RNAi in Pest Control: Critical Factors Affecting dsRNA Efficacy
Simple Summary
Abstract
1. Introduction
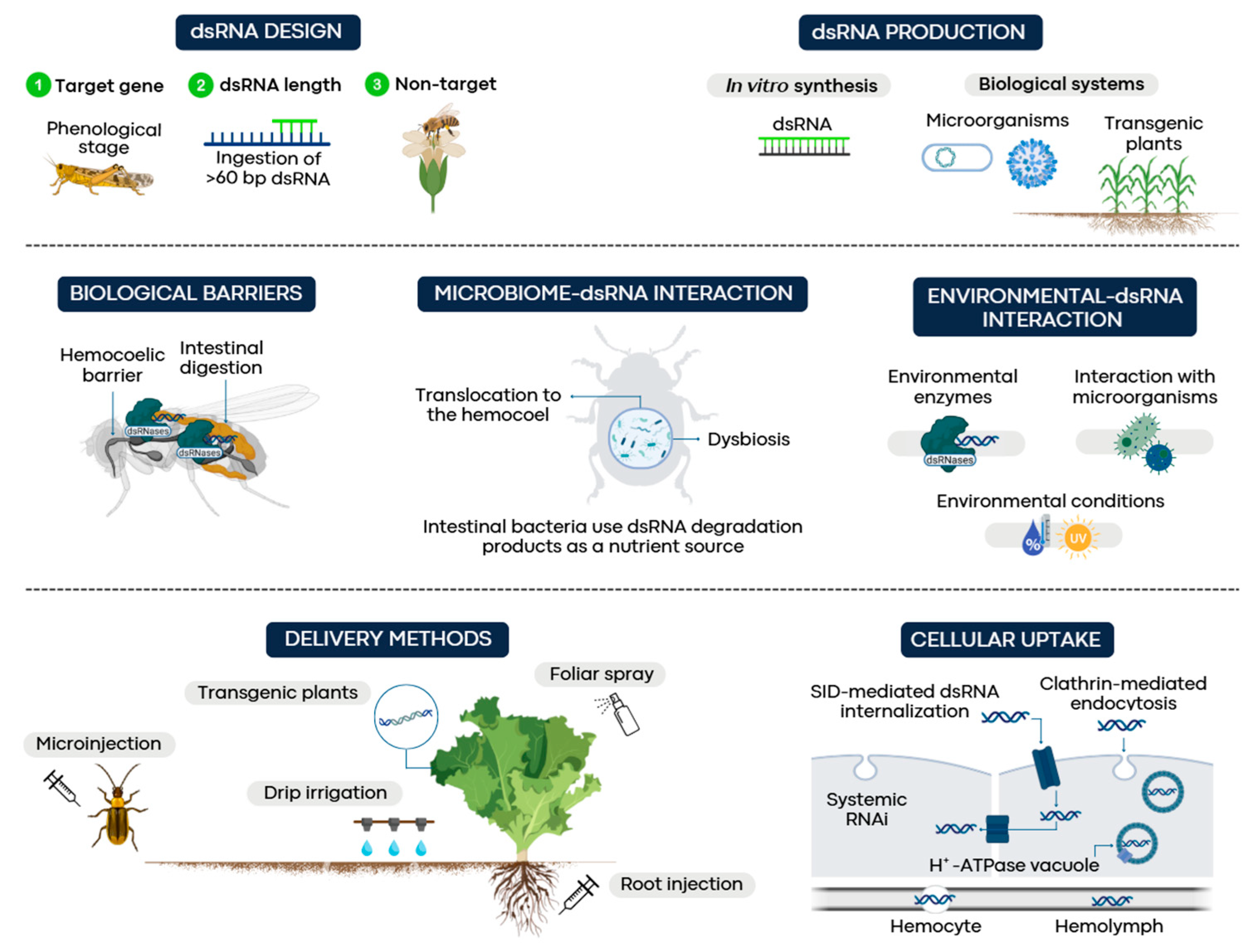
2. Design of Double-Stranded RNA for Gene Silencing
2.1. dsRNA Length
2.2. Target Sequence
2.3. Non-Target Effects
3. dsRNA Production Systems
4. Delivery, Uptake, and dsRNA Transport
4.1. Delivery Methods
4.2. Cellular Uptake
4.3. Intracellular dsRNA Transport and Systemic RNAi
5. Biological Barriers in Insects
5.1. Intestinal Digestion
5.2. Hemocoelic Barrier
5.3. Immune Response
6. Other Factors Affecting RNAi Efficiency
6.1. Environmental Interactions
6.2. Interaction of the Microbiome with dsRNA
6.3. Risks of Resistance Development
7. Challenges and Opportunities
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fróna, D.; Szenderák, J.; Harangi-Rákos, M. The challenge of feeding the world. Sustainability 2019, 11, 5816. [Google Scholar] [CrossRef]
- Junaid, M.; Gokce, A. Global agricultural losses and their causes. Bull. Biol. Allied Sci. Res. 2024, 9, 66. [Google Scholar] [CrossRef]
- Sawicka, B.; Egbuna, C. Pests of agricultural crops and control measures. In Natural Remedies for Pest, Disease and Weed Control; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–16. [Google Scholar]
- Baweja, P.; Kumar, S.; Kumar, G. Fertilizers and pesticides: Their impact on soil health and environment. In Soil Health; Springer: Cham, Switzerland, 2020; pp. 265–285. [Google Scholar]
- Mahmood, I.; Imadi, S.R.; Shazadi, K.; Gul, A.; Hakeem, K.R. Effects of pesticides on environment. In Plant, Soil and Microbes; Springer: Cham, Switzerland, 2016; Volume 1, pp. 253–269. [Google Scholar]
- Asghar, U.; Malik, M.; Javed, A. Pesticide exposure and human health: A review. J. Ecosyst. Ecography 2016, 5, 5. [Google Scholar] [CrossRef]
- Tudi, M.; Li, H.; Li, H.; Wang, L.; Lyu, J.; Yang, L.; Tong, S.; Yu, Q.J.; Ruan, H.D.; Atabila, A. Exposure routes and health risks associated with pesticide application. Toxics 2022, 10, 335. [Google Scholar] [CrossRef] [PubMed]
- Eddleston, M. Poisoning by pesticides. Medicine 2020, 48, 214–217. [Google Scholar] [CrossRef]
- Braak, N.; Neve, R.; Jones, A.K.; Gibbs, M.; Breuker, C.J. The effects of insecticides on butterflies–A review. Environ. Pollut. 2018, 242, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, N.; Chen, K.H.; Huang, J.C.; Lai, H.T.; Uapipatanakul, B.; Roldan, M.J.M.; Macabeo, A.P.G.; Ger, T.R.; Hsiao, C.D. Physiological effects of neonicotinoid insecticides on non-target aquatic animals—An updated review. Int. J. Mol. Sci. 2021, 22, 9591. [Google Scholar] [CrossRef] [PubMed]
- Ricupero, M.; Desneux, N.; Zappalà, L.; Biondi, A. Target and non-target impact of systemic insecticides on a polyphagous aphid pest and its parasitoid. Chemosphere 2020, 247, 125728. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Cohen, Z.P.; Bueno, E.M.; Christensen, B.M.; Schoville, S.D. Rapid evolution of insecticide resistance in the Colorado Potato Beetle, Leptinotarsa decemlineata. Curr. Opin. Insect Sci. 2023, 55, 101000. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Chung, H. New and emerging mechanisms of insecticide resistance. Curr. Opin. Insect Sci. 2024, 63, 101184. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Wang, Z.; Chung, H. Climate change and the genetics of insecticide resistance. Pest Manag. Sci. 2020, 76, 846–852. [Google Scholar] [CrossRef]
- APRD. Arthropod Pesticide Resistance Database (APRD). Available online: https://www.pesticideresistance.org/ (accessed on 2 April 2025).
- Zhang, J.; Khan, S.A.; Hasse, C.; Ruf, S.; Heckel, D.G.; Bock, R. Pest control. Full crop protection from an insect pest by expression of long double-stranded RNAs in plastids. Science 2015, 347, 991–994. [Google Scholar] [CrossRef] [PubMed]
- Askew, W.T.; Edwards, M.G.; Gatehouse, A.M. Ex vivo delivery of dsRNA targeting ryanodine receptors for control of Tuta absoluta. Pest Manag. Sci. 2024, 80, 6400–6408. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.Y.; Yang, L.; Peng, Y.Y.; Chang, T.Y.; Ye, C.; Shang, F.; Niu, J.Z.; Wang, J.J. RNA-sequencing of a citrus bud-feeder, Podagricomela weisei (Coleoptera: Chrysomelidae), reveals xenobiotic metabolism/core RNAi machinery-associated genes and conserved miRNAs. Comp. Biochem. Physiol. Part D Genom. Proteom. 2019, 29, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Song, H.; Abbas, M.; Wang, Y.; Liu, X.; Li, T.; Ma, E.; Zhu, K.Y.; Zhang, J. The stability and sequence cleavage preference of dsRNA are key factors differentiating RNAi efficiency between migratory locust and Asian corn borer. Insect Biochem. Mol. Biol. 2022, 143, 103738. [Google Scholar] [CrossRef] [PubMed]
- Flynt, A.S. Insecticidal RNA interference, thinking beyond long dsRNA. Pest Manag. Sci. 2021, 77, 2179–2187. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Chen, R.; Wang, J.J. RNA interference in insects: The link between antiviral defense and pest control. Insect Sci. 2024, 31, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Ortolá, B.; Cordero, T.; Hu, X.; Daròs, J.A. Intron-assisted, viroid-based production of insecticidal circular double-stranded RNA in Escherichia coli. RNA Biol. 2021, 18, 1846–1857. [Google Scholar] [CrossRef] [PubMed]
- Powell, M.; Pyati, P.; Cao, M.; Bell, H.; Gatehouse, J.A.; Fitches, E. Insecticidal effects of dsRNA targeting the Diap1 gene in dipteran pests. Sci. Rep. 2017, 7, 15147. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.B.; Dhandapani, R.K.; Duan, J.J.; Palli, S.R. RNA interference in the Asian longhorned beetle: Identification of key RNAi genes and reference genes for RT-qPCR. Sci. Rep. 2017, 7, 8913. [Google Scholar] [CrossRef] [PubMed]
- Ai, X.; Wei, Y.; Huang, L.; Zhao, J.; Wang, Y.; Liu, X. Developmental control of Helicoverpa armigera by ingestion of bacteria expressing dsRNA targeting an arginine kinase gene. Biocontrol Sci. Technol. 2018, 28, 253–267. [Google Scholar] [CrossRef]
- Keppanan, R.; Karuppannasamy, A.; Nagaraja, B.; Thiruvengadam, V.; Kesavan, S.; Dhawane, Y.; Ramasamy, A. Effectiveness of chitosan nano hydrogel mediated pickering encapsulation of EcR dsRNA against the Whitefly, Bemisia Tabaci Asia-I (Gennedius)(Hemiptera: Aleyordidae). Pestic. Biochem. Physiol. 2024, 198, 105712. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhao, L.; Shen, Q.; Xie, G.; Wang, S.; Tang, B. Knockdown of two trehalose-6-phosphate synthases severely affects chitin metabolism gene expression in the brown planthopper Nilaparvata lugens. Pest Manag. Sci. 2017, 73, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Adesanya, A.W.; Cardenas, A.; Lavine, M.D.; Walsh, D.B.; Lavine, L.C.; Zhu, F. RNA interference of NADPH-cytochrome P450 reductase increases susceptibilities to multiple acaricides in Tetranychus urticae. Pestic. Biochem. Physiol. 2020, 165, 104550. [Google Scholar] [CrossRef] [PubMed]
- Adeyinka, O.S.; Nasir, I.A.; Riaz, S.; Yousaf, I.; Toufiq, N.; Okiki, A.P.; Tabassum, B. A protective dsRNA is crucial for optimum RNAi gene silencing in Chilo partellus. Int. J. Agric. Biol. 2021, 25, 1238–1248. [Google Scholar] [CrossRef]
- Ali, M.W.; Zhang, Z.-y.; Xia, S.; Zhang, H. Biofunctional analysis of Vitellogenin and Vitellogenin receptor in citrus red mites, Panonychus citri by RNA interference. Sci. Rep. 2017, 7, 16123. [Google Scholar] [CrossRef] [PubMed]
- Badillo, I.E.; Rotenberg, D.; Schneweis, B.A.; Whitfield, A.E. RNA interference tools for the western flower thrips, Frankliniella occidentalis. Insect Physiol. 2015, 76, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Xu, S.; Cong, Z.; Li, L.; Ya, L.; Xing, G.; Dong-Mei, C.; Zhang, P.; MIng-Wang, S.; Xi, C. Silencing of cytochrome P450 in Spodoptera frugiperda (Lepidoptera: Noctuidae) by RNA interference enhances susceptibility to chlorantraniliprole. Insect Sci. 2020, 20, 12. [Google Scholar] [CrossRef]
- Faisal, M.; Abdel-Salam, E.M.; Alatar, A.A.; Saquib, Q.; Alwathnani, H.A.; Canto, T. Genetic transformation and siRNA-mediated gene silencing for aphid resistance in tomato. Agronomy 2019, 9, 893. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, H.; Zhang, X.; Ma, E.; Guo, Y.; Zhu, K.Y.; Zhang, J. RNA interference of cytochrome P450 CYP6F subfamily genes affects susceptibility to different insecticides in Locusta migratoria. Pest Manag. Sci. 2016, 72, 2154–2165. [Google Scholar] [CrossRef] [PubMed]
- Kishk, A.; Hijaz, F.; Anber, H.A.; AbdEl-Raof, T.K.; El-Sherbeni, A.-H.D.; Hamed, S.; Killiny, N. RNA interference of acetylcholinesterase in the Asian citrus psyllid, Diaphorina citri, increases its susceptibility to carbamate and organophosphate insecticides. Pestic. Biochem. Physiol. 2017, 143, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Wei, P.; Zhao, L.; Shi, Z.; Shen, Q.; Yang, M.; Xie, G.; Wang, S. Knockdown of five trehalase genes using RNA interference regulates the gene expression of the chitin biosynthesis pathway in Tribolium castaneum. BMC Biotechnol. 2016, 16, 67. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Gul, H.; Tariq, K.; Hafeez, M.; Desneux, N.; Gao, X.; Song, D. RNA interference-mediated silencing of ecdysone receptor (EcR) gene causes lethal and sublethal effects on melon aphid, Aphis gossypii. Entomol. Gen. 2022, 42, 791–797. [Google Scholar] [CrossRef]
- Xue, Q.; Samakovli, D.; Swevers, L.; Taning, C.N.T. Drosophila X virus-like particles as efficient dsRNA carriers for improved RNAi against the invasive species, Drosophila suzukii. Pest Sci. 2024, 97, 429–443. [Google Scholar] [CrossRef]
- Yogindran, S.; Rajam, M.V. Artificial miRNA-mediated silencing of ecdysone receptor (EcR) affects larval development and oogenesis in Helicoverpa armigera. Insect Biochem. Mol. Biol. 2016, 77, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Khajuria, C.; Rangasamy, M.; Gandra, P.; Fitter, M.; Geng, C.; Woosely, A.; Hasler, J.; Schulenberg, G.; Worden, S. Long dsRNA but not siRNA initiates RNAi in western corn rootworm larvae and adults. Appl. Entomol. 2015, 139, 432–445. [Google Scholar] [CrossRef]
- Wang, K.; Peng, Y.; Fu, W.; Shen, Z.; Han, Z. Key factors determining variations in RNA interference efficacy mediated by different double-stranded RNA lengths in Tribolium castaneum. Insect Mol. Biol. 2019, 28, 235–245. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Xu, W.; Xu, L.; Fu, K.; Guo, W.; Bock, R.; Zhang, J. Length-dependent accumulation of double-stranded RNAs in plastids affects RNA interference efficiency in the Colorado Potato Beetle. J. Exp. Bot. 2020, 71, 2670–2677. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, R.; Ramaseshadri, P.; Anderson, J.; Bachman, P.; Clinton, W.; Flannagan, R.; Ilagan, O.; Lawrence, C.; Levine, S.; Moar, W. Characterizing the mechanism of action of double-stranded RNA activity against western corn rootworm (Diabrotica virgifera virgifera LeConte). PLoS ONE 2012, 7, e47534. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Peng, Y.; Zhang, H.; Wang, K.; Zhao, C.; Zhu, G.; Reddy Palli, S.; Han, Z. Off-target effects of RNAi correlate with the mismatch rate between dsRNA and non-target mRNA. RNA Biol. 2021, 18, 1747–1759. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, E.C.; Hunter, W.B. RNA interference–natural gene-based technology for highly specific pest control (HiSPeC). In RNA Interference; IntechOpen: London, UK, 2016; p. 391. [Google Scholar]
- Zhu, F.; Xu, J.; Palli, R.; Ferguson, J.; Palli, S.R. Ingested RNA interference for managing the populations of the Colorado Potato Beetle, Leptinotarsa decemlineata. Pest Manag. Sci. 2011, 67, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Campuzano, C.; Martínez-Ramírez, A.C.; Contreras, E.; Rausell, C.; Real, M.D. Prohibitin, an essential protein for Colorado Potato Beetle larval viability, is relevant to Bacillus thuringiensis Cry3Aa toxicity. Pestic. Biochem. Physiol. 2013, 107, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Liu, X.-P.; Wan, P.-J.; Shi, X.-Q.; Guo, W.-C.; Li, G.-Q. The P450 enzyme Shade mediates the hydroxylation of ecdysone to 20-hydroxyecdysone in the Colorado Potato Beetle, Leptinotarsa decemlineata. Insect Mol. Biol. 2014, 23, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.-C.; Liu, X.-P.; Fu, K.-Y.; Shi, J.-F.; Lü, F.-G.; Li, G.-Q. Functions of nuclear receptor HR3 during larval-pupal molting in Leptinotarsa decemlineata (Say) revealed by in vivo RNA interference. Insect Biochem. Mol. Biol. 2015, 63, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Aksoy, E.; Çalışkan, M.E.; Bakhsh, A. Transgenic potato lines expressing hairpin RNAi construct of molting-associated EcR gene exhibit enhanced resistance against Colorado Potato Beetle (Leptinotarsa decemlineata, Say). Transgenic Res. 2019, 28, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.-Y.; Guo, W.-C.; Ahmat, T.; Li, G.-Q. Knockdown of a nutrient amino acid transporter gene LdNAT1 reduces free neutral amino acid contents and impairs Leptinotarsa decemlineata pupation. Sci. Rep. 2015, 5, 18124. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.-T.; Jia, S.; Wan, P.-J.; Kong, Y.; Guo, W.-C.; Ahmat, T.; Li, G.-Q. RNA interference of a putative S-adenosyl-L-homocysteine hydrolase gene affects larval performance in Leptinotarsa decemlineata (Say). J. Insect Physiol. 2013, 59, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-H.; Jin, L.; Fu, K.-Y.; Guo, W.-C.; Li, G.-Q. RNA interference targeting Ras GTPase gene Ran causes larval and adult lethality in Leptinotarsa decemlineata. Pest Manag. Sci. 2022, 78, 3849–3858. [Google Scholar] [CrossRef]
- Julian-Chávez, B.; Siqueiros-Cendón, T.S.; Torres-Castillo, J.A.; Sinagawa-García, S.R.; Abraham-Juárez, M.J.; González-Barriga, C.D.; Rascón-Cruz, Q.; Siañez-Estrada, L.I.; Arévalo-Gallegos, S.; Espinoza-Sánchez, E.A. Silencing ACE1 gene with dsRNA of different lengths impairs larval development in Leptinotarsa decemlineata. Insects 2024, 15, 1000. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yang, Y.; Wang, Z.; Wu, M.; Fu, J.; Guo, J.; Chang, L.; Zhang, J. Inaccessibility to double-stranded RNAs in plastids restricts RNA interference in (whitefly). Pest Manag. Sci. 2020, 76, 3168–3176. [Google Scholar] [CrossRef] [PubMed]
- Bally, J.; McIntyre, G.J.; Doran, R.L.; Lee, K.; Perez, A.; Jung, H.; Naim, F.; Larrinua, I.M.; Narva, K.E.; Waterhouse, P.M. In-plant protection against Helicoverpa armigera by production of long hpRNA in chloroplasts. Front. Plant Sci. 2016, 7, 1453. [Google Scholar] [CrossRef] [PubMed]
- Fakhr, E.; Zare, F.; Teimoori-Toolabi, L. Precise and efficient siRNA design: A key point in competent gene silencing. Cancer Gene Ther. 2016, 23, 73–82. [Google Scholar] [CrossRef]
- Chatterjee, M.; Yadav, J.; Rathinam, M.; Karthik, K.; Chowdhary, G.; Sreevathsa, R.; Rao, U. Amenability of Maruca vitrata (Lepidoptera: Crambidae) to gene silencing through exogenous administration and host-delivered dsRNA in pigeonpea (Cajanus cajan L.). Physiol. Mol. Biol. Plants 2022, 28, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Hasnain, A.; Wang, Q.; Liu, D.; Xu, Z.; Zhan, X.; Liu, X.; Pu, J.; Sun, M.; Wang, X. Eco-friendly deacetylated chitosan base siRNA biological-nanopesticide loading cyromazine for efficiently controlling Spodoptera frugiperda. Int. J. Biol. Macromol. 2023, 241, 124575. [Google Scholar] [CrossRef] [PubMed]
- Cagliari, D.; Dias, N.P.; Dos Santos, E.Á.; Rickes, L.N.; Kremer, F.S.; Farias, J.R.; Lenz, G.; Galdeano, D.M.; Garcia, F.R.M.; Smagghe, G. First transcriptome of the Neotropical pest Euschistus heros (Hemiptera: Pentatomidae) with dissection of its siRNA machinery. Sci. Rep. 2020, 10, 4856. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.W.; Wang, Y.C.; Zhang, X.X.; Iqbal, J.; Du, Y.-Z. RNA interference of genes encoding the vacuolar-ATPase in Liriomyza trifolii. Insects 2021, 12, 41. [Google Scholar] [CrossRef] [PubMed]
- Majidiani, S.; PourAbad, R.F.; Laudani, F.; Campolo, O.; Zappalà, L.; Rahmani, S.; Mohammadi, S.A.; Palmeri, V. RNAi in Tuta absoluta management: Effects of injection and root delivery of dsRNAs. Pest Sci. 2019, 92, 1409–1419. [Google Scholar] [CrossRef]
- Hafeez, M.; Liu, S.; Yousaf, H.K.; Jan, S.; Wang, R.-L.; Fernández-Grandon, G.M.; Li, X.; Gulzar, A.; Ali, B.; Rehman, M. RNA interference-mediated knockdown of a cytochrome P450 gene enhanced the toxicity of α-cypermethrin in xanthotoxin-fed larvae of Spodoptera exigua (Hübner). Pestic. Biochem. Physiol. 2020, 162, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Gupta, M.; Pandher, S.; Kaur, G.; Goel, N.; Rathore, P.; Palli, S.R. RNA sequencing, selection of reference genes and demonstration of feeding RNAi in Thrips tabaci (Lind.) (Thysanoptera: Thripidae). BMC Mol. Biol. 2019, 20, 6. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Dong, Y.; Zhang, Q.; Li, S.; Chang, L.; Loiacono, F.V.; Ruf, S.; Zhang, J.; Bock, R. Efficient control of western flower thrips by plastid-mediated RNA interference. Proc. Natl. Acad. Sci. USA 2022, 119, e2120081119. [Google Scholar] [CrossRef] [PubMed]
- Taning, C.N.T.; Christiaens, O.; Berkvens, N.; Casteels, H.; Maes, M.; Smagghe, G. Oral RNAi to control Drosophila suzukii: Laboratory testing against larval and adult stages. Pest Sci. 2016, 89, 803–814. [Google Scholar] [CrossRef]
- Willow, J.; Sulg, S.; Taning, C.N.T.; Silva, A.I.; Christiaens, O.; Kaasik, R.; Prentice, K.; Lövei, G.L.; Smagghe, G.; Veromann, E. Targeting a coatomer protein complex-I gene via RNA interference results in effective lethality in the pollen beetle Brassicogethes aeneus. Pest Sci. 2021, 94, 703–712. [Google Scholar] [CrossRef]
- Wang, G.; Gou, Y.; Guo, S.; Zhou, J.-J.; Liu, C. RNA interference of trehalose-6-phosphate synthase and trehalase genes regulates chitin metabolism in two color morphs of Acyrthosiphon pisum Harris. Sci. Rep. 2021, 11, 948. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Long, G.Y.; Jin, D.C.; Yang, H.; Zhou, C.; Yang, X.B. Knockdown of two trehalase genes by RNA interference is lethal to the white-backed planthopper Sogatella furcifera (Horváth) (Hemiptera: Delphacidae). Biomolecules 2022, 12, 1699. [Google Scholar] [CrossRef] [PubMed]
- Camargo, R.A.; Barbosa, G.O.; Possignolo, I.P.; Peres, L.E.; Lam, E.; Lima, J.E.; Figueira, A.; Marques-Souza, H. RNA interference as a gene silencing tool to control Tuta absoluta in tomato (Solanum lycopersicum). PeerJ 2016, 4, e2673. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Liu, Z.; Chen, J.; Sun, G.; Jiang, Y.; Li, M.; Xiong, L.; Chen, S.; Zhou, Y.; Asad, M. Silencing arginine kinase/integrin β1 subunit by transgenic plant expressing dsRNA inhibits the development and survival of Plutella xylostella. Pest Manag. Sci. 2020, 76, 1761–1771. [Google Scholar] [CrossRef] [PubMed]
- Rasool, K.G.; Mehmood, K.; Tufail, M.; Husain, M.; Alwaneen, W.S.; Aldawood, A.S. Silencing of vitellogenin gene contributes to the promise of controlling red palm weevil, Rhynchophorus ferrugineus (Olivier). Sci. Rep. 2021, 11, 21695. [Google Scholar] [CrossRef] [PubMed]
- Guan, R.; Li, H.; Miao, X. RNAi pest control and enhanced BT insecticidal efficiency achieved by dsRNA of chymotrypsin-like genes in Ostrinia furnacalis. Pest Sci. 2017, 90, 745–757. [Google Scholar] [CrossRef]
- Shi, J.F.; Mu, L.L.; Chen, X.; Guo, W.C.; Li, G.Q. RNA interference of chitin synthase genes inhibits chitin biosynthesis and affects larval performance in Leptinotarsa decemlineata (Say). Int. J. Biol. Sci. 2016, 12, 1319. [Google Scholar] [CrossRef] [PubMed]
- Girard, M.; Berthaud, V.; Martin, E.; Vallon, L.; Rebollo, R.; Vallier, A.; Vigneron, A.; Hay, A.-E.; Valiente Moro, C.; Minard, G. Evaluation of non-invasive dsRNA delivery methods for the development of RNA interference in the Asian tiger mosquito Aedes albopictus. Pest Sci. 2024, 98, 581–596. [Google Scholar] [CrossRef]
- Gredell, J.A.; Berger, A.K.; Walton, S.P. Impact of target mRNA structure on siRNA silencing efficiency: A large-scale study. Biotechnol. Bioeng. 2008, 100, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Larsson, E.; Sander, C.; Marks, D. mRNA turnover rate limits siRNA and microRNA efficacy. Mol. Syst. Biol. 2010, 6, 433. [Google Scholar] [CrossRef]
- Elbashir, S.M.; Harborth, J.; Weber, K.; Tuschl, T. Analysis of gene function in somatic mammalian cells using small interfering RNAs. Methods 2002, 26, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Schubert, S.; Grünweller, A.; Erdmann, V.A.; Kurreck, J. Local RNA target structure influences siRNA efficacy: Systematic analysis of intentionally designed binding regions. Mol. Biol. 2005, 348, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.Y.; Carmack, C.S.; Long, D.D.; Maliyekkel, A.; Shao, Y.; Roninson, I.B.; Ding, Y. A structural interpretation of the effect of GC-content on efficiency of RNA interference. BMC Bioinform. 2009, 10, S33. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.; Leake, D.; Boese, Q.; Scaringe, S.; Marshall, W.S.; Khvorova, A. Rational siRNA design for RNA interference. Nat. Biotechnol. 2004, 22, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, Y.; Sinakevitch, I.; Lei, H.; Smith, B.H. Comparison of RNAi knockdown effect of tyramine receptor 1 induced by dsRNA and siRNA in brains of the honey bee, Apis mellifera. Insect Physiol. 2018, 111, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Asokan, R.; Chandra, G.S.; Manamohan, M.; Kumar, N.K. Effect of diet delivered various concentrations of double-stranded RNA in silencing a midgut and a non-midgut gene of Helicoverpa armigera. Bull. Entomol. Res. 2013, 103, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Camargo, R.d.A.; Herai, R.H.; Santos, L.N.; Bento, F.M.; Lima, J.E.; Marques, S.H.; Figueira, A. De novo transcriptome assembly and analysis to identify potential gene targets for RNAi-mediated control of the tomato leafminer (Tuta absoluta). BMC Genom. 2015, 16, 635. [Google Scholar] [CrossRef] [PubMed]
- Peng, J. Gene redundancy and gene compensation: An updated view. Genet. Genom. 2019, 46, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Arraes, F.B.M.; Martins-de-Sa, D.; Noriega Vasquez, D.D.; Melo, B.P.; Faheem, M.; de Macedo, L.L.P.; Morgante, C.V.; Barbosa, J.A.R.G.; Togawa, R.C.; Moreira, V.J.V. Dissecting protein domain variability in the core RNA interference machinery of five insect orders. RNA Biol. 2021, 18, 1653–1681. [Google Scholar] [CrossRef] [PubMed]
- Guan, R.B.; Li, H.C.; Miao, X.X. Prediction of effective RNA interference targets and pathway-related genes in lepidopteran insects by RNA sequencing analysis. Insect Sci. 2018, 25, 356–367. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Zhang, F.; Huang, S.; Merchant, A.; Zhou, X.; Li, Z. Over-expression of RNA interference (RNAi) core machinery improves susceptibility to RNAi in silkworm larvae. Insect Mol. Biol. 2020, 29, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Singh, S.; Kaur, G.; Pandher, S.; Kaur, N.; Goel, N.; Kaur, R.; Rathore, P. Transcriptome analysis unravels RNAi pathways genes and putative expansion of CYP450 gene family in cotton leafhopper Amrasca biguttula (Ishida). Mol. Biol. Rep. 2021, 48, 4383–4396. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zhou, Q.; Xiao, J.; Qin, X.; Zhang, Y.; Li, X.; Zheng, W.; Zhang, H. Nanoparticle LDH enhances RNAi efficiency of dsRNA in piercing-sucking pests by promoting dsRNA stability and transport in plants. Nanobiotechnology 2024, 22, 544. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Gupta, M.; Pandher, S.; Kaur, G.; Goel, N.; Rathore, P. Using de novo transcriptome assembly and analysis to study RNAi in Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae). Sci. Rep. 2019, 9, 13710. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.A.; Tabuloc, C.A.; Cervantes, K.R.; Chiu, J. Ingestion of genetically modified yeast symbiont reduces fitness of an insect pest via RNA interference. Sci. Rep. 2016, 6, 22587. [Google Scholar] [CrossRef] [PubMed]
- Bachman, P.M.; Huizinga, K.M.; Jensen, P.D.; Mueller, G.; Tan, J.; Uffman, J.P.; Levine, S.L. Ecological risk assessment for DvSnf7 RNA: A plant-incorporated protectant with targeted activity against western corn rootworm. Regul. Toxicol. Pharmacol. 2016, 81, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Boeckman, C.J.; Anderson, J.A.; Linderblood, C.; Olson, T.; Roper, J.; Sturtz, K.; Walker, C.; Woods, R. Environmental risk assessment of the DvSSJ1 dsRNA and the IPD072Aa protein to non-target organisms. GM Crops Food 2021, 12, 459–478. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.L.; Zimmermann, C.S.; Carlson, A.B.; Mathesius, C.A.; Mukerji, P.; McNaughton, J.L.; Walker, C.A.; Roper, J.M. Evaluation of the safety and nutritional equivalency of maize grain with genetically modified event DP-Ø23211-2. GM Crops Food 2021, 12, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Vélez, A.M.; Jurzenski, J.; Matz, N.; Zhou, X.; Wang, H.; Ellis, M.; Siegfried, B.D. Developing an in vivo toxicity assay for RNAi risk assessment in honey bees, Apis mellifera L. Chemosphere 2016, 144, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Zheng, W.; Zheng, W.; Zhang, H. The effects of RNA interference targeting Bactrocera dorsalis ds-Bdrpl19 on the gene expression of rpl19 in non-target insects. Ecotoxicology 2015, 24, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Jarosch, A.; Moritz, R.F.A. RNA interference in honeybees: Off-target effects caused by dsRNA. Apidologie 2012, 43, 128–138. [Google Scholar] [CrossRef]
- Petrick, J.S.; Moore, W.M.; Heydens, W.F.; Koch, M.S.; Sherman, J.H.; Lemke, S.L. A 28-day oral toxicity evaluation of small interfering RNAs and a long double-stranded RNA targeting vacuolar ATPase in mice. Regul. Toxicol. Pharmacol. 2015, 71, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Petrick, J.S.; Frierdich, G.E.; Carleton, S.M.; Kessenich, C.R.; Silvanovich, A.; Zhang, Y.; Koch, M.S. Corn rootworm-active RNA DvSnf7: Repeat dose oral toxicology assessment in support of human and mammalian safety. Regul. Toxicol. Pharmacol. 2016, 81, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.L.; Bartz, S.R.; Schelter, J.; Kobayashi, S.V.; Burchard, J.; Mao, M.; Li, B.; Cavet, G.; Linsley, P.S. Expression profiling reveals off-target gene regulation by RNAi. Nat. Biotechnol. 2003, 21, 635–637. [Google Scholar] [CrossRef] [PubMed]
- Mogren, C.L.; Lundgren, J.G. In silico identification of off-target pesticidal dsRNA binding in honey bees (Apis mellifera). PeerJ 2017, 5, e4131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hou, D.; Chen, X.; Li, D.; Zhu, L.; Zhang, Y.; Li, J.; Bian, Z.; Liang, X.; Cai, X.; et al. Exogenous plant MIR168a specifically targets mammalian LDLRAP1: Evidence of cross-kingdom regulation by microRNA. Cell Res. 2012, 22, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Y.; Liu, Y.; Liu, H.; Wang, H.; Jin, W.; Zhang, Y.; Zhang, C.; Xu, D. Role of plant microRNA in cross-species regulatory networks of humans. BMC Syst. Biol. 2016, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Nanda, S.; Zhang, Y.; Zhou, X.; Yang, C.; Pan, H. Risk assessment of RNAi-based biopesticides. New Crops 2024, 1, 100019. [Google Scholar] [CrossRef]
- Marques, J.T.; Williams, B.R. Activation of the mammalian immune system by siRNAs. Nat. Biotechnol. 2005, 23, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Robbins, M.; Judge, A.; MacLachlan, I. siRNA and Innate Immunity. Oligonucleotides 2009, 19, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, P.A.; Anderson, E.; Lary, J.; Cole, J.L. Mechanism of PKR Activation by dsRNA. J. Mol. Biol. 2008, 381, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Olejniczak, M.; Galka, P.; Krzyzosiak, W.J. Sequence-non-specific effects of RNA interference triggers and microRNA regulators. Nucleic Acids Res. 2009, 38, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, J.G.; Duan, J.J. RNAi-based insecticidal crops: Potential effects on nontarget species. BioScience 2013, 63, 657–665. [Google Scholar] [CrossRef]
- Grimm, D.; Streetz, K.L.; Jopling, C.L.; Storm, T.A.; Pandey, K.; Davis, C.R.; Marion, P.; Salazar, F.; Kay, M.A. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature 2006, 441, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Karpala, A.J.; Doran, T.J.; Bean, A.G.D. Immune responses to dsRNA: Implications for gene silencing technologies. Immunol. Cell Biol. 2005, 83, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Gantier, M.P.; Williams, B.R. The response of mammalian cells to double-stranded RNA. Cytokine Growth Factor Rev. 2007, 18, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, H.; Yang, X.; Chen, G.; Du, L.; Chen, H.; Li, Y.; Peng, Y.; Han, L. Consumption of miRNA-mediated insect-resistant transgenic rice pollen does not harm Apis mellifera adults. Agric. Food Chem. 2021, 69, 4234–4242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, J.; Gao, J.; Zhang, Q.; Liu, X.; Han, Z. New insights into transmission pathways and possible off-target effects of insecticidal dsRNA released by treated plants. Pestic. Biochem. Physiol. 2022, 188, 105281. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, N.L.; Smagghe, G.; Taning, C.N.T.; Oliveira, E.E.; Christiaens, O. Risk assessment of RNAi-based pesticides to non-target organisms: Evaluating the effects of sequence similarity in the parasitoid wasp Telenomus podisi. Sci. Total Environ. 2022, 832, 154746. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.; Mingels, L.; Vogel, E.; Wang, L.; Christiaens, O.; Cappelle, K.; Wynant, N.; Gansemans, Y.; Van Nieuwerburgh, F.; Smagghe, G.; et al. Generation of virus- and dsRNA-derived siRNAs with species-dependent length in insects. Viruses 2019, 11, 738. [Google Scholar] [CrossRef] [PubMed]
- Nunes, F.M.F.; Aleixo, A.C.; Barchuk, A.R.; Bomtorin, A.D.; Grozinger, C.M.; Simões, Z.L.P. Non-target effects of green fluorescent protein (GFP)-derived double-stranded RNA (dsRNA-GFP) used in honey bee RNA interference (RNAi) assays. Insects 2013, 4, 90–103. [Google Scholar] [CrossRef]
- Taning, C.N.T.; Gui, S.; De Schutter, K.; Jahani, M.; Castellanos, N.L.; Christiaens, O.; Smagghe, G. A sequence complementarity-based approach for evaluating off-target transcript knockdown in Bombus terrestris, following ingestion of pest-specific dsRNA. Pest Sci. 2021, 94, 487–503. [Google Scholar] [CrossRef]
- Kulkarni, M.M.; Booker, M.; Silver, S.J.; Friedman, A.; Hong, P.; Perrimon, N.; Mathey-Prevot, B. Evidence of off-target effects associated with long dsRNAs in Drosophila melanogaster cell-based assays. Nat. Methods 2006, 3, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Cao, J.; He, Y.; Yang, S.; Zhang, J. Assessment on effects of transplastomic potato plants expressing Colorado Potato Beetle β-Actin double-stranded RNAs for three non-target pests. Pestic. Biochem. Physiol. 2021, 178, 104909. [Google Scholar] [CrossRef] [PubMed]
- Hanning, J.E.; Saini, H.K.; Murray, M.J.; van Dongen, S.; Davis, M.P.A.; Barker, E.M.; Ward, D.M.; Scarpini, C.G.; Enright, A.J.; Pett, M.R.; et al. Lack of correlation between predicted and actual off-target effects of short-interfering RNAs targeting the human papillomavirus type 16 E7 oncogene. Br. J. Cancer 2013, 108, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Whyard, S.; Singh, A.D.; Wong, S. Ingested double-stranded RNAs can act as species-specific insecticides. Insect Biochem. Mol. Biol. 2009, 39, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.R.; Zapata, F.; Dubelman, S.; Mueller, G.M.; Jensen, P.D.; Levine, S.L. Characterizing a novel and sensitive method to measure dsRNA in soil. Chemosphere 2016, 161, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Carstens, K.; Anderson, J.; Bachman, P.; De Schrijver, A.; Dively, G.; Federici, B.; Hamer, M.; Gielkens, M.; Jensen, P.; Lamp, W.; et al. Genetically modified crops and aquatic ecosystems: Considerations for environmental risk assessment and non-target organism testing. Transgenic Res. 2012, 21, 813–842. [Google Scholar] [CrossRef] [PubMed]
- Bachman, P.; Fischer, J.; Song, Z.; Urbanczyk-Wochniak, E.; Watson, G. Environmental fate and dissipation of applied dsRNA in soil, aquatic systems, and plants. Front. Plant Sci. 2020, 11, 21. [Google Scholar] [CrossRef]
- Zhang, J.; Khan, S.A.; Heckel, D.G.; Bock, R. Next-generation insect-resistant plants: RNAi-mediated crop protection. Trends Biotechnol. 2017, 35, 871–882. [Google Scholar] [CrossRef]
- Hashiro, S.; Yasueda, H. RNA interference-based pesticides and antiviral agents: Microbial overproduction systems for double-stranded RNA for applications in agriculture and aquaculture. Appl. Sci. 2022, 12, 2954. [Google Scholar] [CrossRef]
- Hough, J.; Howard, J.D.; Brown, S.; Portwood, D.E.; Kilby, P.M.; Dickman, M.J. Strategies for the production of dsRNA biocontrols as alternatives to chemical pesticides. Front. Bioeng. Biotechnol. 2022, 10, 980592. [Google Scholar] [CrossRef] [PubMed]
- Guan, R.; Chu, D.; Han, X.; Miao, X.; Li, H. Advances in the development of microbial double-stranded RNA production systems for application of RNA interference in agricultural pest control. Front. Bioeng. Biotechnol. 2021, 9, 753790. [Google Scholar] [CrossRef] [PubMed]
- Nwokeoji, A.O.; Nwokeoji, E.A.; Chou, T.; Togola, A. A novel sustainable platform for scaled manufacturing of double-stranded RNA biopesticides. Bioresour. Bioprocess. 2022, 9, 107. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Huang, Y.; Tang, X. RNAi-based pest control: Production, application and the fate of dsRNA. Front. Bioeng. Biotechnol. 2022, 10, 1080576. [Google Scholar] [CrossRef] [PubMed]
- Lezzerini, M.; van de Ven, K.; Veerman, M.; Brul, S.; Budovskaya, Y.V. Specific RNA interference in Caenorhabditis elegans by ingested dsRNA expressed in Bacillus subtilis. PLoS ONE 2015, 10, e0124508. [Google Scholar] [CrossRef] [PubMed]
- Park, M.G.; Kim, W.J.; Choi, J.Y.; Kim, J.H.; Park, D.H.; Kim, J.Y.; Wang, M.; Je, Y.H. Development of a Bacillus thuringiensis based dsRNA production platform to control sacbrood virus in Apis cerana. Pest Manag. Sci. 2020, 76, 1699–1704. [Google Scholar] [CrossRef] [PubMed]
- Hashiro, S.; Chikami, Y.; Kawaguchi, H.; Krylov, A.A.; Niimi, T.; Yasueda, H. Efficient production of long double-stranded RNAs applicable to agricultural pest control by Corynebacterium glutamicum equipped with coliphage T7-expression system. Appl. Microbiol. Biotechnol. 2021, 105, 4987–5000. [Google Scholar] [CrossRef] [PubMed]
- Abrieux, A.; Chiu, J.C. Oral delivery of dsRNA by microbes: Beyond pest control. Commun. Integr. Biol. 2016, 9, 22587. [Google Scholar] [CrossRef] [PubMed]
- Niehl, A.; Soininen, M.; Poranen, M.M.; Heinlein, M. Synthetic biology approach for plant protection using dsRNA. Plant Biotechnol. J. 2018, 16, 1679–1687. [Google Scholar] [CrossRef] [PubMed]
- Taracena, M.L.; Oliveira, P.L.; Almendares, O.; Umaña, C.; Lowenberger, C.; Dotson, E.M.; Paiva-Silva, G.O.; Pennington, P.M. Genetically modifying the insect gut microbiota to control Chagas disease vectors through systemic RNAi. PLoS Negl. Trop. Dis. 2015, 9, e0003358. [Google Scholar] [CrossRef]
- Leonard, S.P.; Powell, J.E.; Perutka, J.; Geng, P.; Heckmann, L.C.; Horak, R.D.; Davies, B.W.; Ellington, A.D.; Barrick, J.E.; Moran, N.A. Engineered symbionts activate honey bee immunity and limit pathogens. Science 2020, 367, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Elston, K.M.; Maeda, G.P.; Perreau, J.; Barrick, J. Addressing the challenges of symbiont-mediated RNAi in aphids. PeerJ 2023, 11, e14961. [Google Scholar] [CrossRef] [PubMed]
- Whitten, M.M.; Facey, P.D.; Del Sol, R.; Fernández-Martínez, L.T.; Evans, M.C.; Mitchell, J.J.; Bodger, O.G.; Dyson, P.J. Symbiont-mediated RNA interference in insects. Proc. R. Soc. B Biol. Sci. 2016, 283, 20160042. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Rajurkar, A.B.; Kumar, K.; Mohankumar, S. Comparative analysis of Chitin SynthaseA dsRNA mediated RNA interference for management of crop pests of different families of Lepidoptera. Front. Plant Sci. 2020, 11, 427. [Google Scholar] [CrossRef] [PubMed]
- Mamta; Reddy, K.; Rajam, M. Targeting chitinase gene of Helicoverpa armigera by host-induced RNA interference confers insect resistance in tobacco and tomato. Plant Mol. Biol. 2016, 90, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, S.; Tabassum, B.; Tariq, M.; Riaz, S.; Yousaf, I.; Jabbar, B.; Khan, A.; Samuel, A.O.; Zameer, M.; Nasir, I.A. Silencing a Myzus persicae macrophage inhibitory factor by plant-mediated RNAi induces enhanced aphid mortality coupled with boosted RNAi efficacy in transgenic potato lines. Mol. Biotechnol. 2022, 64, 1152–1163. [Google Scholar] [CrossRef] [PubMed]
- Head, G.P.; Carroll, M.W.; Evans, S.P.; Rule, D.M.; Willse, A.R.; Clark, T.L.; Storer, N.P.; Flannagan, R.D.; Samuel, L.W.; Meinke, L.J. Evaluation of SmartStax and SmartStax PRO maize against western corn rootworm and northern corn rootworm: Efficacy and resistance management. Pest Manag. Sci. 2017, 73, 1883–1899. [Google Scholar] [CrossRef] [PubMed]
- Gordon, K.H.J.; Waterhouse, P.M. RNAi for insect-proof plants. Nat. Biotechnol. 2007, 25, 1231–1232. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhang, Q.; Dong, Y.; Wang, Z.; Zhan, W.; Ke, Z.; Li, S.; He, L.; Ruf, S.; Bock, R.; et al. Transplastomic tomatoes expressing double-stranded RNA against a conserved gene are efficiently protected from multiple spider mites. New Phytol. 2023, 237, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Burke, W.; Kaplanoglu, E.; Kolotilin, I.; Menassa, R.; Donly, C. RNA interference in the Tobacco Hornworm, Manduca sexta, using plastid-encoded long double-stranded RNA. Front. Plant Sci. 2019, 10, 313. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Singh, N.D.; Li, L.; Zhang, X.; Daniell, H. Engineered chloroplast dsRNA silences cytochrome p450 monooxygenase, V-ATPase and chitin synthase genes in the insect gut and disrupts Helicoverpa armigera larval development and pupation. Plant Biotechnol. J. 2015, 13, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Mamta, B.; Rajam, M. RNAi technology: A new platform for crop pest control. Physiol. Mol. Biol. Plants 2017, 23, 487–501. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, J.; Dao, V.A.; Majumdar, U.; Schmitt-Engel, C.; Schwirz, J.; Schultheis, D.; Ströhlein, N.; Troelenberg, N.; Grossmann, D.; Richter, T. Large scale RNAi screen in Tribolium reveals novel target genes for pest control and the proteasome as prime target. BMC Genom. 2015, 16, 674. [Google Scholar] [CrossRef] [PubMed]
- Biedenkopf, D.; Will, T.; Knauer, T.; Jelonek, L.; Furch, A.C.U.; Busche, T.; Koch, A. Systemic spreading of exogenous applied RNA biopesticides in the crop plant Hordeum vulgare. ExRNA 2020, 2, 12. [Google Scholar] [CrossRef]
- Pampolini, F.; Rieske, L.K. Foliar application of dsRNA to induce gene silencing in emerald ash borer: Systemic distribution, persistence, and bioactivity. Forests 2023, 14, 1853. [Google Scholar] [CrossRef]
- Willow, J.; Soonvald, L.; Sulg, S.; Kaasik, R.; Silva, A.I.; Taning, C.N.T.; Christiaens, O.; Smagghe, G.; Veromann, E. First evidence of bud feeding-induced RNAi in a crop pest via exogenous application of dsRNA. Insects 2020, 11, 769. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xu, H.; Chen, M.; Tang, P.; Wang, K. Spray-induced gene silencing for postharvest protection: dsRNA stability and insecticidal efficacy. Agric. Food Chem. 2025, 73, 10778–10786. [Google Scholar] [CrossRef] [PubMed]
- EPA. U.S. Environmental Protection Agency: EPA Registers Novel Pesticide Technology for Potato Crops. Available online: https://www.epa.gov/pesticides/epa-registers-novel-pesticide-technology-potato-crops (accessed on 7 July 2025).
- Rodrigues, T.B.; Mishra, S.K.; Sridharan, K.; Barnes, E.R.; Alyokhin, A.; Tuttle, R.; Kokulapalan, W.; Garby, D.; Skizim, N.J.; Tang, Y.-w.; et al. First sprayable double-stranded RNA-based biopesticide product targets proteasome subunit beta type-5 in Colorado Potato Beetle (Leptinotarsa decemlineata). Front. Plant Sci. 2021, 12, 728652. [Google Scholar] [CrossRef] [PubMed]
- The Donald Danforth Plant Science Center. New Technology Gaining Traction. Available online: https://www.danforthcenter.org/news/new-technology-gaining-traction/?highlight=Diamondback%20moth (accessed on 12 July 2025).
- Monsanto. Topical Application of dsRNA for Pest Management. Available online: https://aapco.files.wordpress.com/2016/09/dsrna.pdf (accessed on 12 July 2025).
- Lu, Y.; Deng, X.; Zhu, Q.; Wu, D.; Zhong, J.; Wen, L.; Yu, X. The dsRNA delivery, targeting and application in pest control. Agronomy 2023, 13, 714. [Google Scholar] [CrossRef]
- Pampolini, F.; Rieske, L.K. Root uptake, translocation and persistence of EAB-specific dsRNA in ash seedlings. Sci. Rep. 2025, 15, 6378. [Google Scholar] [CrossRef] [PubMed]
- Wise, J.C.; Wise, A.G.; Rakotondravelo, M.; Vandervoort, C.; Seeve, C.; Fabbri, B. Trunk injection delivery of dsRNA for RNAi-based pest control in apple trees. Pest Manag. Sci. 2022, 78, 3528–3533. [Google Scholar] [CrossRef]
- Ghosh, S.K.B.; Hunter, W.B.; Park, A.L.; Gundersen-Rindal, D.E. Double-stranded RNA oral delivery methods to induce RNA interference in phloem and plant-sap-feeding hemipteran insects. Vis. Exp. 2018, 4, 57390. [Google Scholar] [CrossRef]
- Dalakouras, A.; Jarausch, W.; Buchholz, G.; Bassler, A.; Braun, M.; Manthey, T.; Krczal, G.; Wassenegger, M. Delivery of hairpin RNAs and small RNAs into woody and herbaceous plants by trunk injection and petiole absorption. Front. Plant Sci. 2018, 9, 1253. [Google Scholar] [CrossRef] [PubMed]
- Martinez, Z.; De Schutter, K.; Van Damme, E.J.; Vogel, E.; Wynant, N.; Vanden Broeck, J.; Christiaens, O.; Smagghe, G. Accelerated delivery of dsRNA in lepidopteran midgut cells by a Galanthus nivalis lectin (GNA)-dsRNA-binding domain fusion protein. Pestic. Biochem. Physiol. 2021, 175, 104853. [Google Scholar] [CrossRef] [PubMed]
- Dhandapani, R.K.; Gurusamy, D.; Palli, S.R. Development of catechin, poly-l-lysine, and double-stranded RNA nanoparticles. Appl. Bio Mater. 2021, 4, 4310–4318. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Kiser, M.R.; Moradipour, M.; Nadeau, E.A.; Ghanim, R.W.; Webb, B.A.; Rankin, S.E.; Knutson, B.L. Effect of confinement in nanopores on RNA interactions with functionalized mesoporous silica nanoparticles. Phys. Chem. B 2020, 124, 8549–8561. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, R.; Li, G.; Jin, D.; Guo, J.; Ochoa, R.; Yi, T. Identification of the fibroin of Stigmaeopsis nanjingensis by a nanocarrier-based transdermal dsRNA delivery system. Exp. Appl. Acarol. 2022, 87, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Li, X.; Li, J.; Yu, C.; Zeng, Q.; Ning, G.; Wan, H.; Li, J.; Ma, K.; He, S. Overcoming resistance in insect pest with a nanoparticle-mediated dsRNA and insecticide co-delivery system. Chem. Eng. J. 2023, 475, 146239. [Google Scholar] [CrossRef]
- Qiao, H.; Zhao, J.; Wang, X.; Xiao, L.; Zhu-Salzman, K.; Lei, J.; Xu, D.; Xu, G.; Tan, Y.; Hao, D. An oral dsRNA delivery system based on chitosan induces G protein-coupled receptor kinase 2 gene silencing for Apolygus lucorum control. Pestic. Biochem. Physiol. 2023, 194, 105481. [Google Scholar] [CrossRef]
- Niño-Sánchez, J.; Sambasivam, P.T.; Sawyer, A.; Hamby, R.; Chen, A.; Czislowski, E.; Li, P.; Manzie, N.; Gardiner, D.M.; Ford, R.; et al. BioClay™ prolongs RNA interference-mediated crop protection against Botrytis cinerea. J. Integr. Plant Biol. 2022, 64, 2187–2198. [Google Scholar] [CrossRef] [PubMed]
- Mitter, N.; Worrall, E.A.; Robinson, K.E.; Li, P.; Jain, R.G.; Taochy, C.; Fletcher, S.J.; Carroll, B.J.; Lu, G.Q.; Xu, Z.P. Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nat. Plants 2017, 3, 16207. [Google Scholar] [CrossRef] [PubMed]
- De Schutter, K.; Verbeke, I.; Kontogiannatos, D.; Dubruel, P.; Swevers, L.; Van Damme, E.J.; Smagghe, G. Use of cell cultures in vitro to assess the uptake of long dsRNA in plant cells. In Vitro Cell. Dev. Biol.-Plant 2022, 58, 511–520. [Google Scholar] [CrossRef]
- Saberi, E.; Mondal, M.; Paredes, J.R.; Nawaz, K.; Brown, J.K.; Qureshi, J.A. Optimal dsRNA concentration for RNA interference in Asian citrus psyllid. Insects 2024, 15, 58. [Google Scholar] [CrossRef] [PubMed]
- Zotti, M.; Dos Santos, E.A.; Cagliari, D.; Christiaens, O.; Taning, C.N.T.; Smagghe, G. RNA interference technology in crop protection against arthropod pests, pathogens and nematodes. Pest Manag. Sci. 2018, 74, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Alatorre, M.; Siqueiros-Cendón, T.S.; Torres-Castillo, J.A.; Sinagawa-García, S.R.; Iglesias-Figueroa, B.F.; Abraham-Juárez, M.J.; González-Barriga, C.D.; Rascón-Cruz, Q.; Siañez-Estrada, L.I.; Espinoza-Sánchez, E.A. Gene silencing of Sarco/Endoplasmic Reticulum Ca2+-ATPase and NADPH–Cytochrome P450 Reductase as a novel approach to Leptinotarsa decemlineata management. Agronomy 2025, 15, 1151. [Google Scholar] [CrossRef]
- Cedden, D.; Güney, G.; Scholten, S.; Rostás, M. Lethal and sublethal effects of orally delivered double-stranded RNA on the cabbage stem flea beetle, Psylliodes chrysocephala. Pest Manag. Sci. 2024, 80, 2282–2293. [Google Scholar] [CrossRef] [PubMed]
- Nishide, Y.; Kageyama, D.; Tanaka, Y.; Yokoi, K.; Jouraku, A.; Futahashi, R.; Fukatsu, T. Effectiveness of orally-delivered double-stranded RNA on gene silencing in the stinkbug Plautia stali. PLoS ONE 2021, 16, e0245081. [Google Scholar] [CrossRef] [PubMed]
- Darif, N.; Vogelsang, K.; Vorgia, E.; Schneider, D.; Deligianni, E.; Geibel, S.; Vontas, J.; Denecke, S. Cell penetrating peptides are versatile tools for enhancing multimodal uptake into cells from pest insects. Pestic. Biochem. Physiol. 2023, 190, 105317. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, D.H.; Velez, A.M.; Fishilevich, E.; Wang, H.; Carneiro, N.P.; Valencia-Jimenez, A.; Valicente, F.H.; Narva, K.E.; Siegfried, B.D. Clathrin-dependent endocytosis is associated with RNAi response in the western corn rootworm, Diabrotica virgifera virgifera LeConte. PLoS ONE 2018, 13, e0201849. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Gao, X.; Xu, J.; Liang, X.; Li, Q.; Yao, J.; Zhu, K.Y. Clathrin-dependent endocytosis plays a predominant role in cellular uptake of double-stranded RNA in the red flour beetle. Insect Biochem. Mol. Biol. 2015, 60, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Hu, X.S.; Wang, Z.W.; Wei, D.; Smagghe, G.; Christiaens, O.; Niu, J.; Wang, J.J. Involvement of clathrin-dependent endocytosis in cellular dsRNA uptake in aphids. Insect Biochem. Mol. Biol. 2021, 132, 103557. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.S.; Gurusamy, D.; Palli, S.R. Accumulation of dsRNA in endosomes contributes to inefficient RNA interference in the fall armyworm, Spodoptera frugiperda. Insect Biochem. Mol. Biol. 2017, 90, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Ulvila, J.; Parikka, M.; Kleino, A.; Sormunen, R.; Ezekowitz, R.A.; Kocks, C.; Ramet, M. Double-stranded RNA is internalized by scavenger receptor-mediated endocytosis in Drosophila S2 cells. Biol. Chem. 2006, 281, 14370–14375. [Google Scholar] [CrossRef] [PubMed]
- Wynant, N.; Santos, D.; Van Wielendaele, P.; Vanden Broeck, J. Scavenger receptor-mediated endocytosis facilitates RNA interference in the desert locust, Schistocerca gregaria. Insect Mol. Biol. 2014, 23, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Shukla, J.N.; Kalsi, M.; Sethi, A.; Narva, K.E.; Fishilevich, E.; Singh, S.; Mogilicherla, K.; Palli, S.R. Reduced stability and intracellular transport of dsRNA contribute to poor RNAi response in lepidopteran insects. RNA Biol. 2016, 13, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhan, C.; Fan, J.; Wu, D.; Zhang, R.; Wu, D.; Chen, X.; Lu, Y.; Li, M.; Lin, M. Structural insights into double-stranded RNA recognition and transport by SID-1. Nat. Struct. Mol. Biol. 2024, 31, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- McEwan, D.L.; Weisman, A.S.; Hunter, C.P. Uptake of extracellular double-stranded RNA by SID-2. Mol. Cell 2012, 47, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Cappelle, K.; de Oliveira, C.F.R.; Van Eynde, B.; Christiaens, O.; Smagghe, G. The involvement of clathrin-mediated endocytosis and two Sid-1-like transmembrane proteins in double-stranded RNA uptake in the Colorado Potato Beetle midgut. Insect Mol. Biol. 2016, 25, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Wang, Z.; Wang, H.; Qi, J.; Hu, M.; Hu, Q. Core RNAi machinery and three Sid-1 related genes in Spodoptera litura (Fabricius). Int. J. Agric. Biol. 2015, 17, 937–944. [Google Scholar] [CrossRef]
- Tomoyasu, Y.; Miller, S.C.; Tomita, S.; Schoppmeier, M.; Grossmann, D.; Bucher, G. Exploring systemic RNA interference in insects: A genome-wide survey for RNAi genes in Tribolium. Genome Biol. 2008, 9, R10. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, T.; Ma, X.F.; Xue, J.; Pan, P.; Zhang, X.; Cheng, J.; Zhang, C. Genome-wide screening for components of small interfering RNA (siRNA) and micro-RNA (miRNA) pathways in the brown planthopper, Nilaparvata lugens (Hemiptera: Delphacidae). Insect Mol. Biol. 2013, 22, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.H.; Christie, C.R.; Masotti, A.; Celluzzi, A.; Caporali, A.; Campbell, E.M. Dendrimer-coated carbon nanotubes deliver dsRNA and increase the efficacy of gene knockdown in the red flour beetle Tribolium castaneum. Sci. Rep. 2020, 10, 12422. [Google Scholar] [CrossRef] [PubMed]
- Thungsatianpun, N.; Mavichak, R.; Nattanan, T.; Unajak, S.; Sinthuvanich, C. Cell-penetrating peptide nanocomplexes enhanced cellular uptake of dsRNA in Sf9 cell line. Curr. Sci. Technol. 2021, 11, 299–310. [Google Scholar] [CrossRef]
- Mingels, L.; Wynant, N.; Santos, D.; Peeters, P.; Gansemans, Y.; Billen, J.; Van Nieuwerburgh, F.; Vanden Broeck, J. Extracellular vesicles spread the RNA interference signal of Tribolium castaneum TcA cells. Insect Biochem. Mol. Biol. 2020, 122, 103377. [Google Scholar] [CrossRef] [PubMed]
- Winston, W.M.; Molodowitch, C.; Hunter, C.P. Systemic RNAi in C. elegans requires the putative transmembrane protein SID-1. Science 2002, 295, 2456–2459. [Google Scholar] [CrossRef] [PubMed]
- Maori, E.; Garbian, Y.; Kunik, V.; Mozes-Koch, R.; Malka, O.; Kalev, H.; Sabath, N.; Sela, I.; Shafir, S. A transmissible RNA pathway in honey bees. Cell Rep. 2019, 27, 1949–1959. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.; Winston, W.; Molodowitch, C.; Feinberg, E.; Shih, J.; Sutherlin, M.; Wright, A.; Fitzgerald, M. Systemic RNAi in Caenorhabditis elegans. Cold Spring Harb. Symp. Quant. Biol. 2006, 71, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Shih, J.D.; Hunter, C.P. SID-1 is a dsRNA-selective dsRNA-gated channel. RNA 2011, 17, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.C.; van Rij, R.P.; Hekele, A.; Gillis, A.; Foley, E.; O’Farrell, P.H.; Andino, R. The endocytic pathway mediates cell entry of dsRNA to induce RNAi silencing. Nat. Cell Biol. 2006, 8, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.; Palli, S.R. StaufenC facilitates utilization of the ERAD pathway to transport dsRNA through the endoplasmic reticulum to the cytosol. Proc. Natl. Acad. Sci. USA 2024, 121, e2322927121. [Google Scholar] [CrossRef] [PubMed]
- Sakashita, K.; Tatsuke, T.; Masaki, Y.; Lee, J.M.; Kawaguchi, Y.; Kusakabe, T. dsRNA binding activity of Silworm Larval hemolymph is mediated by lipophorin complex. Fac. Agric. Kyushu Univ. 2009, 54, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.M.; Silver, K.; Zhang, J.; Park, Y.; Zhu, K.Y. Molecular mechanisms influencing efficiency of RNA interference in insects. Pest Manag. Sci. 2019, 75, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Dhahbi, J.M.; Atamna, H.; Li, R.; Yamakawa, A.; Guerrero, N.; Lam, H.T.; Mote, P.; Spindler, S.R. MicroRNAs circulate in the hemolymph of Drosophila and accumulate relative to tissue microRNAs in an age-dependent manner. Genom. Insights 2016, 9, GEI.S38147. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Kwon, K.; Suh, H.-W.; Lee, S.; Park, K.-H.; Kwon, O.-Y.; Choi, J.-Y. Exosome isolation from hemolymph of Korean rhinoceros beetle, Allomyrina dichotoma (Coleoptera: Scarabaeidae). Entomol. Res. 2015, 45, 339–344. [Google Scholar] [CrossRef]
- Yoon, J.-S.; Kim, K.; Palli, S.R. Double-stranded RNA in exosomes: Potential systemic RNA interference pathway in the Colorado Potato Beetle, Leptinotarsa decemlineata. J. Asia-Pac. Entomol. 2020, 23, 1160–1164. [Google Scholar] [CrossRef]
- Karlikow, M.; Goic, B.; Mongelli, V.; Salles, A.; Schmitt, C.; Bonne, I.; Zurzolo, C.; Saleh, M.-C. Drosophila cells use nanotube-like structures to transfer dsRNA and RNAi machinery between cells. Sci. Rep. 2016, 6, 27085. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Gatehouse, J.A.; Fitches, E.C. A systematic study of RNAi effects and dsRNA stability in Tribolium castaneum and Acyrthosiphon pisum, following injection and ingestion of analogous dsRNAs. Int. J. Mol. Sci. 2018, 19, 1079. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Z.; Jiang, Y.X.; Li, M.W.; Li, J.W.; Zha, B.H.; Yang, G. Double-stranded RNA-degrading enzymes reduce the efficiency of RNA interference in Plutella xylostella. Insects 2021, 12, 712. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shi, Y.; Xue, Q.; Smagghe, G.; De Schutter, K.; Taning, C.N.T. Identification and functional analysis of gut dsRNases in the beet armyworm Spodoptera exigua. Insect Biochem. Mol. Biol. 2024, 175, 104206. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Taning, C.N.T.; Smagghe, G.; Christiaens, O. Silencing of double-stranded ribonuclease improves oral RNAi efficacy in southern green stinkbug Nezara viridula. Insects 2021, 12, 115. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wang, B.; Lei, G.; Chen, G.; Liu, D. Advances in nanocarriers to improve the stability of dsRNA in the environment. Front. Bioeng. Biotechnol. 2022, 10, 974646. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.L.; Walker, W.B. Saliva of Lygus lineolaris digests double stranded ribonucleic acids. Insect Physiol. 2012, 58, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Christiaens, O.; Swevers, L.; Smagghe, G. DsRNA degradation in the pea aphid (Acyrthosiphon pisum) associated with lack of response in RNAi feeding and injection assay. Peptides 2014, 53, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Reis, M.A.; Noriega, D.D.; dos Santos Alves, G.; Coelho, R.R.; Grossi-de-Sa, M.F.; Antonino, J.D. Why is oral-induced RNAi inefficient in Diatraea saccharalis? A possible role for DsREase and other nucleases. Pestic. Biochem. Physiol. 2022, 186, 105166. [Google Scholar] [CrossRef]
- Song, H.; Zhang, J.; Li, D.; Cooper, A.M.; Silver, K.; Li, T.; Liu, X.; Ma, E.; Zhu, K.Y.; Zhang, J. A double-stranded RNA degrading enzyme reduces the efficiency of oral RNA interference in migratory locust. Insect Biochem. Mol. Biol. 2017, 86, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Peng, Y.; Chen, J.; Peng, Y.; Wang, X.; Shen, Z.; Han, Z. Comparison of efficacy of RNAi mediated by various nanoparticles in the rice striped stem borer (Chilo suppressalis). Pestic. Biochem. Physiol. 2020, 165, 104467. [Google Scholar] [CrossRef] [PubMed]
- Terra, W.R.; Ferreira, C. Evolutionary trends of digestion and absorption in the major insect orders. Arthropod Struct. Dev. 2020, 56, 100931. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, P.; Buer, B.; Ilias, A.; Kaforou, S.; Aivaliotis, M.; Orfanoudaki, G.; Douris, V.; Geibel, S.; Vontas, J.; Denecke, S. A spatiotemporal atlas of the lepidopteran pest Helicoverpa armigera midgut provides insights into nutrient processing and pH regulation. BMC Genom. 2022, 23, 75. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wang, K.; Fu, W.; Sheng, C.; Han, Z. Biochemical comparison of dsRNA degrading nucleases in four different insects. Front. Physiol. 2018, 9, 624. [Google Scholar] [CrossRef] [PubMed]
- Mo, M.; Yang, G.; Du, J.; Zhou, Y.; Khan, A.; Li, S.; Hu, C. Identification and functional analyses of the Cmdsrnase5 and Cmdsrnase6 Genes in rice leaffolder Cnaphalocrocis Medinalis. Int. J. Mol. Sci. 2022, 23, 140079. [Google Scholar] [CrossRef] [PubMed]
- Volpe, G.; Mazzucchiello, S.M.; Rosati, N.; Lucibelli, F.; Varone, M.; Baccaro, D.; Mattei, I.; Di Lelio, I.; Becchimanzi, A.; Giordano, E. Simultaneous silencing of gut nucleases and a vital target gene by adult dsRNA feeding enhances RNAi efficiency and mortality in Ceratitis capitata. Insects 2024, 15, 717. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, Y.; Yang, Q.; Lin, X.; Liu, Y.; Li, Z.; Swevers, L. Successful oral RNA interference efficiency in the silkworm Bombyx mori through nanoparticle-shielded dsRNA delivery. J. Insect Physiol. 2025, 161, 104749. [Google Scholar] [CrossRef] [PubMed]
- Sandal, S.; Singh, S.; Bansal, G.; Kaur, R.; Mogilicherla, K.; Pandher, S.; Roy, A.; Kaur, G.; Rathore, P.; Kalia, A. Nanoparticle-shielded dsRNA delivery for enhancing RNAi efficiency in Cotton Spotted Bollworm Earias vittella (Lepidoptera: Nolidae). Int. J. Mol. Sci. 2023, 24, 9161. [Google Scholar] [CrossRef] [PubMed]
- Spit, J.; Philips, A.; Wynant, N.; Santos, D.; Plaetinck, G.; Vanden Broeck, J. Knockdown of nuclease activity in the gut enhances RNAi efficiency in the Colorado Potato Beetle, Leptinotarsa decemlineata, but not in the desert locust, Schistocerca gregaria. Insect Biochem. Mol. Biol. 2017, 81, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Peng, Y.; Pu, J.; Fu, W.; Wang, J.; Han, Z. Variation in RNAi efficacy among insect species is attributable to dsRNA degradation in vivo. Insect Biochem. Mol. Biol. 2016, 77, 1–9. [Google Scholar] [CrossRef]
- Liu, J.; Smagghe, G.; Swevers, L. Transcriptional response of BmToll9-1 and RNAi machinery genes to exogenous dsRNA in the midgut of Bombyx mori. Insect Physiol. 2013, 59, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kolliopoulou, A.; Smagghe, G.; Swevers, L. Modulation of the transcriptional response of innate immune and RNAi genes upon exposure to dsRNA and LPS in silkmoth-derived Bm5 cells overexpressing BmToll9-1 receptor. Insect Physiol. 2014, 66, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Flenniken, M.L.; Andino, R. Non-specific dsRNA-mediated antiviral response in the honey bee. PLoS ONE 2013, 8, e77263. [Google Scholar] [CrossRef] [PubMed]
- Dubelman, S.; Fischer, J.; Zapata, F.; Huizinga, K.; Jiang, C.; Uffman, J.; Levine, S.; Carson, D. Environmental fate of double-stranded RNA in agricultural soils. PLoS ONE 2014, 9, e93155. [Google Scholar] [CrossRef] [PubMed]
- Joaquim, M.E.S.; Belchior, G.G.; José, M.O.d.M.A.; Zapata, F.; Jiang, C.; Fischer, J.; Berger, G.U. Dissipation of DvSnf7 double-stranded RNA in Brazilian soils. Agric. Environ. Lett. 2019, 4, 190016. [Google Scholar] [CrossRef]
- Fischer, J.R.; Zapata, F.; Dubelman, S.; Mueller, G.M.; Uffman, J.P.; Jiang, C.; Jensen, P.D.; Levine, S.L. Aquatic fate of a double-stranded RNA in a sediment–water system following an over-water application. Environ. Toxicol. Chem. 2017, 36, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Albright, V.C., III; Wong, C.R.; Hellmich, R.L.; Coats, J.R. Dissipation of double-stranded RNA in aquatic microcosms. Environ. Toxicol. Chem. 2016, 36, 1249–1253. [Google Scholar] [CrossRef] [PubMed]
- San Miguel, K.; Scott, J.G. The next generation of insecticides: dsRNA is stable as a foliar-applied insecticide. Pest Manag. Sci. 2016, 72, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Guan, R.; Guo, H.; Miao, X. New insights into an RNAi approach for plant defence against piercing-sucking and stem-borer insect pests. Plant Cell Environ. 2015, 38, 2277–2285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Hodge, J.; Chatterjee, A.; Moon, T.S.; Parker, K.M. Duplex structure of double-stranded RNA provides stability against hydrolysis relative to single-stranded RNA. Environ. Sci. Technol. 2021, 55, 8045–8053. [Google Scholar] [CrossRef] [PubMed]
- Parker, K.M.; Barragán Borrero, V.; Van Leeuwen, D.M.; Lever, M.A.; Mateescu, B.; Sander, M. Environmental fate of RNA interference pesticides: Adsorption and degradation of double-stranded RNA molecules in agricultural soils. Environ. Sci. Technol. 2019, 53, 3027–3036. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Niño-Sánchez, J.; Hamby, R.; Capriotti, L.; Chen, A.; Mezzetti, B.; Jin, H. Artificial nanovesicles for dsRNA delivery in spray-induced gene silencing for crop protection. Plant Biotechnol. J. 2023, 21, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Dalakouras, A.; Wassenegger, M.; Dadami, E.; Ganopoulos, I.; Pappas, M.L.; Papadopoulou, K. Genetically modified organism-free RNA interference: Exogenous application of RNA molecules in plants. Plant Physiol. 2019, 182, 38–50. [Google Scholar] [CrossRef]
- Xu, L.; Xu, S.; Sun, L.; Zhang, Y.; Luo, J.; Bock, R.; Zhang, J. Synergistic action of the gut microbiota in environmental RNA interference in a leaf beetle. Microbiome 2021, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ke, Z.; Xu, L.; Yang, Y.; Chang, L.; Zhang, J. A faster killing effect of plastid-mediated RNA interference on a leaf beetle through induced dysbiosis of the gut bacteria. Plant Commun. 2024, 5, 100974. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Li, S.; Zhang, W.; Xia, Y. RNAi-knockdown of the Locusta migratoria nuclear export factor protein results in insect mortality and alterations in gut microbiome. Pest Manag. Sci. 2019, 75, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Khajuria, C.; Ivashuta, S.; Wiggins, E.; Flagel, L.; Moar, W.; Pleau, M.; Miller, K.; Zhang, Y.; Ramaseshadri, P.; Jiang, C. Development and characterization of the first dsRNA-resistant insect population from western corn rootworm, Diabrotica virgifera virgifera LeConte. PLoS ONE 2018, 13, e0197059. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Dee, J.; Moar, W.; Dufner-Beattie, J.; Baum, J.; Dias, N.P.; Alyokhin, A.; Buzza, A.; Rondon, S.I.; Clough, M. Selection for high levels of resistance to double-stranded RNA (dsRNA) in Colorado Potato Beetle (Leptinotarsa decemlineata Say) using non-transgenic foliar delivery. Sci. Rep. 2021, 11, 6523. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Zhang, M.; Zhang, J. Characterization and potential mechanism of resistance to double-stranded RNA in willow leaf beetle, Plagiodera versicolora. Pest Sci. 2024, 97, 2217–2226. [Google Scholar] [CrossRef]
- Pinto, M.M.D.; Ferreira dos Santos, R.; De Bortoli, S.A.; Moar, W.; Jurat-Fuentes, J.L. Lack of fitness costs in dsRNA-resistant Leptinotarsa decemlineata ([Coleoptera]: [Chrysomelidae]). J. Econ. Entomol. 2023, 116, 1352–1359. [Google Scholar] [CrossRef] [PubMed]
- Galli, M.; Feldmann, F.; Vogler, U.K.; Kogel, K.-H. Can biocontrol be the game-changer in integrated pest management? A review of definitions, methods and strategies. J. Plant Dis. Prot. 2024, 131, 265–291. [Google Scholar] [CrossRef]
- CAST. RNA interference in agriculture: Methods, applications, and governance. Counc. Agric. Sci. Technol. Ames Iowa 2024, 1, 1–20. [Google Scholar] [CrossRef]
- De Schutter, K.; Taning, C.N.T.; Van Daele, L.; Van Damme, E.J.M.; Dubruel, P.; Smagghe, G. RNAi-based biocontrol products: Market status, regulatory aspects, and risk assessment. Front. Insect Sci. 2022, 1, 818037. [Google Scholar] [CrossRef] [PubMed]
- Dietz-Pfeilstetter, A.; Mendelsohn, M.; Gathmann, A.; Klinkenbuß, D. Considerations and regulatory approaches in the USA and in the EU for dsRNA-based externally applied pesticides for plant protection. Front. Plant Sci. 2021, 12, 682387. [Google Scholar] [CrossRef] [PubMed]
- Leahy, J.; Mendelsohn, M.; Kough, J.; Jones, R.; Berckes, N. Biopesticide oversight and registration at the U.S. Environmental Protection Agency. In Biopesticides: State of the Art and Future Opportunities; Gros, A.D., Coats, J.R., Duke, S.O., Seiber, J.N., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2014; Volume 1172, pp. 3–18. [Google Scholar]
- Pallis, S.; Alyokhin, A.; Manley, B.; Rodrigues, T.B.; Barnes, E.; Narva, K. Baseline susceptibility to a novel dsRNA-based insecticide across us populations of Colorado Potato Beetle. Agriculture 2023, 13, 2283. [Google Scholar] [CrossRef]
- Mishra, S.; Moar, W.; Jurat-Fuentes, J.L. Larvae of Colorado Potato Beetle (Leptinotarsa decemlineata Say) resistant to double-stranded RNA (dsRNA) remain susceptible to small-molecule pesticides. Pest Manag. Sci. 2024, 80, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Moar, W.; Khajuria, C.; Pleau, M.; Ilagan, O.; Chen, M.; Jiang, C.; Price, P.; McNulty, B.; Clark, T.; Head, G. Cry3Bb1-resistant Western Corn Rootworm, Diabrotica virgifera virgifera (LeConte) does not exhibit cross-resistance to DvSnf7 dsRNA. PLoS ONE 2017, 12, e0169175. [Google Scholar] [CrossRef] [PubMed]
- Oberemok, V.V.; Laikova, K.V.; Gal’chinsky, N.V.; Useinov, R.Z.; Novikov, I.A.; Temirova, Z.Z.; Shumskykh, M.N.; Krasnodubets, A.M.; Repetskaya, A.I.; Dyadichev, V.V. DNA insecticide developed from the Lymantria dispar 5.8 S ribosomal RNA gene provides a novel biotechnology for plant protection. Sci. Rep. 2019, 9, 6197. [Google Scholar] [CrossRef] [PubMed]


| Gene | Function | mRNA Size (kb) | Degree of Conservation | Maximum Knockdown Reported | Phenotypic Effect | References |
|---|---|---|---|---|---|---|
| Ion transport and calcium regulation | ||||||
| V-ATPase | Ion and nutrient transport; regulation of cellular homeostasis. | Subunit A, ~2.0–2.5 kb; Subunit B, ~1.5–1.8 kb; Subunit D, ~1.0–1.2 kb; Subunit E, ~0.7–0.9 kb. | Highly preserved | 80% | Decreased survival and fertility of female thrips and reduced number of offspring. | [29,31,38,60,61] |
| RyR | Release of calcium (Ca2+) from the sarcoplasmic reticulum into the cytosol during muscle contraction. | ~15.5 kb | Highly preserved | 75% | Reduction in survival and emergence of adults. | [17,62] |
| Hormonal and neuromuscular signaling | ||||||
| ACE | Hydrolysis of acetylcholine, a neurotransmitter released at the synapse to facilitate signal transmission between neurons. | ~1.4–2.3 kb | Highly preserved | 70% | Reduced weight gain, larval, nymphal, and adult mortality, and increased pesticide susceptibility. | [29,33,35,54] |
| EcR | Primary receptor for ecdysone, a steroid hormone that regulates the molting, metamorphosis, and reproduction processes. | ~1.5–2.8 kb | Highly preserved | 90% | Reduction in weight, longevity, fertility, egg laying, failure to hatch, morbidity, and mortality. | [26,37,39,50] |
| Detoxification and xenobiotic metabolism | ||||||
| CYP450 | Compounds’ bioactivation and xenobiotic metabolism. | ~1.5–2.0 kb | Highly preserved | 90% | Weight decreased, reduced enzymatic CPR activity, and increased pesticide susceptibility. | [12,28,32,34,63] |
| Cytoskeleton, cell motility, and vesicular trafficking | ||||||
| SNF | Vesicular transport, formation of multivesicular vesicles, protein degradation, and cell division. | ~1.2–1.5 kb | Highly preserved | 94% | Larval and adult mortality. | [64,65] |
| ACT | Cell structure, movement, and division. | ~1.5–2.0 kb | Highly preserved | 71% | 100% mortality, cessation of feeding, decreased larval weight, and altered actin filaments. | [16,46] |
| TPM | Muscle function and maintenance of cytoskeletal integrity. | ~1.2–2.2 kb | Highly preserved | 76% | Reduction in feeding; mortality. | [58] |
| α-COP | Vesicular transport between different compartments of the endomembrane system. | ~10–15 kb | Highly preserved | 82% | Mortality | [66,67] |
| Energy metabolism | ||||||
| TRE1 | Catalyzes the hydrolysis of trehalose involved in energy metabolism, chitin synthesis, and metamorphosis. | ~1.9–2.9 kb | Moderately preserved | 85% | Abnormal phenotypes, wing and molt deformities, alteration of genes involved in chitin biosynthesis, soft and transparent cuticle, weight loss, and mortality. | [27,68,69] |
| ArgK | Catalysis of the transfer of phosphates to arginine, participating in energy metabolism. | ~0.3–1.1 kb | Highly preserved | 80% | Length, weight, and pupation rate reduction, melanization, forewings and antenna malformation, and mortality. | [25,29,70,71] |
| Synthesis and macromolecule processing | ||||||
| Vg | Provision of nutrients to oocytes and embryo development. | ~5.0–5.5 kb | Moderately preserved | 99% | Atrophy of oogenesis, decreased size of ovaries and eggs, low egg production, delayed oviposition periods, and lack of hatching. | [30,72] |
| Chy | Digestive proteolysis in the intestine. | ~0.7–0.9 kb | Highly preserved | 87% | Length and weight reduction, mortality. | [29,73] |
| CHS | Chitin biosynthesis, vital for structure, protection, and mobility. | ~4.2–4.7 kb | Highly preserved | 90% | Decreased chitin content, ecdysis inhibition, delayed larval growth, abnormal pupation, and mortality. | [36,59,74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendoza-Alatorre, M.; Julian-Chávez, B.; Solano-Ornelas, S.; Siqueiros-Cendón, T.S.; Torres-Castillo, J.A.; Sinagawa-García, S.R.; Abraham-Juárez, M.J.; González-Barriga, C.D.; Rascón-Cruz, Q.; Siañez-Estrada, L.I.; et al. RNAi in Pest Control: Critical Factors Affecting dsRNA Efficacy. Insects 2025, 16, 737. https://doi.org/10.3390/insects16070737
Mendoza-Alatorre M, Julian-Chávez B, Solano-Ornelas S, Siqueiros-Cendón TS, Torres-Castillo JA, Sinagawa-García SR, Abraham-Juárez MJ, González-Barriga CD, Rascón-Cruz Q, Siañez-Estrada LI, et al. RNAi in Pest Control: Critical Factors Affecting dsRNA Efficacy. Insects. 2025; 16(7):737. https://doi.org/10.3390/insects16070737
Chicago/Turabian StyleMendoza-Alatorre, Maribel, Brenda Julian-Chávez, Stephanie Solano-Ornelas, Tania Samanta Siqueiros-Cendón, Jorge Ariel Torres-Castillo, Sugey Ramona Sinagawa-García, María Jazmín Abraham-Juárez, Carmen Daniela González-Barriga, Quintín Rascón-Cruz, Luis Ignacio Siañez-Estrada, and et al. 2025. "RNAi in Pest Control: Critical Factors Affecting dsRNA Efficacy" Insects 16, no. 7: 737. https://doi.org/10.3390/insects16070737
APA StyleMendoza-Alatorre, M., Julian-Chávez, B., Solano-Ornelas, S., Siqueiros-Cendón, T. S., Torres-Castillo, J. A., Sinagawa-García, S. R., Abraham-Juárez, M. J., González-Barriga, C. D., Rascón-Cruz, Q., Siañez-Estrada, L. I., & Espinoza-Sánchez, E. A. (2025). RNAi in Pest Control: Critical Factors Affecting dsRNA Efficacy. Insects, 16(7), 737. https://doi.org/10.3390/insects16070737







