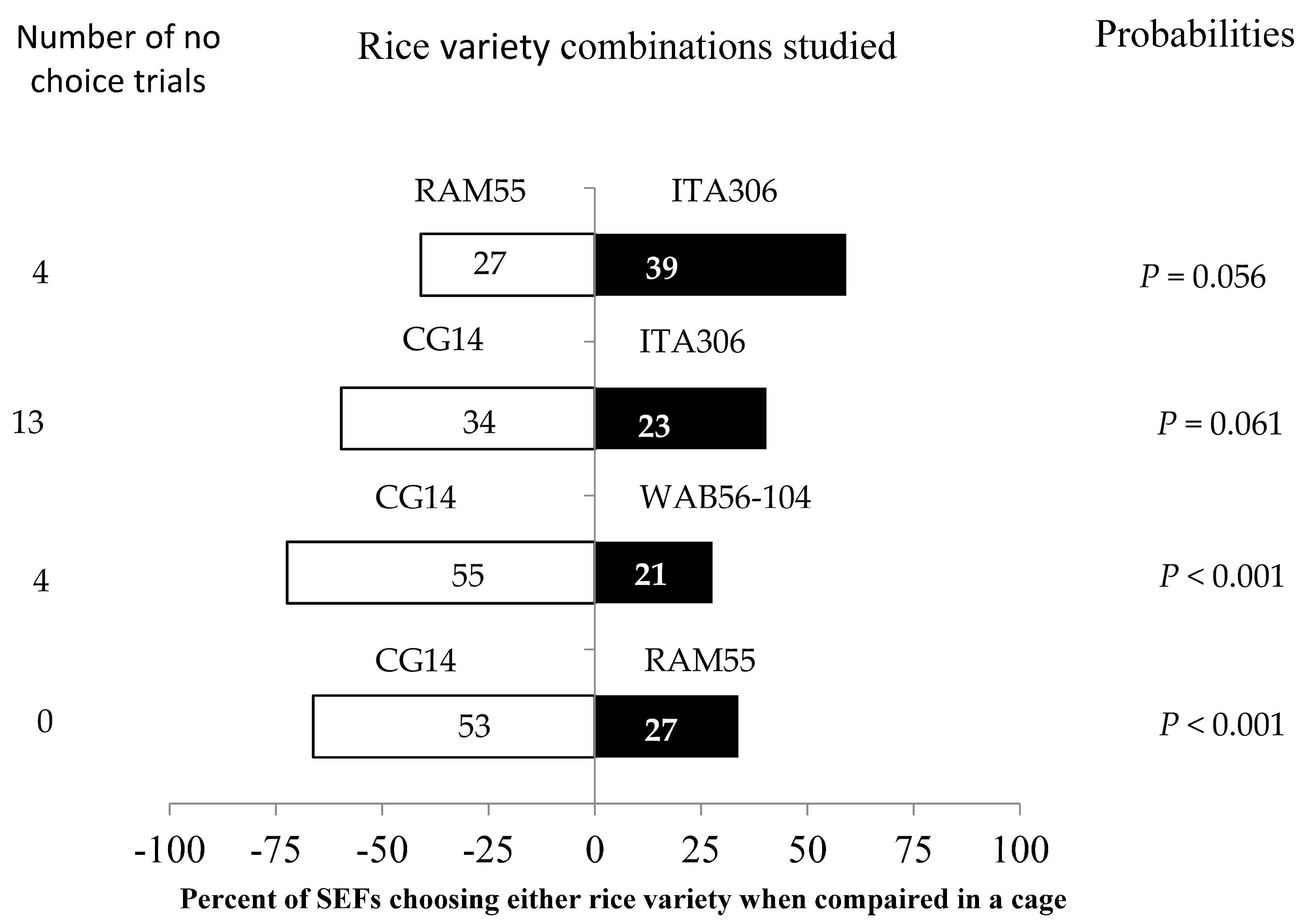
3.4. SEF Response to Potted Rice Plants When Two Varieties Were Paired in Cage
3.5. SEF Response to Potted Rice Plants When Four Varieties Were Paired in Cage
3.6. SEF Time Spent Before Reaching the Host Plants in the Screen Cage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, Z.; Tang, H.; Cai, Y.; Zeng, B.; Zhao, J.; Tang, X.; Lu, M.; Wang, H.; Zhu, X.; Wu, X.; et al. Natural variation of HTH5 from wild rice, Oryza rufipogon Griff., is involved in conferring high-temperature tolerance at the heading stage. Plant Biotechnol. J. 2022, 20, 1591–1605. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.M.; Ibrahim, A.; Dossou-Yovo, E.R.; Senthilkumar, K.; Tsujimoto, Y.; Asai, H.; Saito, K. Inorganic fertilizer use and its association with rice yield gaps in sub-Saharan Africa. Glob. Food Secur. 2023, 38, 100708. [Google Scholar] [CrossRef] [PubMed]
- USAID. Global Food Security Response: West Africa Rice Value Chain Analysis. MicroReport 161; United States Agency for International Development: Washington, DC, USA, 2009. [Google Scholar]
- Ibrahim, A.; Saito, K.; Kokou, A.; Johnson, J.-M.; Diagne, M.; Fagnombo, D.J.; Felix, F.; Sylvia, B.O.; Martial, H. Seizing opportunity towards sustainable rice cultivation in sub-Saharan Africa. Environ. Sustain. Indic. 2022, 15, 100189. [Google Scholar] [CrossRef]
- van Ittersum, M.K.; van Bussel, L.G.; Wolf, J.; Grassini, P.; van Wart, J.; Guilpart, N.; Claessens, L.; de Groot, H.; Wiebe, K.; Mason-D’Croz, D.; et al. Can sub-Saharan Africa feed itself? Proc. Natl. Acad. Sci. USA 2016, 113, 14964–14969. [Google Scholar] [CrossRef] [PubMed]
- Arouna, A.; Fatognon, I.A.; Saito, K.; Futakuchi, K. Moving toward rice self-sufficiency in sub-Saharan Africa by 2030: Lessons learned from 10 years of the Coalition for African Rice Development. World Dev. Perspect. 2021, 21, 100291. [Google Scholar] [CrossRef] [PubMed]
- Togola, A.; Nwilene, F.E.; Agbaka, A.; Degila, F.; Tolulope, A.; Chougourou, D. Screening Upland Varieties of NERICA and its Parents for Resistance to Stalk-eyed Fly, Diopsis sp. (Diptera, Diopsidae) in Benin. J. Appl. Sci. 2011, 11, 145–150. [Google Scholar] [CrossRef]
- Bocco, R.; Gandonou, C.B.; Amoussou, P.-L.; Togola, A.; Dieng, I.; Ndjiondjop, M.-N.; Seck, P.A.; Tamo, M. Rapid phenotyping for identification of rice resistant varieties to Diopsis apicalis (Diptera: Diopsidae) Westwood. Cogent Biol. 2019, 5, 1649851. [Google Scholar] [CrossRef]
- Appert, J.; Deuse, J. Insectes Nuisibles aux Cultures Vivrières et Maraîchères; Edition Maisonneuve & Larose: Paris, France, 1988; 105p. [Google Scholar]
- Banwo, O.O. Management of major insect pests of rice in Tanzania. Plant Prot. Sci. 2002, 38, 108–113. [Google Scholar] [CrossRef]
- Heinrichs, E.A.; Barrion, A.T. Rice-Feeding Insects and Selected Natural Enemies in West Africa Biology, Ecology, Identification; IIRI/WARDA: New York, NY, USA, 2004. [Google Scholar]
- Conde, S.; Catarino, S.; Ferreira, S.; Temudo, M.P.; Monteiro, F. Rice Pests and Diseases Around the World: Literature-Based Assessment with Emphasis on Africa and Asia. Agriculture 2025, 15, 667. [Google Scholar] [CrossRef]
- Pimentel, D.; Zuniga, R.; Morrison, D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ. 2005, 52, 273–288. [Google Scholar] [CrossRef]
- Yao, N.; Lee, C.-R.; Semagn, K.; Sow, M.; Nwilene, F.; Kolade, O.; Bocco, R.; Oyetunji, O.; Mitchell-Olds, T.; Ndjiondjop, M.-N.; et al. QTL mapping in three rice populations uncovers major genomic regions associated with African rice gall midge resistance. PLoS ONE 2016, 11, e0160749. [Google Scholar] [CrossRef] [PubMed]
- Makkar, G.S.; Bentur, J.S. Breeding for Stem Borer and Gall Midge Resistance in Rice. In Breeding Insect Resistant Crops for Sustainable Agriculture; Arora, R., Sandhu, S., Eds.; Springer: Singapore, 2017. [Google Scholar] [CrossRef]
- Divya, D.; Madhavi, K.R.; Dass, M.A.; Maku, R.V.; Mallikarjuna, G.; Sundaram, R.M.; Laha, G.S.; Padmakumari, A.P.; Patel, H.K.; Prasad, M.S.; et al. Expression Profile of Defense Genes in Rice Lines Pyramided with Resistance Genes Against Bacterial Blight, Fungal Blast and Insect Gall Midge. Rice 2018, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhai, K.; Xie, Z.; Yang, D.; Zhu, X.; Liu, J.; Wang, X.; Qin, P.; Yang, Y.; Zhang, G. Epigenetic regulation of antagonistic receptors confers rice blast resistance with yield balance. Science 2017, 355, 962–965. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, P.; Mei, L.; He, X.; Chen, L.; Liu, H.; Shen, S.; Ji, Z.; Zheng, X.; Zhang, Y.; et al. Xa7, a new executor R gene that confers durable and broad-spectrum resistance to bacterial blight disease in rice. Plant Commun. 2021, 2, 100143. [Google Scholar] [CrossRef] [PubMed]
- Bocco, R.; Lorieux, M.; Futakuchi, K.; Manneh, B.; Baimey, H.; Ndjiondjop, M.N. (2012) Agro-morphological characterization of a population of introgression lines derived from crosses between IR 64 (O. sativa indica) and TOG 5681 (O. glaberrima) for drought tolerance. Plant Sci. 2014, 183, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Tripathi, S.; Singh, S.P.; Prasad, A.; Akter, F.; Syed, M.A.; Badri, J.; Das, S.P.; Bhattarai, R.; Natividad, M.A.; et al. Rice breeding for yield under drought has selected for longer flag leaves and lower stomatal density. J. Exp. Bot. 2021, 72, 4981–4992. [Google Scholar] [CrossRef] [PubMed]
- Sahu, N.; Gadratagi, B.G.; Guru-Pirasanna-Pandi, G.; Patil, N.B.; Basak, N.; Rath, P.C.; Anilkumar, C.; Rath, L.K. Antixenosis and antibiosis mechanisms of resistance to Asian rice gall midge, Orseolia oryzae (Wood-Mason) in rice land races. Ann. Appl. Biol. 2024, 185, 183–194. [Google Scholar] [CrossRef]
- Rawat, N.; Sinha, D.K.; Rajendrakumar, P.; Shrivastava, N.S.; Bentur, J.S. Role of pathogenesis-interactions. Curr. Sci. 2010, 99, 1361–1368. [Google Scholar]
- Liu, X.; Williams, C.E.; Nemacheck, J.A.; Wang, H.; Subramanyam, S.; Zheng, C.; Chen, M.-S. Reactive oxygen species are involved in plant defense against a gall midge. Plant Physiol. 2010, 152, 985–999. [Google Scholar] [CrossRef] [PubMed]
- Tumlinson, J.H.; Lewis, W.J.; Vet, L.E.M. How parasitic wasps find their hosts. Sci. Am. 1993, 268, 100–106. [Google Scholar] [CrossRef]
- Borrero-Echeverry, F. Social and Environmental Olfactory Signals Mediated Insect Behavioral Ecology and Evolution. Ph.D. Thesis, Swedish University of Agricultural Sciences, Alnarp, Sweden, 2016; 69p. [Google Scholar]
- Held, D.W.; Gonsiska, P.; Potter, D.A. Evaluating companion planting and non-host masking odors for protecting roses from the Japanese beetle (Coleoptera: Scarabaeidae). J. Econ. Entomol. 2003, 96, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Bruce, T.J.A.; Wadhams, L.J.; Woodcock, C.M. Insect host location: A volatile situation. Trends Plant Sci. 2005, 10, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Blight, M.M.; Pickett, J.A.; Wadhams, L.J.; Woodcock, C.M. Antennal perception of oilseed rape, Brassica napus (Brassicaceae), volatiles by the cabbage seed weevil Ceutorhynchus assimilis (Coleoptera, Curculionidae). J. Chem. Ecol. 1995, 21, 1649–1664. [Google Scholar] [CrossRef] [PubMed]
- Bartlet, E.; Blight, M.M.; Lane, P.; Williams, I.H. The responses of the cabbage seed weevil Ceutorhynchus assimilis to volatile compounds from oilseed rape in a linear track olfactometer. Entomol. Exp. Appl. 1997, 85, 257–262. [Google Scholar] [CrossRef]
- Barata, E.N.; Pickett, J.A.; Wadhams, L.J.; Woodcock, C.M.; Mustaparta, H. Identification of host and nonhost semiochemicals of eucalyptus woodborer Phoracantha semipunctata by gas chromatography electroantennography. J. Chem. Ecol. 2000, 26, 1877–1895. [Google Scholar] [CrossRef]
- van Tol, R.W.H.M.; Visser, J.H. Olfactory antennal responses of the vine weevil Otiorhynchus sulcatus to plant volatiles. Entomol. Exp. Appl. 2002, 102, 49–64. [Google Scholar] [CrossRef]
- Wright, G.A.; Smith, B.H. Variation in complex olfactory stimuli and its influence on odour recognition. Proc. R. Soc. Lond. B 2004, 271, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Janssen, A. Plants with spider-mite prey attract more predatory mites than clean plants under greehouse conditions. Entomol. Exp. Appl. 1999, 90, 191–198. [Google Scholar] [CrossRef]
- Gnanvossou, D.; Hanna, R.; Dicke, M. Infochemical-Mediated Niche Use by the Predatory Mites Typhlodromalus manihoti and, T. aripo (Acari: Phytoseiidae). J. Insect Behav. 2003, 16, 523–535. [Google Scholar]
- Dannon, E.A.; Tamò, M.; Van Huis, A.; Dicke, M. Effects of Volatiles from Maruca vitrata Larvae and Caterpillar-Infested Flowers of Their Host Plant Vigna unguiculata on the Foraging Behavior of the Parasitoid Apanteles taragamae. J. Chem. Ecol. 2010, 36, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Ranjith, A.M. An inexpensive olfactometer and wind tunnel for Trichogramma chilonis Ishii (Trichogrammatidae: Hymenoptera). J. Trop. Agric. 2007, 45, 63–65. [Google Scholar]
- Piesik, D.; Weaver, D.K.; Runyon, J.B.; Buteler, M.; Peck, G.E.; Morrill, W.L. Behavioural responses of wheat stem sawflies to wheat volatiles. Agric. For. Entomol. 2008, 10, 245–253. [Google Scholar] [CrossRef]
- Dicke, M. Behavioural and community ecology of plants that cry for help. Plant Cell Environ. 2009, 32, 654–665. [Google Scholar] [CrossRef] [PubMed]
- Zakir, A.; Sadek, M.M.; Bengtsson, M.; Hansson, B.S.; Witzgall, P.; Anderson, P. Herbivore-induced plant volatiles provide associational resistance against an ovipositing herbivore. J. Ecol. 2013, 101, 410–417. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025. [Google Scholar]
- Yao, N.K. A Genetic Study for Resistance to African Rice Gall Midge in West African Cultivars. Ph.D. Thesis, University of KwaZulu-Natal, KwaZulu-Natal, South Africa, 2012; 227p. [Google Scholar]
- Bocco, R.; Gandonou, C.B.; Gbaguidi, F.; Ahouansou, A.C. Phytochemical screening and quantitative variation of some secondary metabolites in five cultivated rice varieties. J. Appl. Biosci. 2017, 113, 11146–11157. [Google Scholar]
- Ayiecho, P.O.; Nyabundi, J.O. Breeding for Resistance to Biotic Stress Factors. In Conventional and Contemporary Practices of Plant Breeding; Springer: Cham, Switzerland, 2025; pp. 283–332. [Google Scholar]
- Alvarez, A.E.; Tjallingii, W.F.; Garzo, E.; Vleeshouwers, V.; Dicke, M.; and Vosman, B. Location of resistance factors in the leaves of potato and wild tuber-bearing Solanum species to the aphid Myzus persicae. Entomol. Exp. Appl. 2006, 121, 145–157. [Google Scholar] [CrossRef]
- Wu, J.X.; Liu, X.M.; Zhang, S.Z.; Zhu, Y.C.; Whitworth, R.J.; Chen, M.S. Differential responses of wheat inhibitor-like genes to hessian fly, Mayetiola destructor, attacks during compatible and incompatible interactions. J. Chem. Ecol. 2008, 34, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Bai, J.; Huang, L.; Zhu, L.; Liu, X.; Weng, N.; Reese, J.C.; Harris, M.; Stuart, J.J.; Chen, M.S. Gene expression of different wheat genotypes during attack by virulent and avirulent Hessian fly (Mayetiola destructor) larvae. J. Chem. Ecol. 2007, 33, 2171–2194. [Google Scholar] [CrossRef] [PubMed]
- Laothawornkitkul, J.; Paul, N.D.; Vickers, C.E.; Possell, M.; Taylor, J.E.; Mullineaux, P.M.; Hewitt, C.N. Isoprene emissions influence herbivore-feeding decisions. Plant Cell Environ. 2008, 31, 1410–1415. [Google Scholar] [CrossRef] [PubMed]
- Unsicker, S.B.; Kunert, G.; Gershenzon, J. Protective perfumes: The role of vegetative volatiles in plant defense against herbivores. Curr. Opin. Plant Biol. 2009, 12, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E. Sites of synthesis, biochemistry and functional role of plant volatiles. S. Afr. J. Bot. 2010, 76, 612–631. [Google Scholar] [CrossRef]
- Latif, S.; Chiapusio, G.; Weston, L.A. Allelopathy and the role of allelochemicals in plant defence. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2017; Volume 82, pp. 19–54. [Google Scholar]
- Kong, C.H.; Xuan, T.D.; Khanh, T.D.; Tran, H.D.; Trung, N.T. Allelochemicals and signaling chemicals in plants. Molecules 2019, 24, 2737. [Google Scholar] [CrossRef] [PubMed]
- Belz, R.G. Allelopathy in crop/weed interactions—An update. Pest Manag. Sci. Former. Pestic. Sci. 2007, 63, 308–326. [Google Scholar] [CrossRef] [PubMed]
- Sain, M.; Kalode, M.B. Greenhouse evaluation of rice cultivars for resistance to gall midge, Orseolia oryzae (Wood-Mason) and studies on mechanism of resistance. Insect Sci. Appl. 1994, 15, 67–74. [Google Scholar] [CrossRef]
- Hilker, M.; Meiners, T. Plants and insect eggs: How do they affect each other? Phytochemistry 2011, 72, 1612–1623. [Google Scholar] [CrossRef] [PubMed]
- Awmack, C.S.; Leather, S.R. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 2002, 47, 817–844. [Google Scholar] [CrossRef] [PubMed]
- Morewood, W.D.; Simmonds, K.E.; Gries, R.; Allison, J.D.; Borden, J.H. Distribution by conophthorin of the kairomonal response of sawyer beetles to bark beetle pheromones. J. Chem. Ecol. 2003, 29, 2115–2129. [Google Scholar] [CrossRef] [PubMed]
- Coley, P.D.; Bateman, L.M.; Kursar, T.A. The effects of plant quality on caterpillar growth and defense against natural enemies. Oikos 2006, 115, 219–228. [Google Scholar] [CrossRef]
- Morrissey, J.P.; Osbourn, A.E. Fungal resistance to plant antibiotics as a mechanism of pathogenesis. Microbiol. Mol. Biol. Rev. 1999, 63, 708–724. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.; Kerem, Z.; Makkar, H.P.S.; Becker, K. The biological action of saponins in animal systems: A review. Br. J. Nutr. 2002, 88, 587–605. [Google Scholar] [CrossRef] [PubMed]
- Togola, A.; Agbaka, A.; Agunbiade, T.A.; Anato, F.; Chougourou, D.C.; Nwilene, F.E. Connaissance paysanne des insectes foreurs de tiges du riz et leurs dégâts dans différentes zones écologiques du Bénin (Afrique de l’Ouest). Cah. d’Agric. 2010, 19, 262–266. [Google Scholar] [CrossRef]
- Wade, M.J. The co-evolutionary genetics of ecological communities. Nat. Rev. Genet. 2007, 8, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Kutschera, U.; Briggs, W.R. From Charles Darwin’s botanical country-house studies to modern plant biology. Plant Biol. 2009, 11, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Suhandono, S.; Kusumawardhani, M.K.; Aditiawati, P. Isolation and molecular identification of endophytic bacteria from Rambutan fruits (Nephelium lappaceum, L.) cultivar Binjai. HAYATI J. Biosci. 2016, 23, 39–44. [Google Scholar] [CrossRef]
- da Silva Ribeiro, A.; Polonio, J.C.; Tenório Costa, A.; dos Santos, C.M.; Rhoden, S.A.; Azevedo, J.L.; Pamphile, J.A. Bioprospection of Culturable Endophytic Fungi Associated with the Ornamental Plant Pachystachys lutea. Curr. Microbiol. 2018, 75, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, L.; Yaakop, A.S.; Salleh, B.; Zakaria, M. Endophytic fungi from paddy. Trop. Life Sci. Res. 2010, 21, 101. [Google Scholar] [PubMed]
- Wambugu, P.W.; Ndjiondjop, M.N.; Henry, R. Genetics and Genomics of African Rice (Oryza glaberrima Steud) Domestication. Rice 2021, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Liu, S.; Kyaing, M.S.; Xu, P.; Tharreau, D.; Deng, W.; Li, X.; Bi, Y.; Zeng, L.; Li, J.; et al. Identification and fine mapping of Pi69(t), a new gene conferring broad-spectrum resistance against Magnaporthe oryzae from Oryza glaberrima steud. Front. Plant Sci. 2020, 11, 1190. [Google Scholar] [CrossRef] [PubMed]
- Petitot, A.-S.; Kyndt, T.; Haidar, R.; Dereeper, A.; Collin, M.; Engler, J.d.A.; Gheysen, G.; Fernandez, D. Transcriptomic and histological responses of African rice (Oryza glaberrima) to Meloidogyne graminicola provide new insights into root-knot nematode resistance in monocots. Ann. Bot. 2017, 119, 85–899. [Google Scholar] [CrossRef] [PubMed]


| Varieties | Species | Ecologies | Reaction to SEF |
|---|---|---|---|
| ITA306 | Oryza sativa | Lowland/Irrigated | Susceptible |
| WAB56-104 | Oryza sativa | Upland | Susceptible |
| CG14 | Oryza glaberrima | Floating | Resistant |
| RAM55 | Oryza glaberrima | Floating | Resistant |
| ITA306 | WAB56-104 | CG14 | RAM55 | Clean Air | p-Values |
|---|---|---|---|---|---|
| 39.98 | 66.30 | 0.007 | |||
| 38.65 | 65.25 | 0.264 | |||
| 28.65 | 42.48 | 0.365 | |||
| 39.71 | 45.15 | 0.773 | |||
| 84.33 | 90.03 | 0.636 | |||
| 59 | 66.16 | 0.393 | |||
| 36.30 | 33.38 | 0.787 | |||
| 73 | 75.33 | 0.752 | |||
| 36.97 | 45.85 | 0.474 | |||
| 57.82 | 56.02 | 0.915 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bocco, R.; Pegalepo, E.; Togola, A.; Nwilene, F.; Gandonou, C.B.; Zoclanclounon, Y.A.B.; Ndjiondjop, M.N.; Sow, M.; Kim, J.J.; Tamò, M. Determination of Rice Accession Status Using Infochemical and Visual Cues Emitted to Sustainably Control Diopsis apicalis Dalman. Insects 2025, 16, 752. https://doi.org/10.3390/insects16080752
Bocco R, Pegalepo E, Togola A, Nwilene F, Gandonou CB, Zoclanclounon YAB, Ndjiondjop MN, Sow M, Kim JJ, Tamò M. Determination of Rice Accession Status Using Infochemical and Visual Cues Emitted to Sustainably Control Diopsis apicalis Dalman. Insects. 2025; 16(8):752. https://doi.org/10.3390/insects16080752
Chicago/Turabian StyleBocco, Roland, Esther Pegalepo, Abou Togola, Francis Nwilene, Christophe Bernard Gandonou, Yedomon Ange Bovys Zoclanclounon, Marie Noelle Ndjiondjop, Mounirou Sow, Jeong Jun Kim, and Manuele Tamò. 2025. "Determination of Rice Accession Status Using Infochemical and Visual Cues Emitted to Sustainably Control Diopsis apicalis Dalman" Insects 16, no. 8: 752. https://doi.org/10.3390/insects16080752
APA StyleBocco, R., Pegalepo, E., Togola, A., Nwilene, F., Gandonou, C. B., Zoclanclounon, Y. A. B., Ndjiondjop, M. N., Sow, M., Kim, J. J., & Tamò, M. (2025). Determination of Rice Accession Status Using Infochemical and Visual Cues Emitted to Sustainably Control Diopsis apicalis Dalman. Insects, 16(8), 752. https://doi.org/10.3390/insects16080752







