Fine-Scale Risk Mapping for Dengue Vector Using Spatial Downscaling in Intra-Urban Areas of Guangzhou, China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
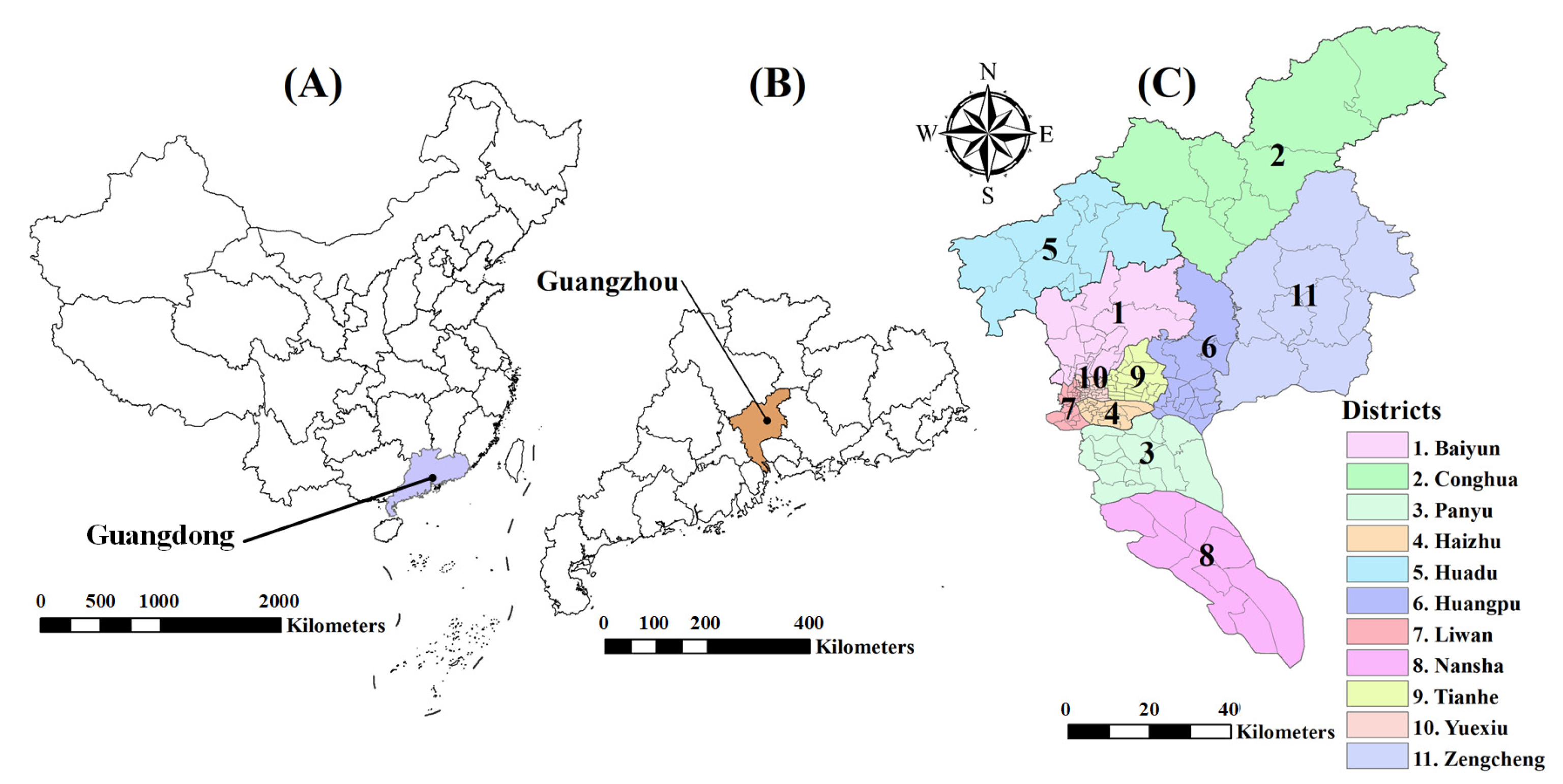
2.1. Study Area
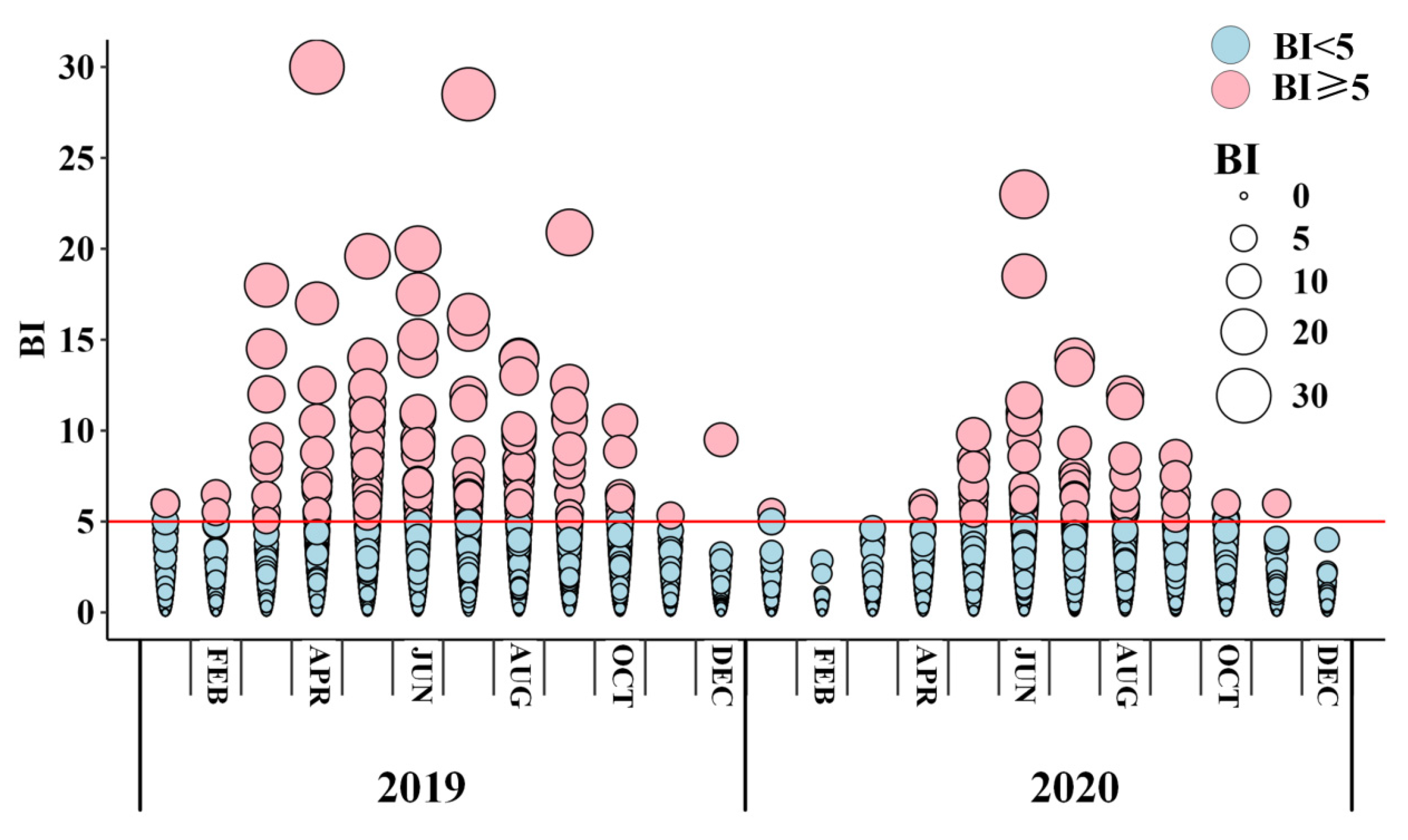
2.2. Entomological Data
2.3. Environmental and Socio-Economic Data
2.3.1. Meteorological Data
2.3.2. Vegetation Data
2.3.3. Land Use Data
2.3.4. Population Density Data
2.4. Methods for Data Analysis
2.4.1. Variables Selection
2.4.2. Data Imbalance and Data Resampling
2.4.3. Random Forest Model and Spatial Downscaling
2.4.4. Model Evaluation
3. Results
3.1. Comparing Model Performance
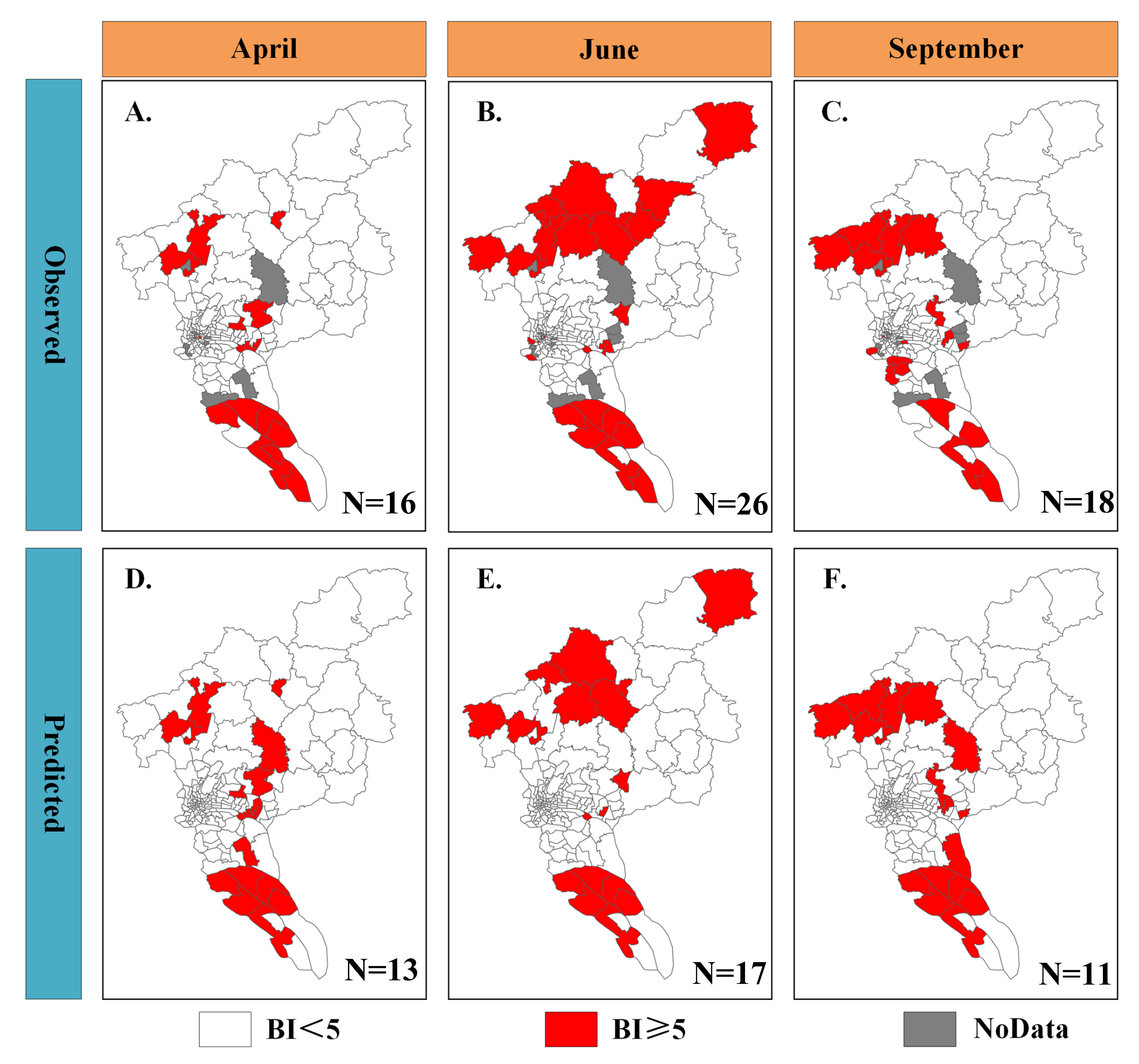
3.2. Risk Mapping at Township Scale
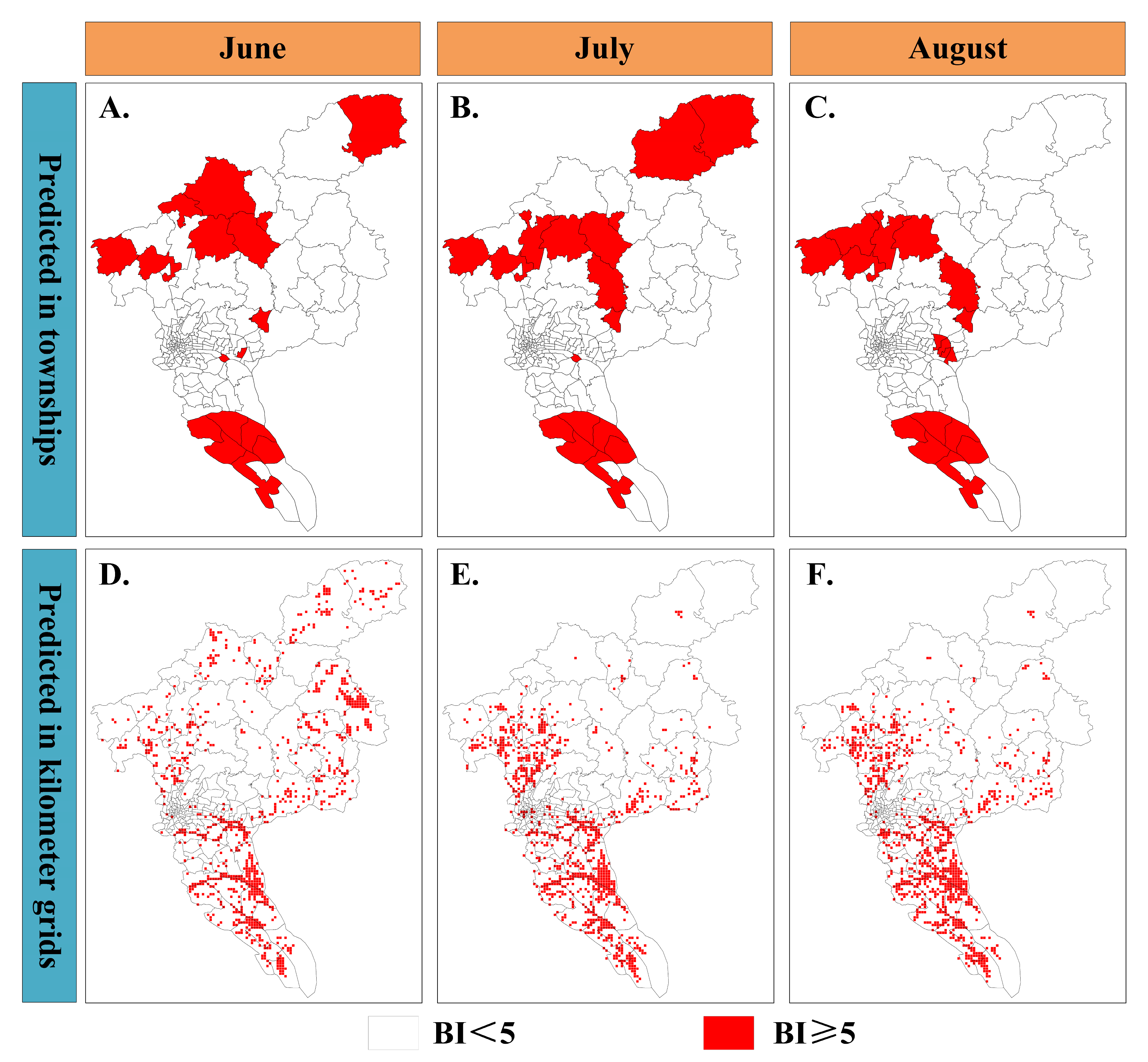
3.3. Risk Mapping at Township and Kilometer Grids Scale
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baldacchino, F.; Marcantonio, M.; Manica, M.; Marini, G.; Zorer, R.; Delucchi, L.; Arnoldi, D.; Montarsi, F.; Capelli, G.; Rizzoli, A.; et al. Mapping of Aedes albopictus Abundance at a Local Scale in Italy. Remote Sens. 2017, 9, 749. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, W.; Lv, C. Modern technologies and solutions to enhance surveillance and response systems for emerging zoonotic diseases. Sci. One Health 2024, 3, 100061. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Hou, X.; Ren, Z.; Lowe, R.; Wang, Y.; Li, R.; Liu, X.; Sun, J.; Lu, L.; Song, X. Climate factors and the East Asian summer monsoon may drive large outbreaks of dengue in China. Environ. Res. 2020, 183, 109190. [Google Scholar] [CrossRef]
- Chen, J.; Ding, R.L.; Liu, K.K.; Xiao, H.; Hu, G.; Xiao, X.; Yue, Q.; Lu, J.H.; Han, Y.; Bu, J.; et al. Collaboration between meteorology and public health: Predicting the dengue epidemic in Guangzhou, China, by meteorological parameters. Front. Cell Infect. Microbiol. 2022, 12, 881745. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Xiao, J.; Liu, T.; Zhang, B.; Hao, Y.; Ma, W. Spatiotemporal analysis of the dengue outbreak in Guangdong Province, China. BMC Infect. Dis. 2019, 19, 493. [Google Scholar] [CrossRef]
- Luo, L.; Li, X.; Xiao, X.; Xu, Y.; Huang, M.; Yang, Z. Identification of Aedes albopictus larval index thresholds in the transmission of dengue in Guangzhou, China. J. Vector Ecol. 2015, 40, 240–246. [Google Scholar] [CrossRef]
- Wang, T.; Fan, Z.-W.; Ji, Y.; Chen, J.-J.; Zhao, G.-P.; Zhang, W.-H.; Zhang, H.-Y.; Jiang, B.-G.; Xu, Q.; Lv, C.-L.; et al. Mapping the Distributions of Mosquitoes and Mosquito-Borne Arboviruses in China. Viruses 2022, 14, 691. [Google Scholar] [CrossRef]
- Mwangangi, J.M.; Mbogo, C.M.; Orindi, B.O.; Muturi, E.J.; Midega, J.T.; Nzovu, J.; Gatakaa, H.; Githure, J.; Borgemeister, C.; Keating, J. Shifts in malaria vector species composition and transmission dynamics along the Kenyan coast over the past 20 years. Malar. J. 2013, 12, 13. [Google Scholar] [CrossRef]
- Youssefi, F.; Zoej, M.J.V.; Hanafi-Bojd, A.A.; Dariane, A.B.; Khaki, M.; Safdarinezhad, A.; Ghaderpour, E. Temporal Monitoring and Predicting of the Abundance of Malaria Vectors Using Time Series Analysis of Remote Sensing Data through Google Earth Engine. Sensors 2022, 22, 1942. [Google Scholar] [CrossRef]
- Wu, S.; Ren, H.; Chen, W.; Li, T. Neglected Urban Villages in Current Vector Surveillance System: Evidences in Guangzhou, China. Int. J. Environ. Res. Public Health 2019, 17, 2. [Google Scholar] [CrossRef]
- Parra, M.C.P.; Lorenz, C.; Dibo, M.R.; de Aguiar Milhim, B.H.G.; Guirado, M.M.; Nogueira, M.L.; Chiaravalloti-Neto, F. Association between densities of adult and immature stages of Aedes aegypti mosquitoes in space and time: Implications for vector surveillance. Parasit. Vectors 2022, 15, 133. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.; Aidil, R.; Nisfariza, M.; Ngui, R.; Lim, Y.; Yusoff, W.; Ruslan, R. Spatial density of Aedes distribution in urban areas: A case study of breteau index in Kuala Lumpur, Malaysia. J. Vector Borne Dis. 2014, 51, 91–96. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Q. Aedes Surveillance and Risk Warnings for Dengue—China, 2016–2019. China CDC Wkly. 2020, 2, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Adde, A.; Roux, E.; Mangeas, M.; Dessay, N.; Nacher, M.; Dusfour, I.; Girod, R.; Briolant, S. Dynamical Mapping of Anopheles darlingi Densities in a Residual Malaria Transmission Area of French Guiana by Using Remote Sensing and Meteorological Data. PLoS ONE 2016, 11, e0164685. [Google Scholar] [CrossRef] [PubMed]
- Scavuzzo, J.M.; Trucco, F.; Espinosa, M.; Tauro, C.B.; Abril, M.; Scavuzzo, C.M.; Frery, A.C. Modeling Dengue vector population using remotely sensed data and machine learning. Acta Trop. 2018, 185, 167–175. [Google Scholar] [CrossRef]
- Machault, V.; Vignolles, C.; Pages, F.; Gadiaga, L.; Tourre, Y.M.; Gaye, A.; Sokhna, C.; Trape, J.F.; Lacaux, J.P.; Rogier, C. Risk mapping of Anopheles gambiae s.l. densities using remotely-sensed environmental and meteorological data in an urban area: Dakar, Senegal. PLoS ONE 2012, 7, e50674. [Google Scholar] [CrossRef]
- Yin, S.; Ren, C.; Shi, Y.; Hua, J.; Yuan, H.-Y.; Tian, L.-W. A systematic review on modeling methods and influential factors for mapping dengue-related risk in urban settings. Int. J. Environ. Res. Public Health 2022, 19, 15265. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Pientong, C.; Zafar, S.; Ekalaksananan, T.; Paul, R.E.; Haque, U.; Rocklöv, J.; Overgaard, H.J. Mapping the spatial distribution of the dengue vector Aedes aegypti and predicting its abundance in northeastern Thailand using machine-learning approach. One Health 2021, 13, 100358. [Google Scholar] [CrossRef]
- Iyaloo, D.P.; Degenne, P.; Elahee, K.B.; Lo Seen, D.; Bheecarry, A.; Tran, A. ALBOMAURICE: A predictive model for mapping Aedes albopictus mosquito populations in Mauritius. SoftwareX 2021, 13, 100638. [Google Scholar] [CrossRef]
- Udayanga, L.; Gunathilaka, N.; Iqbal, M.C.M.; Abeyewickreme, W. Climate change induced vulnerability and adaption for dengue incidence in Colombo and Kandy districts: The detailed investigation in Sri Lanka. Infect. Dis. Poverty 2020, 9, 102. [Google Scholar] [CrossRef]
- Tran, A.; Ponçon, N.; Toty, C.; Linard, C.; Guis, H.; Ferré, J.-B.; Lo Seen, D.; Roger, F.; de la Rocque, S.; Fontenille, D.; et al. Using remote sensing to map larval and adult populations of Anopheles hyrcanus (Diptera: Culicidae) a potential malaria vector in Southern France. Int. J. Health Geogr. 2008, 7, 9. [Google Scholar] [CrossRef]
- Uusitalo, R.; Siljander, M.; Culverwell, C.L.; Mutai, N.C.; Forbes, K.M.; Vapalahti, O.; Pellikka, P.K.E. Predictive mapping of mosquito distribution based on environmental and anthropogenic factors in Taita Hills, Kenya. Int. J. Appl. Earth Obs. Geoinf. 2019, 76, 84–92. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, H.; Leng, P.; Zhu, J.; Yao, S.; Zhu, Y.; Wu, H. Analysis of the spatial distribution of Aedes albopictus in an urban area of Shanghai, China. Parasit. Vectors 2021, 14, 501. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Fu, J.; Jiang, D.; Hao, M.; Lin, G. Mapping the spatial distribution of Aedes aegypti and Aedes albopictus. Acta Trop. 2018, 178, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Altamiranda-Saavedra, M.; Porcasi, X.; Scavuzzo, C.M.; Correa, M.M. Downscaling incidence risk mapping for a Colombian malaria endemic region. Trop. Med. Int. Health 2018, 23, 1101–1109. [Google Scholar] [CrossRef]
- Daliakopoulos, I.N.; Katsanevakis, S.; Moustakas, A. Spatial Downscaling of Alien Species Presences Using Machine Learning. Front. Earth Sci. 2017, 5, 60. [Google Scholar] [CrossRef]
- Venkateshwarprasad, K.; Sashirekha, K. Efficient prediction of cyber crime breaches using decision tree compared with Naïve Bayes with improved accuracy. In Proceedings of the AIP Conference Proceedings, Trichy, India, 28–29 March 2024; p. 020065. [Google Scholar]
- Xiong, Y.; Ma, Y.; Ruan, L.; Li, D.; Lu, C.; Huang, L.; National Traditional Chinese Medicine Medical, T. Comparing different machine learning techniques for predicting COVID-19 severity. Infect. Dis. Poverty 2022, 11, 19. [Google Scholar] [CrossRef]
- Kofidou, M.; de Courcy Williams, M.; Nearchou, A.; Veletza, S.; Gemitzi, A.; Karakasiliotis, I. Applying Remotely Sensed Environmental Information to Model Mosquito Populations. Sustainability 2021, 13, 7655. [Google Scholar] [CrossRef]
- Mudele, O.; Bayer, F.M.; Zanandrez, L.F.R.; Eiras, A.E.; Gamba, P. Modeling the Temporal Population Distribution of $Ae.~aegypti$ Mosquito Using Big Earth Observation Data. IEEE Access 2020, 8, 14182–14194. [Google Scholar] [CrossRef]
- Zhang, M.-z.; Ren, Z.-p.; Fan, J.-f.; Xiao, J.-p.; Zhang, Y.-t. Fine-Scale Dengue Transmission Risk Prediction Based on Multi-Source Geographic Data in Guangzhou, China; National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention: Beijing, China, 2023; Volume 34, pp. 654–663. [Google Scholar]
- Sang, S.; Yin, W.; Bi, P.; Zhang, H.; Wang, C.; Liu, X.; Chen, B.; Yang, W.; Liu, Q. Predicting Local Dengue Transmission in Guangzhou, China, through the Influence of Imported Cases, Mosquito Density and Climate Variability. PLoS ONE 2014, 9, e102755. [Google Scholar] [CrossRef]
- Li, Y.; An, Q.; Sun, Z.; Gao, X.; Wang, H. Distribution areas and monthly dynamic distribution changes of three Aedes species in China: Aedes aegypti, Aedes albopictus and Aedes vexans. Parasites Vectors 2023, 16, 297. [Google Scholar] [CrossRef]
- Jing, Y.; Wang, X.; Tang, S.; Wu, J. Data informed analysis of 2014 dengue fever outbreak in Guangzhou: Impact of multiple environmental factors and vector control. J. Theor. Biol. 2017, 416, 161–179. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jia, X.; Wang, Y.; Xu, C. Spatial variation of population, density, and composition of mosquitoes in mainland China. Sci. Data 2025, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-J.; Liang, X.-Y.; He, S.-Y.; Jiang, Y.-M.; Zhou, J.-H.; Li, X.-N.; Chen, Z.-Q.; Yuan, J. Surveillance on dengue vector Aedes albopictus in Guangzhou from 2019 to 2021. Chin. J. Hyg. Insectic. Equip. 2022, 28, 426–430. [Google Scholar] [CrossRef]
- Tsantalidou, A.; Arvanitakis, G.; Georgoulias, A.K.; Akritidis, D.; Zanis, P.; Fornasiero, D.; Wohlgemuth, D.; Kontoes, C. A Data Driven Approach for Analyzing the Effect of Climate Change on Mosquito Abundance in Europe. Remote Sens. 2023, 15, 5649. [Google Scholar] [CrossRef]
- Peng, S.; Ding, Y.; Liu, W.; Li, Z. 1 km monthly temperature and precipitation dataset for China from 1901 to 2017. Earth System Sci. Data 2019, 11, 1931–1946. [Google Scholar] [CrossRef]
- Chen, S.; Whiteman, A.; Li, A.; Rapp, T.; Delmelle, E.; Chen, G.; Brown, C.L.; Robinson, P.; Coffman, M.J.; Janies, D.; et al. An operational machine learning approach to predict mosquito abundance based on socioeconomic and landscape patterns. Landsc. Ecol. 2019, 34, 1295–1311. [Google Scholar] [CrossRef]
- Lin, C.H.; Wen, T.H. Using geographically weighted regression (GWR) to explore spatial varying relationships of immature mosquitoes and human densities with the incidence of dengue. Int. J. Environ. Res. Public Health 2011, 8, 2798–2815. [Google Scholar] [CrossRef]
- Ren, Z.; Wang, D.; Ma, A.; Hwang, J.; Bennett, A.; Sturrock, H.J.; Fan, J.; Zhang, W.; Yang, D.; Feng, X. Predicting malaria vector distribution under climate change scenarios in China: Challenges for malaria elimination. Sci. Rep. 2016, 6, 20604. [Google Scholar] [CrossRef]
- Cheng, J.; Bambrick, H.; Yakob, L.; Devine, G.; Frentiu, F.D.; Williams, G.; Li, Z.; Yang, W.; Hu, W. Extreme weather conditions and dengue outbreak in Guangdong, China: Spatial heterogeneity based on climate variability. Environ. Res. 2021, 196, 110900. [Google Scholar] [CrossRef]
- Akter, R.; Hu, W.; Gatton, M.; Bambrick, H.; Cheng, J.; Tong, S. Climate variability, socio-ecological factors and dengue transmission in tropical Queensland, Australia: A Bayesian spatial analysis. Environ. Res. 2021, 195, 110285. [Google Scholar] [CrossRef]
- Liu, X.; Song, C.; Ren, Z.; Wang, S. Predicting the Geographical Distribution of Malaria-Associated Anopheles dirus in the South-East Asia and Western Pacific Regions Under Climate Change Scenarios. Front. Environ. Sci. 2022, 10, 841966. [Google Scholar] [CrossRef]
- Khalilia, M.; Chakraborty, S.; Popescu, M. Predicting disease risks from highly imbalanced data using random forest. BMC Med. Inform. Decis. Mak. 2011, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, B. Learning from imbalanced data: Open challenges and future directions. Prog. Artif. Intell. 2016, 5, 221–232. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Joo, G.; Song, Y.; Im, H.; Park, J. Clinical Implication of Machine Learning in Predicting the Occurrence of Cardiovascular Disease Using Big Data (Nationwide Cohort Data in Korea). IEEE Access 2020, 8, 157643–157653. [Google Scholar] [CrossRef]
- Liu, S.; Roemer, F.; Ge, Y.; Bedrick, E.J.; Li, Z.-M.; Guermazi, A.; Sharma, L.; Eaton, C.; Hochberg, M.C.; Hunter, D.J. Comparison of evaluation metrics of deep learning for imbalanced imaging data in osteoarthritis studies. Osteoarthr. Cartil. 2023, 31, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.; Chong, D.; Batarseh, F.A. Foundations of data imbalance and solutions for a data democracy. In Data Democracy: At the Nexus of Artificial Intelligence, Software Development, and Knowledge Engineering, 1st ed.; Batarseh, F.A., Yang, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 83–106. [Google Scholar]
- Li, Q.; Ren, H.; Zheng, L.; Cao, W.; Zhang, A.; Zhuang, D.; Lu, L.; Jiang, H. Ecological Niche Modeling Identifies Fine-Scale Areas at High Risk of Dengue Fever in the Pearl River Delta, China. Int. J. Environ. Res. Public Health 2017, 14, 619. [Google Scholar] [CrossRef]
- Estallo, E.L.; Sangermano, F.; Grech, M.; Ludueña-Almeida, F.; Frías-Cespedes, M.; Ainete, M.; Almirón, W.; Livdahl, T. Modelling the distribution of the vector Aedes aegypti in a central Argentine city. Med. Vet. Entomol. 2018, 32, 451–461. [Google Scholar] [CrossRef]
- Baylis, M.; Slater, H.; Michael, E. Predicting the Current and Future Potential Distributions of Lymphatic Filariasis in Africa Using Maximum Entropy Ecological Niche Modelling. PLoS ONE 2012, 7, e32202. [Google Scholar] [CrossRef]
- Marston, C.; Rowland, C.; O’Neil, A.; Irish, S.; Wat’senga, F.; Martín-Gallego, P.; Aplin, P.; Giraudoux, P.; Strode, C. Developing the Role of Earth Observation in Spatio-Temporal Mosquito Modelling to Identify Malaria Hot-Spots. Remote Sens. 2022, 15, 43. [Google Scholar] [CrossRef]
- Machault, V.; Yébakima, A.; Etienne, M.; Vignolles, C.; Palany, P.; Tourre, Y.; Guérécheau, M.; Lacaux, J.-P. Mapping Entomological Dengue Risk Levels in Martinique Using High-Resolution Remote-Sensing Environmental Data. ISPRS Int. J. Geo-Inf. 2014, 3, 1352–1371. [Google Scholar] [CrossRef]
- Kang, Q.; Chen, X.; Li, S.; Zhou, M. A Noise-Filtered Under-Sampling Scheme for Imbalanced Classification. IEEE Trans. Cybern. 2017, 47, 4263–4274. [Google Scholar] [CrossRef] [PubMed]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic minority over-sampling technique. J. Artif. Int. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Galar, M.; Fernandez, A.; Barrenechea, E.; Bustince, H.; Herrera, F. A Review on Ensembles for the Class Imbalance Problem: Bagging-, Boosting-, and Hybrid-Based Approaches. IEEE Trans. Syst. Man Cybern. Part C (Appl. Rev.) 2012, 42, 463–484. [Google Scholar] [CrossRef]
- Bi, Y.; Jiao, X.; Lee, Y.L.; Zhou, T. Inconsistency among evaluation metrics in link prediction. Proc. Natl. Acad. Sci. USA Nexus 2024, 3, pgae498. [Google Scholar] [CrossRef]
- Lin, H.; Liu, T.; Song, T.; Lin, L.; Xiao, J.; Lin, J.; He, J.; Zhong, H.; Hu, W.; Deng, A.; et al. Community Involvement in Dengue Outbreak Control: An Integrated Rigorous Intervention Strategy. PLOS Neglected Trop. Dis. 2016, 10, e0004919. [Google Scholar] [CrossRef]
- Grillet, M.-E.; Barrera, R.; Martínez, J.-E.; Berti, J.; Fortin, M.-J. Disentangling the effect of local and global spatial variation on a mosquito-borne infection in a neotropical heterogeneous environment. Am. J. Trop. Med. Hyg. 2010, 82, 194. [Google Scholar] [CrossRef]
- Chou-Chen, S.W.; Barboza, L.A.; Vásquez, P.; García, Y.E.; Calvo, J.G.; Hidalgo, H.G.; Sanchez, F. Bayesian spatio-temporal model with INLA for dengue fever risk prediction in Costa Rica. Environ. Ecol. Stat. 2023, 30, 687–713. [Google Scholar] [CrossRef]
- Hu, H.Z.; Yan, Z.Q.; Jiang, Y.M.; Li, C.L.; Wu, H.Y.; Mai, W.L.; Hu, S.C.; Long, Z.M. Analysis on the dengue fever and its vector control in Guangzhou city in recent years. Chin. J. Hyg. Insectic. Equip. 2009, 15, 375–378. [Google Scholar]
| Variables | Temporal Resolution | Spatial Resolution | Sources |
|---|---|---|---|
| Mean temperature | Monthly | 1000 m | https://data.tpdc.ac.cn/ (accessed on 24 June 2025). |
| Cumulative rainfall | Monthly | 1000 m | https://data.tpdc.ac.cn/ (accessed on 24 June 2025). |
| Mean NDVI | Monthly | 10 m | https://developers.google.com/earth-engine/datasets/ (accessed on 24 June 2025). |
| SHEI | Year | 2.4 m | http://geoscape.pku.edu.cn/dataproject.html (accessed on 24 June 2025). |
| Population densities | Year | 100 m | https://hub.worldpop.org/ (accessed on 24 June 2025). |
| Models | ROC-AUC | Specificity | Recall | G-Means |
|---|---|---|---|---|
| Model1 | 0.8643 | 0.9892 | 0.2469 | 0.4911 |
| Model2 * | 0.8468 | 0.7682 | 0.7977 | 0.7821 |
| Model3 | 0.8614 | 0.9689 | 0.3903 | 0.6124 |
| Model4 | 0.8609 | 0.9396 | 0.5604 | 0.7244 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.; Ren, Z.; Fan, J.; Xiao, J.; Zhang, Y.; Liu, X. Fine-Scale Risk Mapping for Dengue Vector Using Spatial Downscaling in Intra-Urban Areas of Guangzhou, China. Insects 2025, 16, 661. https://doi.org/10.3390/insects16070661
Shen Y, Ren Z, Fan J, Xiao J, Zhang Y, Liu X. Fine-Scale Risk Mapping for Dengue Vector Using Spatial Downscaling in Intra-Urban Areas of Guangzhou, China. Insects. 2025; 16(7):661. https://doi.org/10.3390/insects16070661
Chicago/Turabian StyleShen, Yunpeng, Zhoupeng Ren, Junfu Fan, Jianpeng Xiao, Yingtao Zhang, and Xiaobo Liu. 2025. "Fine-Scale Risk Mapping for Dengue Vector Using Spatial Downscaling in Intra-Urban Areas of Guangzhou, China" Insects 16, no. 7: 661. https://doi.org/10.3390/insects16070661
APA StyleShen, Y., Ren, Z., Fan, J., Xiao, J., Zhang, Y., & Liu, X. (2025). Fine-Scale Risk Mapping for Dengue Vector Using Spatial Downscaling in Intra-Urban Areas of Guangzhou, China. Insects, 16(7), 661. https://doi.org/10.3390/insects16070661







