Synergistic Larvicidal and Pupicidal Effects of Monoterpene Mixtures Against Aedes aegypti with Low Toxicity to Guppies and Honeybees
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mosquitoes
2.2. Chemicals and Treatment Formulations
2.3. Toxicity Bioassay
2.4. Microscopic of Morphological Changes
2.5. Toxicity Bioassay of Non-Target Species
2.5.1. Bioassay of Guppies
2.5.2. Bioassay of Honeybees
2.6. Statistical Analysis
3. Results
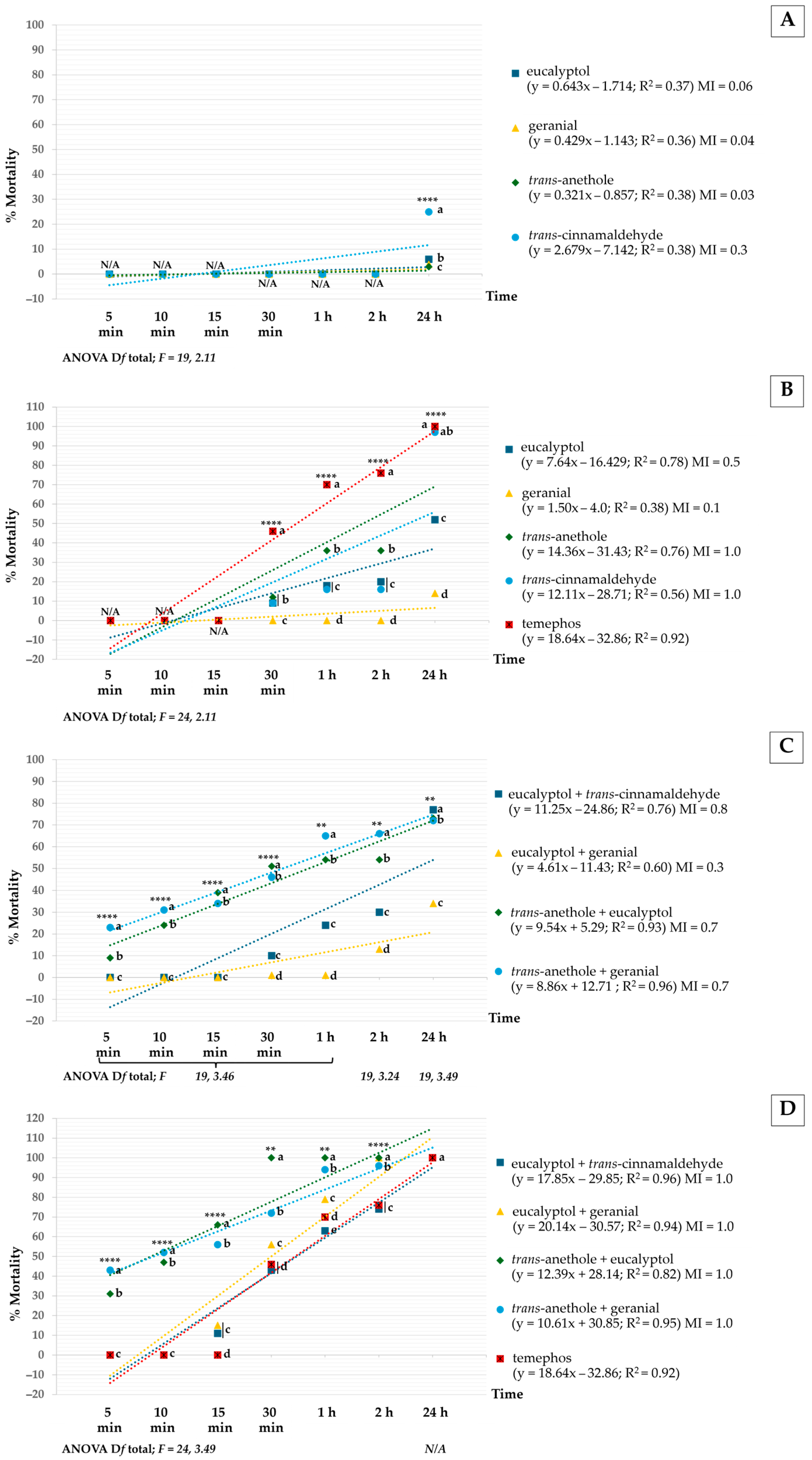
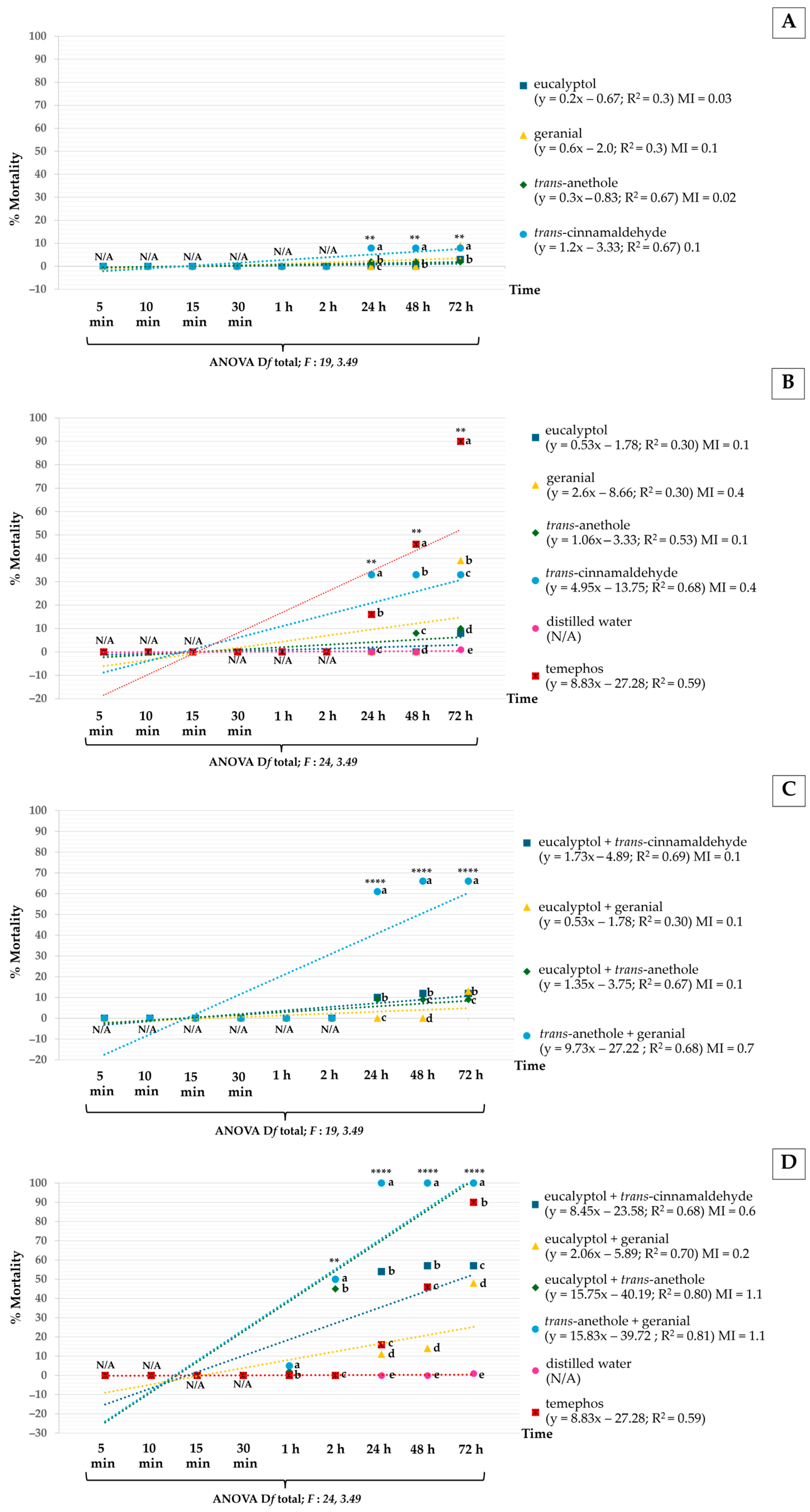
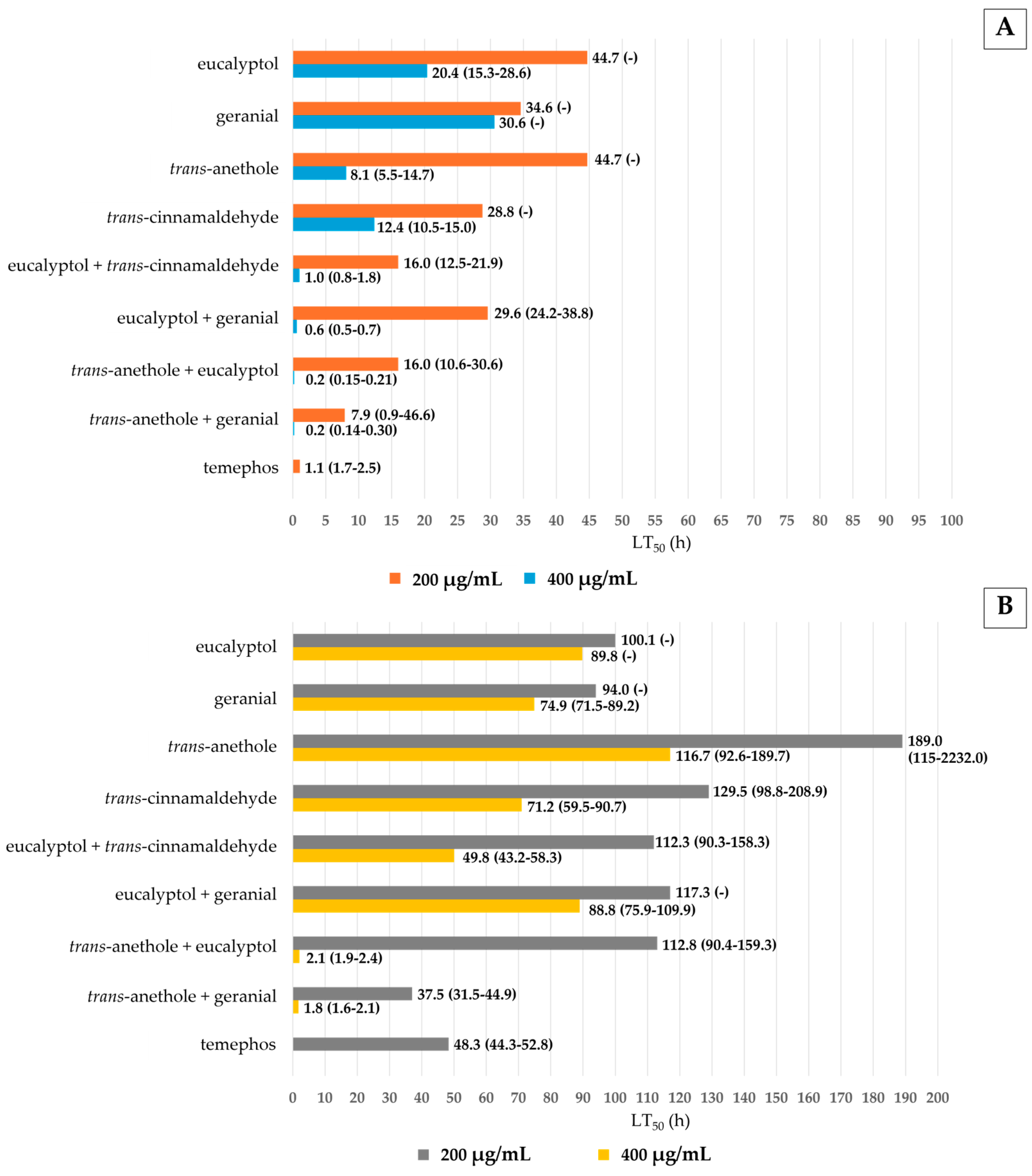
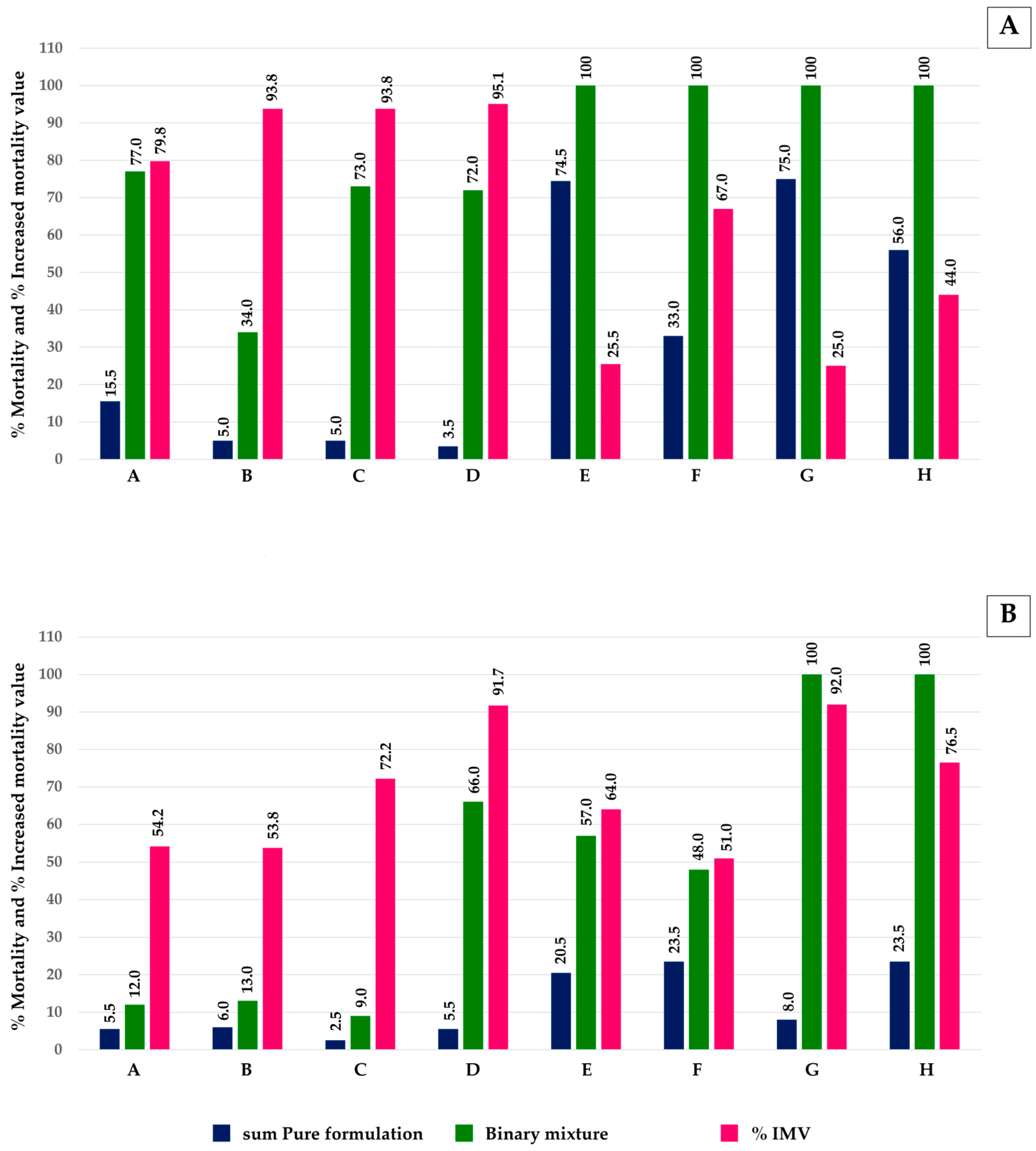
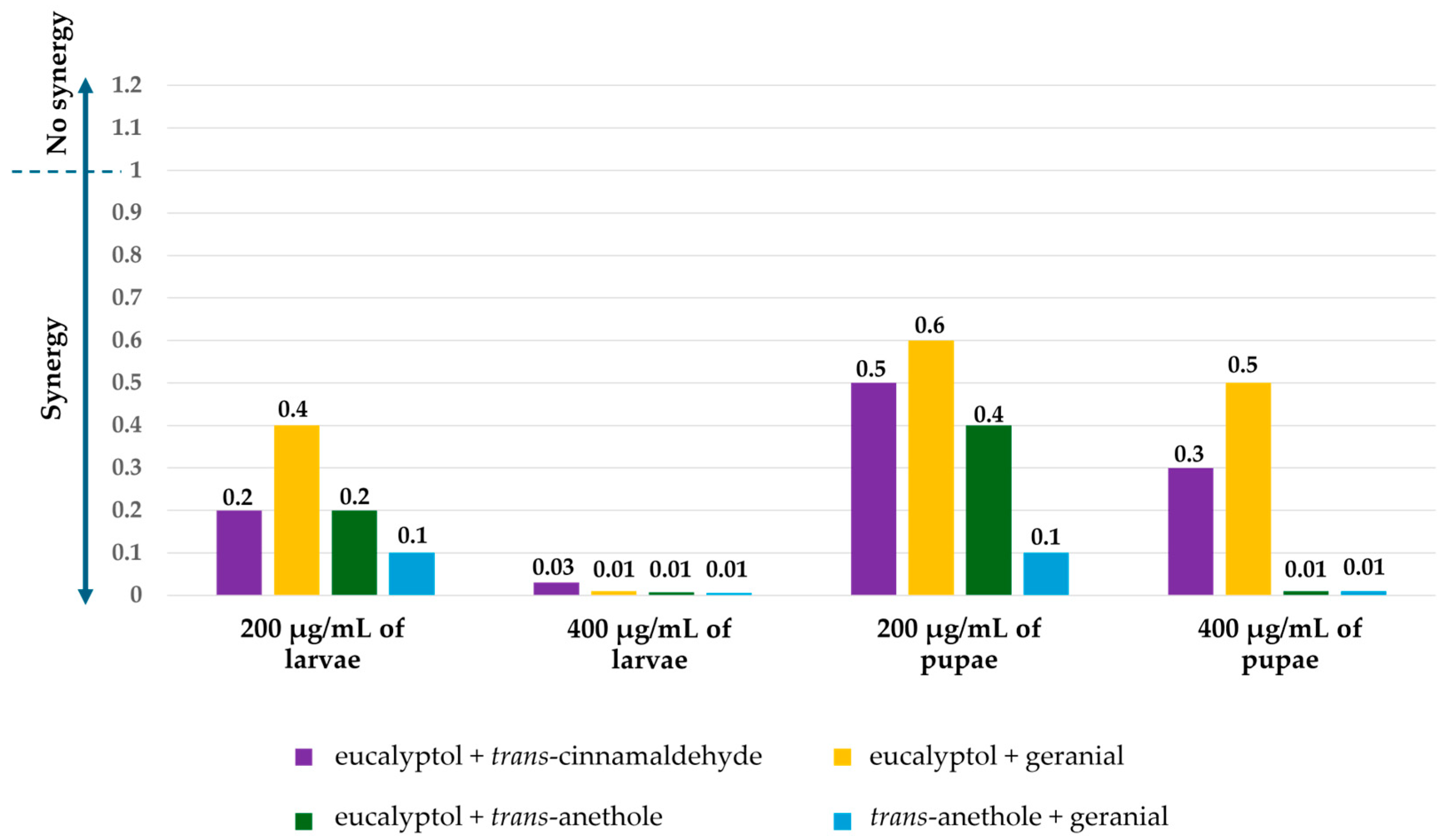
3.1. Toxicity of Pure and Mixtures of Monoterpenes Against Ae. aegypti Larvae and Pupae
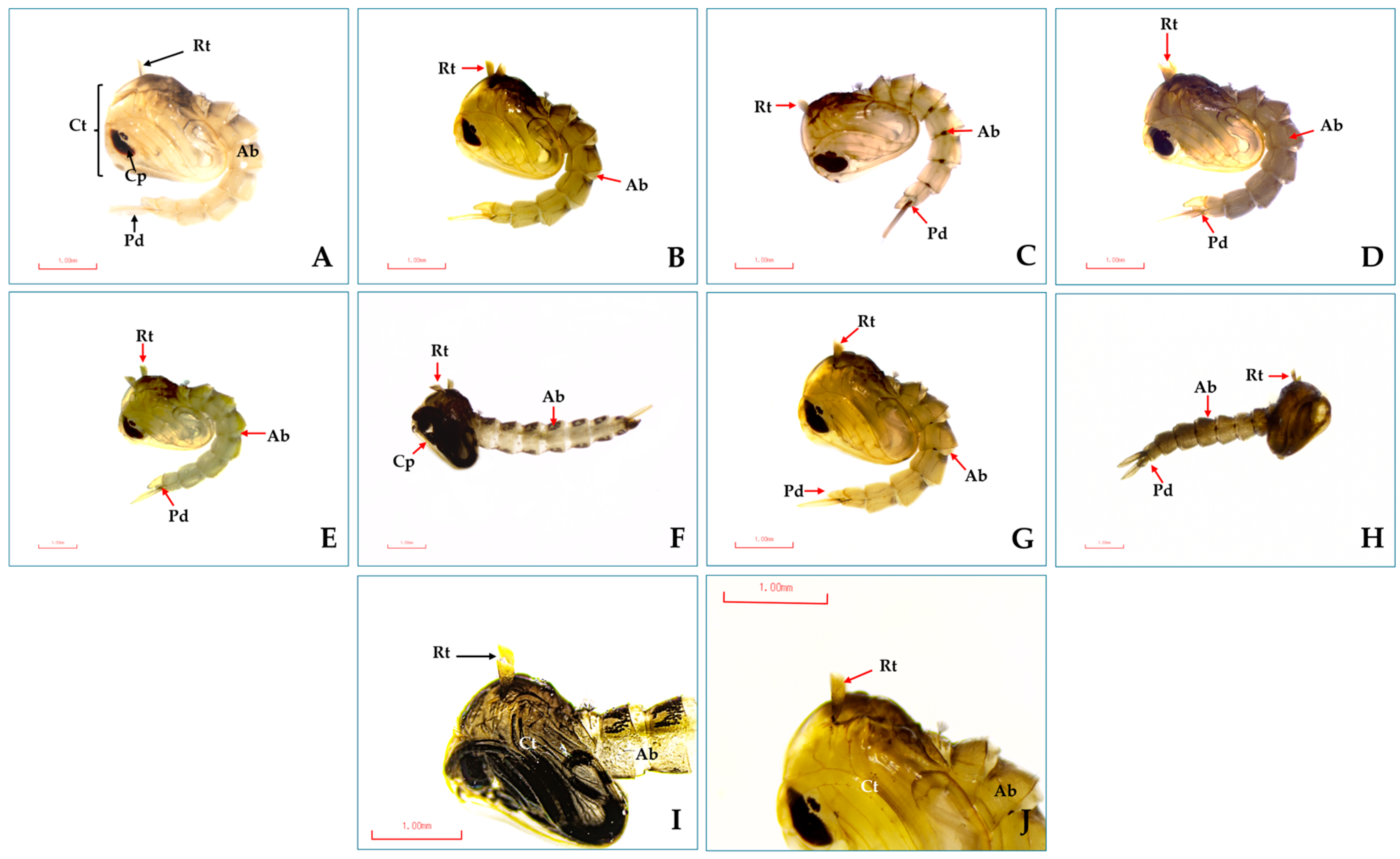
3.2. Morphological Changes After Treatment with Monoterpenes
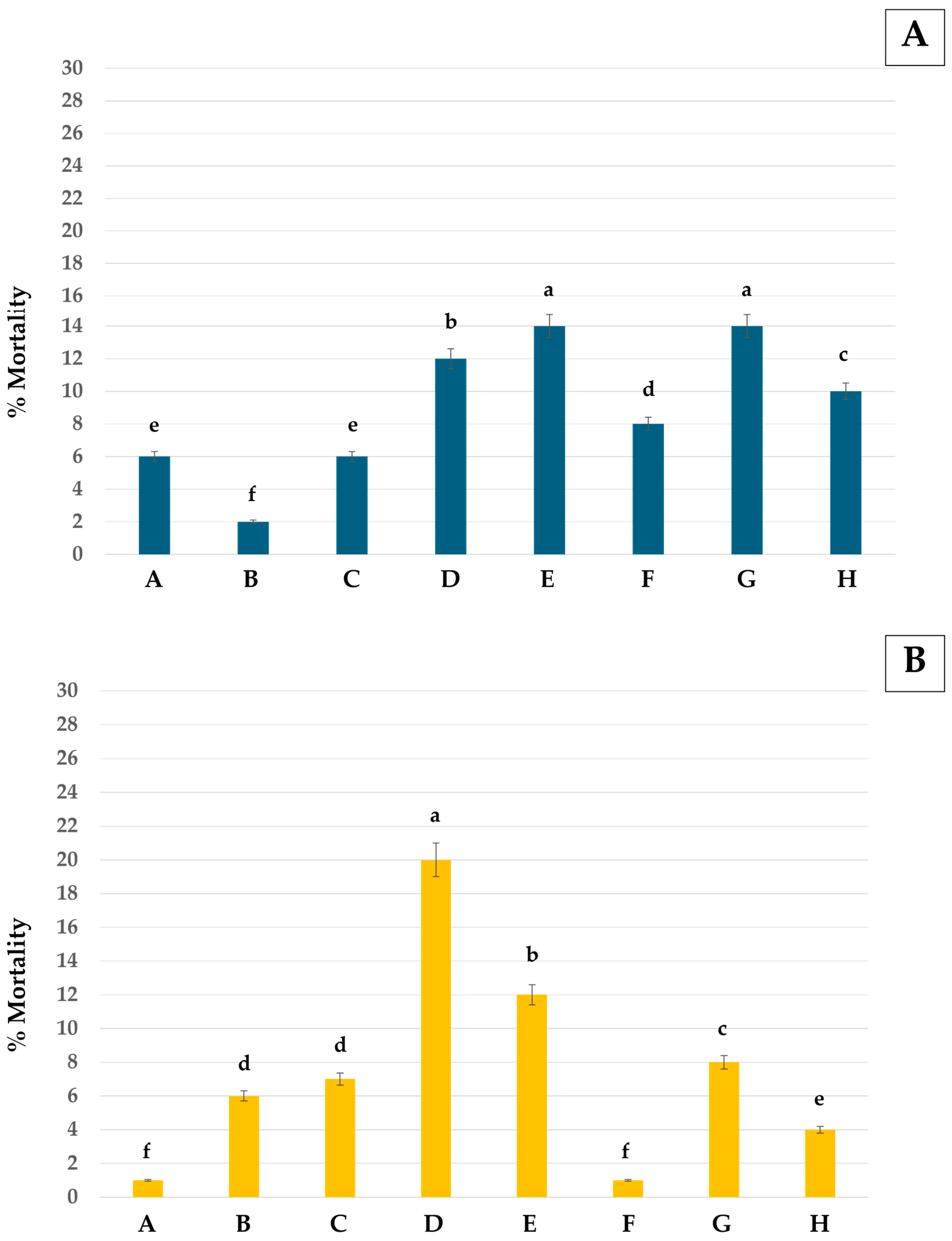
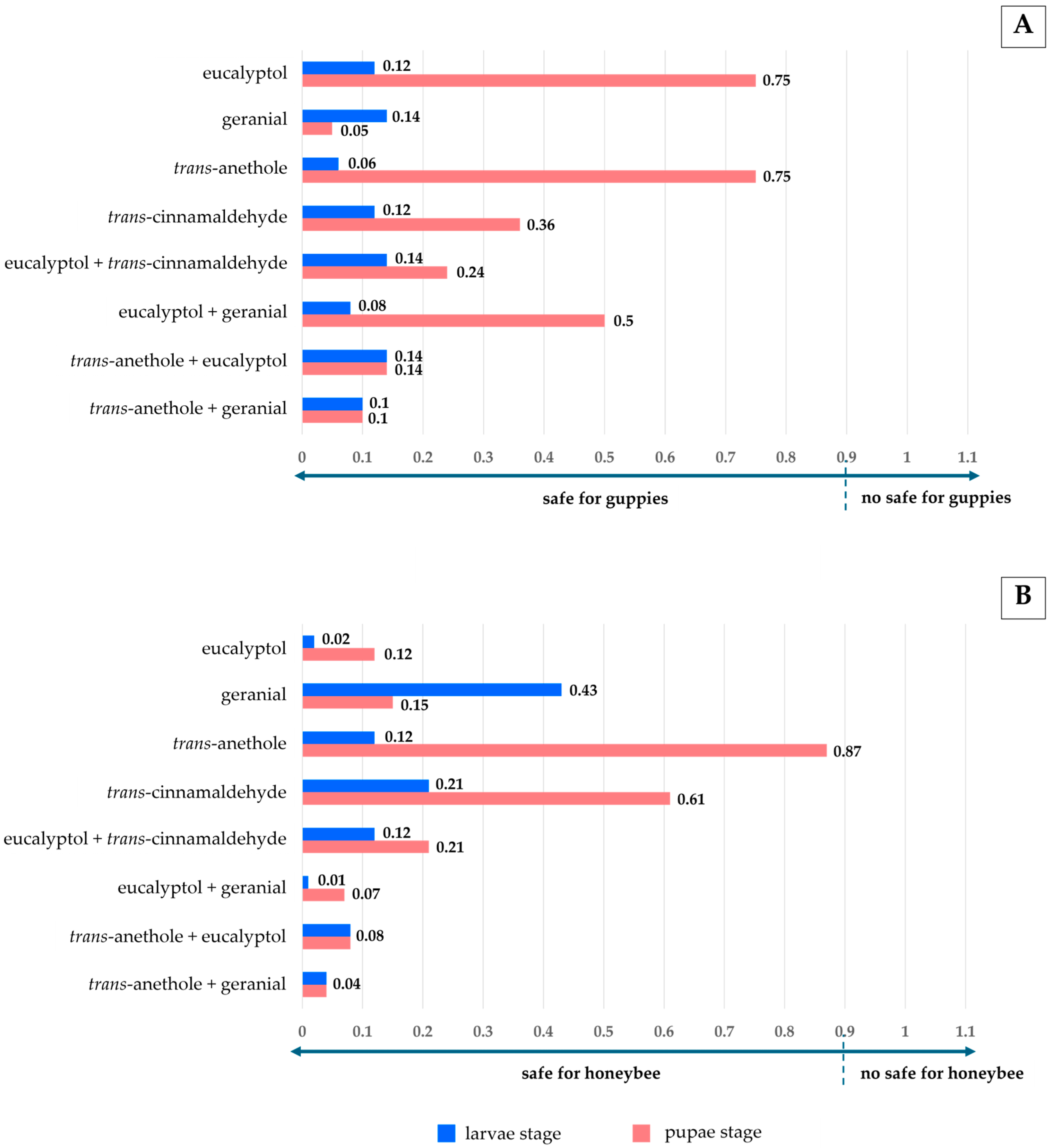
3.3. Efficacy on Non-Target Species
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Saputra, F.R.; Wahid, I.; Supriyono, S.; Hadi, U.K. Abundance of adult Aedes aegypti and Ae. albopictus (Culicidae: Diptera) across six settlements in South Sulawesi, Indonesia. Biodiversitas 2025, 26, 509–519. [Google Scholar] [CrossRef]
- Facchinelli, L.; Badolo, A.; McCall, P.J. Biology and behaviour of Aedes aegypti in the human environment: Opportunities for vector control of arbovirus transmission. Viruses 2023, 15, 636. [Google Scholar] [CrossRef] [PubMed]
- Leta, S.; Beyene, T.J.; De Clercq, E.M.; Amenu, K.; Kraemer, M.U.G.; Revie, C.W. Global risk mapping for major diseases transmitted by Aedes aegypti and Aedes albopictus. Int. J. Infect. Dis. 2018, 67, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Thailand Ministry of Public Health. Preventing Dengue Through Eliminating Mosquito Breeding Sites. 2023. Available online: https://ddc.moph.go.th/uploads/publish/1449920230712042416.pdf (accessed on 15 May 2025).
- WHO. Vector-Borne Diseases. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (accessed on 10 May 2025).
- WHO. Brazil Is the Country Most Affected by New Dengue Outbreak in the Americas. Available online: https://news.un.org/pt/story/2023/07/1817882 (accessed on 10 May 2025).
- Jacobs, E.; Chrissian, C.; Rankin-Turner, S.; Wear, M.; Camacho, E.; Broderick, N.A.; McMeniman, C.J.; Stark, R.E.; Casadevall, A. Cuticular profiling of insecticide resistant Aedes aegypti. Sci. Rep. 2023, 13, 10154. [Google Scholar] [CrossRef] [PubMed]
- Kasai, S.; Itokawa, K.; Uemura, N.; Takaoka, A.; Furutani, S.; Maekawa, Y.; Kobayashi, D.; Imanishi-Kobayashi, N.; Amoa-Bosompem, M.; Murota, K.; et al. Discovery of super–insecticide-resistant dengue mosquitoes in Asia: Threats of concomitant knockdown resistance mutations. Sci. Adv. 2022, 8, eabq7345. [Google Scholar] [CrossRef] [PubMed]
- Naqqash, M.N.; Gökçe, A.; Bakhsh, A.; Salim, M. Insecticide resistance and its molecular basis in urban insect pests. Parasitol. Res. 2016, 115, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Jobe, N.B.; Huijben, S.; Paaijmans, K.P. Non-target effects of chemical malaria vector control on other biological and mechanical infectious disease vectors. Lancet Planet. Health 2023, 7, e706–e717. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Mercado, J.P.; Sierra-Santoyo, A.; Verdín-Betancourt, F.A.; Rojas-García, A.E.; Quintanilla-Vega, B. Temephos, an organophosphate larvicide for residential use: A review of its toxicity. Crit. Rev. Toxicol. 2022, 52, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R.; Benelli, G. Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Laurentino, A.O.M.; de Medeiros, F.D.; de Oliveira, J.; da Rosa, N.; Gomes, T.M.; Peretti, E.d.M.; Prophiro, J.S.; Fortunato, J.J. Effects of prenatal exposure to temephos on behavior and social interaction. Neuropsych. Dis. Treat. 2019, 15, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Bae, J.-W.; Kim, D.-H.; Jeong, D.-J.; Ha, J.J.; Yi, J.K.; Kwon, W.-S. Detrimental effects of temephos on male fertility: An in vitro study on a mouse model. Reprod. Toxicol. 2020, 96, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R. Essential oils for the development of eco-friendly mosquito larvicides: A review. Ind. Crops Prod. 2015, 76, 174–187. [Google Scholar] [CrossRef]
- Soonwera, M.; Moungthipmalai, T.; Aungtikun, J.; Sittichok, S. Combinations of plant essential oils and their major compositions inducing mortality and morphological abnormality of Aedes aegypti and Aedes albopictus. Heliyon 2022, 8, e09346. [Google Scholar] [CrossRef] [PubMed]
- Sittichok, S.; Passara, H.; Sinthusiri, J.; Moungthipmalai, T.; Puwanard, C.; Murata, K.; Soonwera, M. Synergistic larvicidal and pupicidal toxicity and the morphological impact of the dengue vector (Aedes aegypti) induced by geranial and trans-cinnamaldehyde. Insects 2024, 15, 714. [Google Scholar] [CrossRef] [PubMed]
- Spinozzi, E.; Ferrati, M.; Cappellacci, L.; Petrelli, R.; Baldassarri, C.; Morshedloo, M.R.; Maggi, F.; Pavela, R. Major monoterpenoids from Dracocephalum moldavica essential oil act as insecticides against Culex quinquefasciatus with synergistic and antagonistic effects. Ind. Crops Prod. 2024, 219, 119060. [Google Scholar] [CrossRef]
- Soonwera, M.; Sinthusiri, J.; Passara, H.; Moungthipmalai, T.; Puwanard, C.; Sittichok, S.; Murata, K. Combinations of lemongrass and star anise essential oils and their main constituent: Synergistic housefly repellency and safety against non-target organisms. Insects 2024, 15, 210. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.L.; Duchon, S.; Di Filippo, L.D.; Chorilli, M.; Corbel, V.; Oliveira, P.L. Larvicidal properties of terpenoid-based nanoemulsions against the dengue vector Aedes aegypti L. and their potential toxicity against non-target organism. PLoS ONE 2024, 19, e0293124. [Google Scholar] [CrossRef] [PubMed]
- Sarma, R.; Adhikari, K.; Mahanta, S.; Khanikor, B. Combinations of plant essential oil based terpene compounds as larvicidal and adulticidal agent against Aedes aegypti (Diptera: Culicidae). Sci. Rep. 2019, 9, 9471. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Ochoa, S.; Correa-Basurto, J.; Rodríguez-Valdez, L.M.; Sánchez-Torres, L.E.; Nogueda-Torres, B.; Nevárez-Moorillón, G.V. In vitro and in silico studies of terpenes, terpenoids and related compounds with larvicidal and pupaecidal activity against Culex quinquefasciatus Say (Diptera: Culicidae). Chem. Cent. J. 2018, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Castillo, A.U.; Rodríguez-Valdez, L.M.; Correa-Basurto, J.; Nogueda-Torres, B.; Andrade-Ochoa, S.; Nevárez-Moorillón, G.V. Terpenic constituents of essential oils with larvicidal activity against Aedes aegypti: A QSAR and docking molecular study. Molecules 2023, 28, 2454. [Google Scholar] [CrossRef] [PubMed]
- Reegan, A.D.; Kumar, P.S.; Asharaja, A.C.; Devi, C.; Jameela, S.; Balakrishna, K.; Ignacimuthu, S. Larvicidal and ovicidal activities of phenyl acetic acid isolated from Streptomyces collinus against Culex quinquefasciatus Say and Aedes aegypti L. (Diptera: Culicidae). Exp. Parasitol. 2021, 226–227, 108120. [Google Scholar] [CrossRef] [PubMed]
- Waliwitiya, R.; Kennedy, C.J.; Lowenberger, C.A. Larvicidal and oviposition-altering activity of monoterpenoids, trans-anethole and rosemary oil to the yellow fever mosquito Aedes aegypti (Diptera: Culicidae). Pest Manag. Sci. 2009, 65, 241–248. [Google Scholar] [CrossRef] [PubMed]
- N’goka, V.; Enoua, G.C.; Kende, G.; Pouambeka, T.W.; Etsatsala, N.G.E.R. Larvicidal and ovicidal activities of some cinnamaldehyde derivatives against Anopheles gambiae, malaria vector agent. Am. J. Chem. 2023, 13, 1–10. [Google Scholar]
- Dhinakaran, S.R.; Mathew, N.; Munusamy, S. Synergistic terpene combinations as larvicides against the dengue vector Aedes aegypti Linn. Drug Dev. Res. 2019, 80, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, G.M.; Annies, V.; de Oliveira, C.F.; Lara, R.A.; Gabriel, M.M.; Betim, F.C.M.; Nadal, J.M.; Farago, P.V.; Dias, J.F.G.; Miguel, O.G.; et al. Evaluation of larvicidal activity and ecotoxicity of linalool, methyl cinnamate and methyl cinnamate/linalool in combination against Aedes aegypti. Ecotoxicol. Environ. Saf. 2017, 139, 238–244. [Google Scholar] [CrossRef]
- Nwanade, C.F.; Wang, M.; Li, H.; Masoudi, A.; Yu, Z.; Liu, J. Individual and synergistic toxicity of cinnamon essential oil constituents against Haemaphysalis longicornis (Acari: Ixodidae) and their potential effects on non-target organisms. Ind. Crops Prod. 2022, 178, 114614. [Google Scholar] [CrossRef]
- de Oliveira, A.C.; Simões, R.C.; Cláudia, P.S.; Tavares, C.P.S.; Costa Sá, I.S.; da Silva, F.M.A.; Figueira, E.A.G.; Nunomura, S.M.; Nunomura, R.C.S.; Roque, R.A. Toxicity of the essential oil from Tetradenia riparia (Hochstetter.) Codd (Lamiaceae) and its principal constituent against malaria and dengue vectors and non-target animals. Pestic. Biochem. Physiol. 2022, 188, 105265. [Google Scholar] [CrossRef] [PubMed]
- Pino-Otín, M.R.; Langa, E.; Val, J.; Mainar, A.M.; Ballestero, D. Impact of citronellol on river and soil environments using non-target model organisms and natural populations. J. Environ. Manag. 2021, 287, 112303. [Google Scholar] [CrossRef] [PubMed]
- Moungthipmalai, T.; Puwanard, C.; Aungtikun, J.; Sittichok, S.; Soonwera, M. Ovicidal toxicity of plant essential oils and their major constituents against two mosquito vectors and their non-target aquatic predators. Sci. Rep. 2023, 13, 2119. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Pavela, R.; Giordani, C.; Casettari, L.; Curzi, G.; Cappellacci, L.; Petrelli, R.; Maggi, F. Acute and sub-lethal toxicity of eight essential oils of commercial interest against the filariasis mosquito Culex quinquefasciatus and the housefly Musca domestica. Ind. Crops Prod. 2018, 112, 668–680. [Google Scholar] [CrossRef]
- Marinov, V.; Valcheva-Kuzmanova, S. Review on the pharmacological activities of anethole. Scr. Sci. Pharm. 2015, 2, 14–19. [Google Scholar] [CrossRef]
- Bhowal, M.; Gopal, M. Eucalyptol: Safety and pharmacological profile. RGUHS J. Pharm. Sci. 2015, 5, 125–131. [Google Scholar] [CrossRef]
- Hacke, A.C.M.; da Silva, F.D.A.; Lima, D.; Vellosa, J.C.R.; Rocha, J.B.T.; Marques, J.A.; Pereira, R.P. Cytotoxicity of Cymbopogon citratus (DC) Stapf fractions, essential oil, citral, and geraniol in human leukocytes and erythrocytes. J. Ethnopharmacol. 2022, 291, 115147. [Google Scholar] [CrossRef]
- Baker, B.P.; Grant, J.A. Cinnamon & Cinnamon Oil Profile Active Ingredient Eligible for Minimum Risk Pesticide Use. Available online: https://ecommons.cornell.edu/server/api/core/bitstreams/700a4eca-b375-4ee8-ba8f-989813fe0fa2/content (accessed on 12 January 2025).
- Pavela, R. Acute toxicity and synergistic and antagonistic effects of the aromatic compounds of some essential oils against Culex quinquefasciatus Say larvae. Parasitol. Res. 2015, 114, 3835–3853. [Google Scholar] [CrossRef]
- Saddiq, A.A.; Khayyat, S.A. Chemical and antimicrobial studies of monoterpene: Citral. Pestic. Biochem. Physiol. 2010, 98, 89–93. [Google Scholar] [CrossRef]
- Muhoza, B.; Qi, B.; Harindintwali, J.D.; Koko, M.Y.F.; Zhang, S.; Li, Y. Encapsulation of cinnamaldehyde: An insight on delivery systems and food applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 2521–2543. [Google Scholar] [CrossRef] [PubMed]
- Suwannapong, G.; Maksong, S.; Seanbualuang, P.; Benbow, M.E. Experimental infection of red dwarf honeybee, Apis florea, with Nosema ceranae. J. Asia. Pac. Entomol. 2010, 13, 361–364. [Google Scholar] [CrossRef]
- WHO. Guidelines for Laboratory and Field Testing of Mosquito Larvicides. 2005. Available online: https://www.who.int/publications/i/item/WHO-CDS-WHOPES-GCDPP-2005.13 (accessed on 1 May 2025).
- WHO. Temephos in Drinking-Water: Use for Vector Control in Drinking-Water Sources and Containers. 2009. Available online: https://www.who.int/docs/default-source/wash-documents/wash-chemicals/temephos-background-document.pdf?sfvrsn=c34fda71_4 (accessed on 1 May 2025).
- Selvi, M.; Sarikaya, R.; Erkoç, F.; Koçak, O. Investigation of acute toxicity of chlorpyrifos-methyl on guppy Poecilia reticulata. Chemospere 2005, 60, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.W.; Park, R.M.; Bailer, A.J. Comparing median lethal concentration values using confidence interval overlap or ratio tests. Environ. Toxicol. Chem. 2006, 25, 1441–1444. [Google Scholar] [CrossRef] [PubMed]
- Candy, S.G. The application of generalized linear mixed models to multi-level sampling for insect population monitoring. Environ. Ecol. Stat. 2000, 7, 217–238. [Google Scholar] [CrossRef]
- Gan, S.J.; Leong, Y.Q.; bin Barhanuddin, M.F.H.; Wong, S.T.; Wong, S.F.; Mak, J.W.; Ahmad, R.B. Dengue fever and insecticide resistance in Aedes mosquitoes in Southeast Asia: A review. Parasites. Vectors 2021, 14, 315. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.R.; Azit, N.A.; Fadzil, S.M.; Abd Gbani, S.R.; Abmad, N.; Nawi, A.M. Insecticidal resistance of dengue vectors in Southeast Asia: A system review. Afr. Health Sci. 2021, 21, 1124–1140. [Google Scholar] [CrossRef] [PubMed]
- Gevao, B.; Semple, K.T.; Jones, K.C. Bound pesticide residues in soils: A review. Environ. Pollut. 2000, 108, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Gai, L.; Harkes, P.; Tan, G.; Ritsema, C.J.; Alcon, F.; Contreras, J.; Abrantes, N.; Campos, I.; Baldi, I.; et al. Pesticide residues with hazard classifications relevant to non-target species including human are omnipresent in the environment and farmer residences. Environ. Inter. 2023, 181, 108280. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B. Botanical insecticides in the twenty-first century-fulfilling their promise? Annu. Rev. Entomol. 2020, 65, 233–249. [Google Scholar] [CrossRef] [PubMed]
- Demirak, M.S.S.; Canpolat, E. Plant-based bioinsecticides for mosquito control: Impact on insecticide resistance and disease transmission. Insects 2022, 13, 162. [Google Scholar] [CrossRef] [PubMed]
- Soonwera, M.; Sittichok, S. Adulticidal activities of Cymbopogon citratus (Stap f.) and Eucalyptus globulus (Labill.) essential oils and their synergistic combination against Aedes aegypti (L.), Aedes albopictus (Skuse), and Musca domestica (L.). Environ. Sci. Pollut. Res. 2020, 27, 20201–20214. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R. Acute and synergistic effects of some monoterpenoid essential oil compounds on the housefly (Musca domestica L.). J. Essent. Oil-Bear. Plants 2008, 11, 451–459. [Google Scholar] [CrossRef]
- Das, N.G.; Dhiman, S.; Talukdar, P.K.; Rabha, B.; Goswami, D.; Veer, V. Synergistic mosquito-repellent activity of Curcuma longa, Pogostemon heyneanus and Zanthoxylum limonella essential oils. J. Infect. Public Health. 2015, 8, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Ramzi, A.; Lalami, A.E.O.; Annemer, S.; Ezzoubi, Y.; Assouguem, A.; Almutairi, M.H.; Peluso, I.; Ercisli, S.; Farah, A. Synergistic effect of bioactive monoterepenes against the mosquito, Culex pipiens (Diptera: Culicidae). Molecules 2022, 27, 4182. [Google Scholar] [CrossRef]
- Soonwera, M.; Moungthipmalai, T.; Puwanard, C.; Sittichok, S.; Sinthusiri, J.; Passara, H. Adulticidal synergy of two plant essential oils and their major constituents against the housefly Musca domestica and bioassay on non-target species. Heliyon 2024, 10, e26910. [Google Scholar] [CrossRef] [PubMed]
- Scalerandi, E.; Flores, G.A.; Palacio, M.; Defago, M.T.; Carpinella, M.C.; Valladares, G.; Bertoni, A.; Palacios, S.M. Uderstranding synergistic toxicity of terpenene as insecticides: Contribution of metabolic detoxification in Musca domestica. Front. Plant Sci. 2018, 9, 1579. [Google Scholar] [CrossRef] [PubMed]
- Moungthipmalai, T.; Soonwera, M. Adulticidal activity against housefly (Musca domestica L.: Muscidae: Diptera) of combinations of Cymbopogon citratus and Eucalyptus globulus essential oils and their major constituents. Int. J. Agric. Technol. 2023, 19, 1127–1134. [Google Scholar]
- Sittichok, S.; Passara, H.; Sinthusiri, J.; Soonwera, M.; Thongsaiklaing, T.; Morris, J.; Moungthipmalai, T.; Puwanard, C.; Jintanasirinurak, S. Plant essential oils, trans-anethole and eugenol, for housefly knock down and mortality. Int. J. Agric. Technol. 2025, 21, 555–562. [Google Scholar] [CrossRef]
- WHO. Temephos in: Pesticide Residues in Food 2006. Joint FAO/WHO Meeting on Pesticide Residues. Available online: https://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/JMPR/JMPRrepor2006.pdf (accessed on 12 April 2025).
- Valle, D.; Bellinato, D.F.; Viana-Medeiros, P.F.; Lima, J.B.P.; Junior, A.J.M. Resistance to temephos and deltamethrin in Aedes aegypti from Brazil between 1985 and 2017. Mem. Inst. Oswaldo Cruz 2019, 114, e180544. [Google Scholar] [CrossRef] [PubMed]
- Braga, I.A.; Lima, J.B.P.; Soares, S.S.; Valle, D. Aedes aegypti resistance to temephos during 2001 in several municipalities in the states of Rio de Janero, Sergipe, and Alagoas, Brazil. Mem. Inst. Oswaldo Cruz 2004, 99, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Davila-Barboza, J.A.; Gutierrez-Rodriguez, S.M.; Juache-Villagrana, A.E.; Lopez-Monroy, B.; Flores, A.E. Widespread resistance to temephos in Aedes aegypti (Diptera: Culicidae) from Mexico. Insects 2024, 15, 120. [Google Scholar] [CrossRef] [PubMed]
- Soonwera, M.; Phasomkusolsil, S. Effect of Cymbopogon citratus (lemongrass) and Syzygium aromaticum (clove) oils on the morphology and mortality of Aedes aegypti and Anopheles dirus larvae. Parasitol. Res. 2016, 115, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Oliveria, F.M.; Wanderley-Texixeria, V.; Cruz, G.S.; Silva, C.T.S.; Dutra, K.A.; Costa, H.N.; Braga, V.A.A.; Silva, E.J.; Guedes, C.A.; Alves, T.J.S.; et al. Histological, histochemical and energy disorders caused by R-limonene on Aedes aegypti larvae (Diptera: Culicidae). Acta Trop. 2021, 221, 105987. [Google Scholar] [CrossRef] [PubMed]
- Regnault-Roger, C.; Vincent, C.; Arnason, J.T. Essential oils in insect control: Low-risk products in a high-strakes world. Annu. Rev. Entomol. 2012, 57, 405–424. [Google Scholar] [CrossRef] [PubMed]
- El-Wakeil, N.E. RETRACTED ARTICLE: Botanical pesticides and their mode of action. Gessunde Pflanz. 2013, 65, 125–149. [Google Scholar] [CrossRef]
- Diaz-Resendiz, K.J.G.; Hermosillo-Escobedo, A.T.; Ventura-Rsmon, G.H.; Toledo-Ibarra, G.A.; Giron-Perez, D.A.; Bueno-Dura, A.Y.; Giron-Perez, M. Death of guppy fish (Poecilia reticulate) leukocytes induced by in vivo exposure to temephos and spinosad. Inter. J. Environ. Health Res. 2020, 32, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.B.; de Campos Junior, E.O. Enzymatic alterrations and genotoxic effects produced by sublethal concentrations of organophosphorous temephos in Poecilia reticulate. J. Toxicol. Environ. Health Part A 2015, 78, 1033–1037. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Chaparro, A.; Verdin-Betancourt, F.A.; Sierra-Santoyo, A. Human biotransformation pathway of temephos using an in silico approach. Chem. Res. Toxicol. 2020, 33, 2765–2774. [Google Scholar] [CrossRef] [PubMed]
- Satriawa, D.; Sindjaja, W.; Richardo, T. Toxicity of the organophosphorus pesticide temephos. Indones. J. Life Sci. 2019, 1, 62–76. Available online: https://journal.i3l.ac.id/index.php/IJLS/article/view/26 (accessed on 1 May 2025). [CrossRef]
- Benitez-Trinidad, A.B.; Herrera-Moreno, J.F.; Vazquez-Estrada, G.; Verdin-Betancourt, F.A.; Sordo, M.; Ostrosky-Wegman, P.; Bernal-Hernandez, Y.Y.; Medina-Diaz, I.M.; Barron-Vivanco, B.S.; Robledo-Mareno, M.L.; et al. Cytostatic and genotoxic effect of temephos in human lymphocytes and HepG2 cells. Toxicol. Vitro 2015, 29, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Khodadadian, R.; Dehkordi, S.B. A comprehensive review of the neurological effect of anethole. IBRO Neurosci. Rep. 2025, 18, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.-M.; Peng, J.-Q.; Chen, Y.; Tao, L.; Zhang, Y.-Y.; Fu, L.-Y.; Long, Q.-D.; Shen, X.-C. 1,8-cineole: A review of source, biological activity, and application. J. Asian Nat. Products. Res. 2020, 23, 938–954. [Google Scholar] [CrossRef] [PubMed]
- Solon, I.G.; Santos, W.S.; Branco, L.G.S. Citral as an anti-inflammatory agents: Mechanisms, therapeutic potential and perspectives. Pharmacol. Res. Nat. Prod. 2025, 7, 100253. [Google Scholar] [CrossRef]
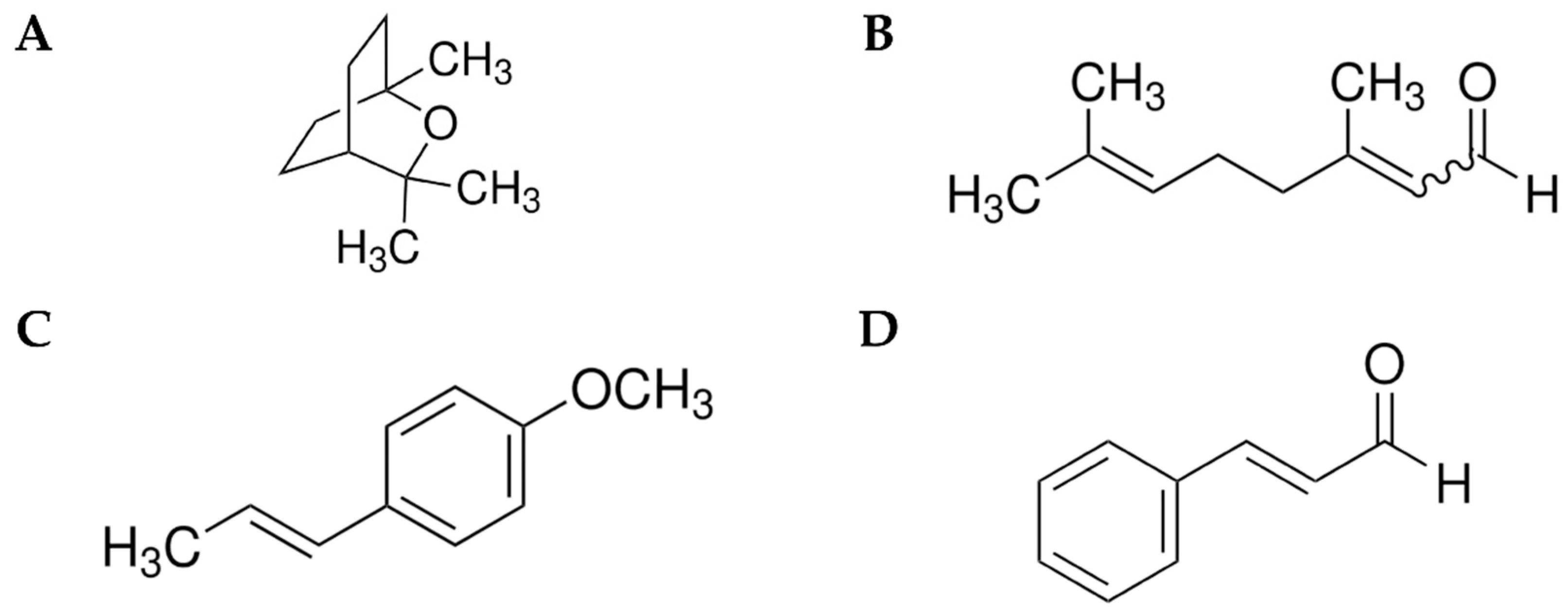

| Pure and Mixed Forms. | Activity Against | Efficiency | Ref. |
|---|---|---|---|
| Eucalyptol | Ae. aegypti larvae | LC50 = 104.5 µg/mL | [21] |
| Culex quinquefasciatus larvae and pupae | LC50 = 44.4 and 92.9 µg/mL | [22] | |
| Geranial | Cx. quinquefasciatus larvae and pupae | LC50 = 53.4 and 193.9 µg/mL | [22] |
| Limonene | Cx. quinquefasciatus larvae and pupae | LC50 = 27.3 and 98.4 µg/mL | [22] |
| Carvacrol | Ae. aegypti larvae | LC50 = 8.8 µg/mL | [23] |
| Phenyl acetic acid | Ae. aegypti larvae | LC50 = 3.81 ppm | [24] |
| Trans-anethole | Ae. aegypti larvae | LC50 = 88.5 mg L−1 | [25] |
| Ae. aegypti larvae and pupae | LC50 = 2.5% and 3.3% | [16] | |
| Trans-cinnamaldehyde | Anopheles gambiae larvae | LC50 = 0.06 g/L | [26] |
| Geranial + trans-cinnamaldehyde (1:1) | Ae. aegypti larvae and pupae | LT50 = 0.2 h | [17] |
| Trans-anethole + γ-terpinene (1:1) | Ae. aegypti larvae | LC50 = 12.4 ppm | [27] |
| Methyl cinnamate + linalool (1:1) | Ae. aegypti larvae | LC50 = 57.7 µg/mL | [28] |
| Treatment | Lethal Concentration(50% or 90%) | Estimated Concentration (µg/mL) | CI95 (µg/mL) | Slope ± SE | ICV |
|---|---|---|---|---|---|
| eucalyptol | LC50 | 382 | 353–416 | 0.011 ± 0.002 | |
| LC90 | 496 | 452–584 | |||
| geranial | LC50 | 672 | 502–1592 | 0.003 ± 0.002 | |
| LC90 | 1047 | 729–2870 | |||
| trans-anethole | LC50 | 299 | 271–328 | 0.021 ± 0.003 | |
| LC90 | 362 | 332–401 | |||
| trans-cinnamaldehyde | LC50 | 267 | 239–295 | 0.012 ± 0.002 | |
| LC90 | 376 | 341–429 | |||
| eucalyptol + trans-cinnamaldehyde | LC50 | 189 | - | 0.023 ± 0.006 | 0.3 |
| LC90 | 244 | - | |||
| eucalyptol + geranial | LC50 | 219 | - | 0.022 ± 0.005 | 0.2 |
| LC90 | 277 | - | |||
| eucalyptol + trans-anethole | LC50 | 176 | - | 0.025 ± 0.005 | 0.3 |
| LC90 | 228 | - | |||
| trans-anethole + geranial | LC50 | 183 | - | 0.024 ± 0.003 | 0.2 |
| LC90 | 236 | - |
| Treatment | Lethal Concentration(50% or 90%) | Estimated Concentration (µg/mL) | CI95 (µg/mL) | Slope ± SE | ICV |
|---|---|---|---|---|---|
| eucalyptol | LC50 | 770 | 539–5066 | 0.003 ± 0.000 | |
| LC90 | 1151 | 746–9035 | |||
| geranial | LC50 | 445 | 393–552 | 0.007 ± 0.002 | |
| LC90 | 642 | 540–898 | |||
| trans-anethole | LC50 | 761 | - | 0.004 ± 0.002 | |
| LC90 | 1095 | - | |||
| trans-cinnamaldehyde | LC50 | 470 | 406–617 | 0.006 ± 0.001 | |
| LC90 | 702 | 574–1042 | |||
| eucalyptol + trans-cinnamaldehyde | LC50 | 364 | 327–413 | 0.007 ± 0.001 | 0.3 |
| LC90 | 535 | 471–656 | |||
| eucalyptol + geranial | LC50 | 518 | 443–815 | 0.007 ± 0.002 | 0.4 |
| LC90 | 714 | 570–1356 | |||
| eucalyptol + trans-anethole | LC50 | 252 | 224–744 | 0.023 ± 0.011 | 0.2 |
| LC90 | 308 | 256–1355 | |||
| trans-anethole + geranial | LC50 | 167 | - | 0.026 ± 0.011 | 0.1 |
| LC90 | 217 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sittichok, S.; Passara, H.; Moungthipmalai, T.; Sinthusiri, J.; Murata, K.; Soonwera, M. Synergistic Larvicidal and Pupicidal Effects of Monoterpene Mixtures Against Aedes aegypti with Low Toxicity to Guppies and Honeybees. Insects 2025, 16, 738. https://doi.org/10.3390/insects16070738
Sittichok S, Passara H, Moungthipmalai T, Sinthusiri J, Murata K, Soonwera M. Synergistic Larvicidal and Pupicidal Effects of Monoterpene Mixtures Against Aedes aegypti with Low Toxicity to Guppies and Honeybees. Insects. 2025; 16(7):738. https://doi.org/10.3390/insects16070738
Chicago/Turabian StyleSittichok, Sirawut, Hataichanok Passara, Tanapoom Moungthipmalai, Jirisuda Sinthusiri, Kouhei Murata, and Mayura Soonwera. 2025. "Synergistic Larvicidal and Pupicidal Effects of Monoterpene Mixtures Against Aedes aegypti with Low Toxicity to Guppies and Honeybees" Insects 16, no. 7: 738. https://doi.org/10.3390/insects16070738
APA StyleSittichok, S., Passara, H., Moungthipmalai, T., Sinthusiri, J., Murata, K., & Soonwera, M. (2025). Synergistic Larvicidal and Pupicidal Effects of Monoterpene Mixtures Against Aedes aegypti with Low Toxicity to Guppies and Honeybees. Insects, 16(7), 738. https://doi.org/10.3390/insects16070738







