1. Introduction
The fall armyworm,
Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae), is native to tropical and subtropical regions in the Americas. In December 2018, the FAW was first detected in Yunnan Province, China [
1], and quickly spread to 26 provinces, autonomous regions, and municipalities, posing a serious economic threat to China’s agricultural production [
2]. The FAW is an obligatory migratory insect with a strong long-distance migration ability, capable of moving hundreds of kilometers between different regions and host plants. It can exist continuously throughout the year in the tropical and subtropical regions of southern China [
3]; however, when the temperature rises in spring, it migrates seasonally to the north with the East Asian and Indian monsoons [
4]. Due to its lack of diapause capacity and sensitivity to cold, it cannot survive at extremely low temperatures and returns south after autumn [
5].
The FAW is a herbivorous pest with a broad host range, including 353 host plant species across 76 plant families, predominantly Gramineae, followed by Compositae and Leguminae [
6]. The FAW evolved into two distinct haplotypes in the Americas, with one type primarily utilizing maize (
Zea mays L.), sorghum [
Sorghum bicolor (L.)], and cotton (
Gossypium hirsutum L.), while the other type utilizes rice (
Oryza sativa L.) and various forage crops [
7,
8]. Based on
COI and
Tpi gene analysis, the invasive Chinese population was identified as the maize type [
9]. At the end of 2018, there were 3609 authorized maize cultivars in China, with a planting area exceeding 41 million hectares in 2019 [
10]. A total of 1.125 million hectares of crops in China were devastated by the FAW in 2019, resulting in economic losses for the country’s maize production ranging from USD 5.4 to 47 billion annually [
11,
12]. The FAW prefers maize as its host plant, causing damage at any stage, particularly the young leaves and growth points. Adults prefer maize for oviposition, and their larvae have significantly higher hatching and survival rates than on other plants [
2].
Host selection by phytophagous insects is a complex process that involves multiple factors. However, many insects tend to prefer natal plant species (on which they have developed) when selecting plants for feeding or oviposition [
13]. Hopkins’ host selection principle (HHSP) states that environmental cues experienced by insects during their early stages influence their behavioral choices in later developmental phases [
14]. For example, the feeding preferences of
Perina nuda larvae are influenced by prior feeding experience, particularly when encountering different host choices [
15]. Insects’ prior experiences with host plants can modify their subsequent feeding and oviposition behaviors through learning [
16,
17]. However, these studies primarily focused on short-term trials (specific developmental stages or a single generation) to explore the effects of host experience on insect preferences. Whether learned behaviors emerge after multiple generations of continuous feeding on the same host, or whether such prolonged feeding experiences induce preference-driven shifts in host selection behavior, has often been overlooked.
Host plant species are an important factor affecting insect population growth and outbreaks. The relative adaptability of FAW populations to different host plants may result in different local population dynamics, and the longevity, fecundity, and survival rates may vary with the host plant on which the larvae feed. The effects of various host plants on FAW fitness and population parameters are detailed in Acharya et al., Li et al., and Lu et al. [
18,
19,
20]. According to various studies, there is a growing distinction in growth and development patterns among successive generations as the number of generations increases [
21,
22]. However, many experiments only show comparisons between the first generation on different host plants or between different generations feeding on the same variety. Nevertheless, the influence of different maize cultivars on the host plant preference (larval feeding and adult oviposition) and developmental parameters (such as stage-specific lifespan, pupal weight, survival rate, and fecundity) of multiple generations of FAW populations has not been adequately addressed. Therefore, it is necessary to conduct studies on multiple generations of FAW populations to reflect the fitness of the FAW on different cultivars and the different traits among the cultivars. This will result in more precise long-term population predictions and will provide critical data for long-term FAW management.
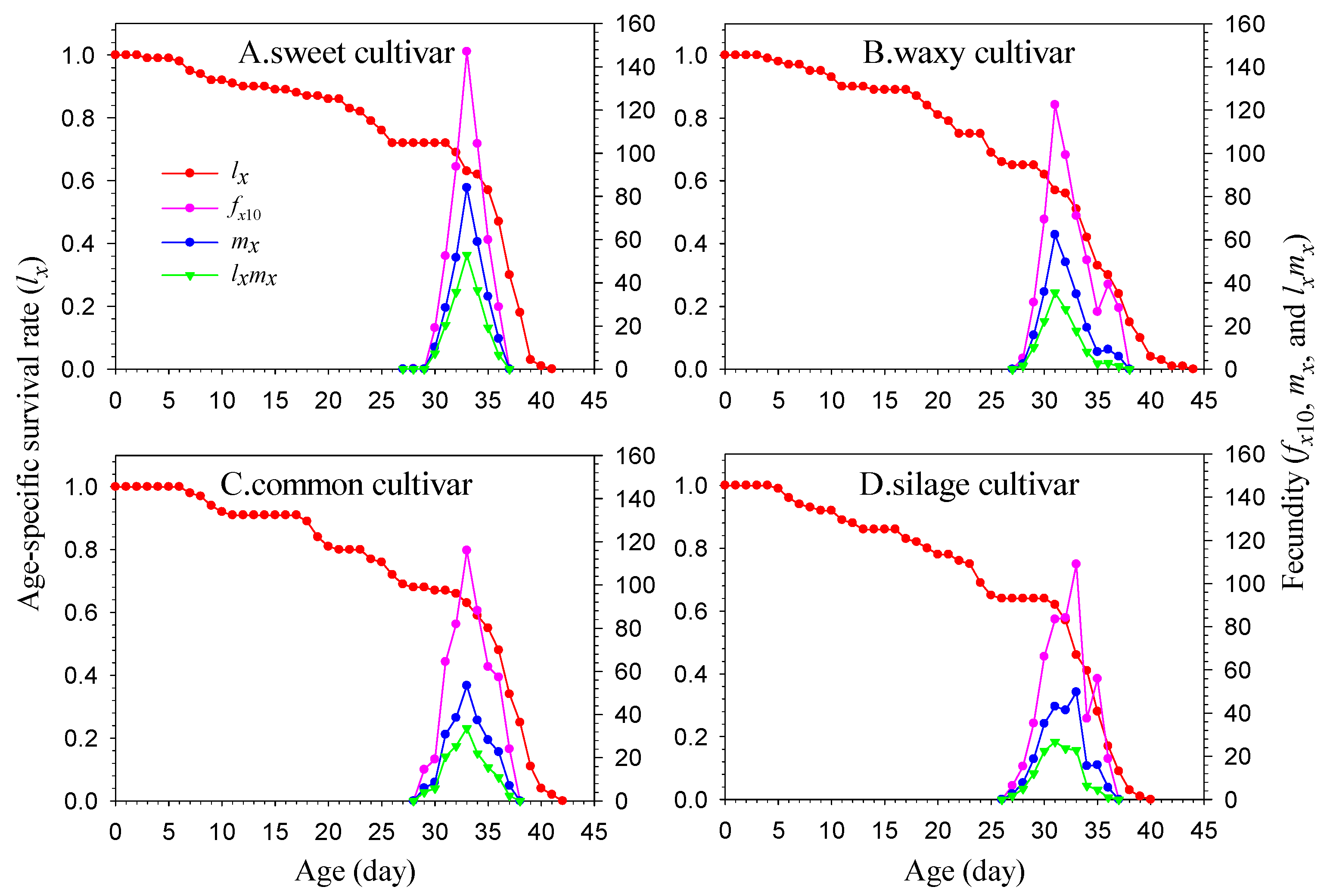
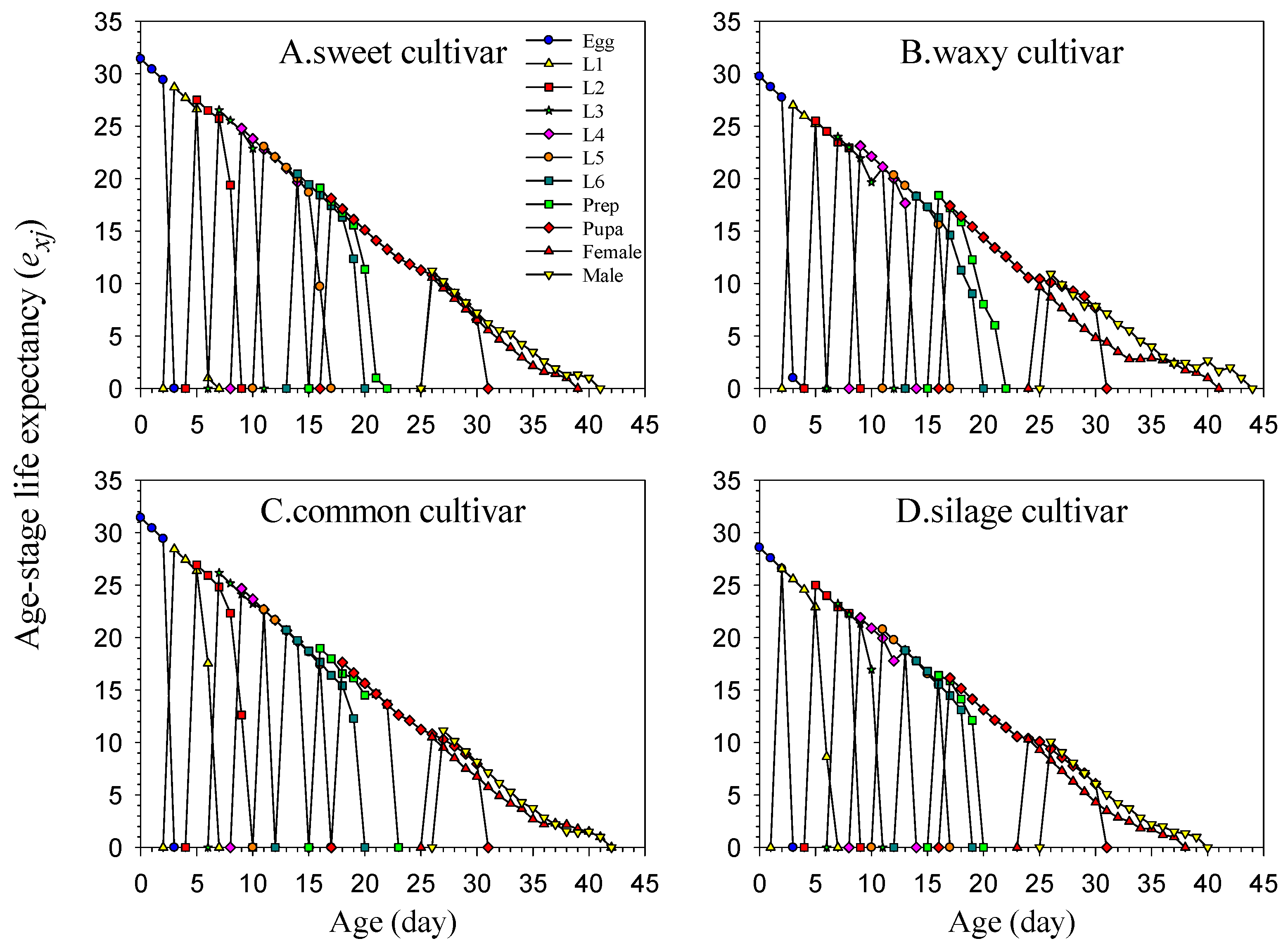
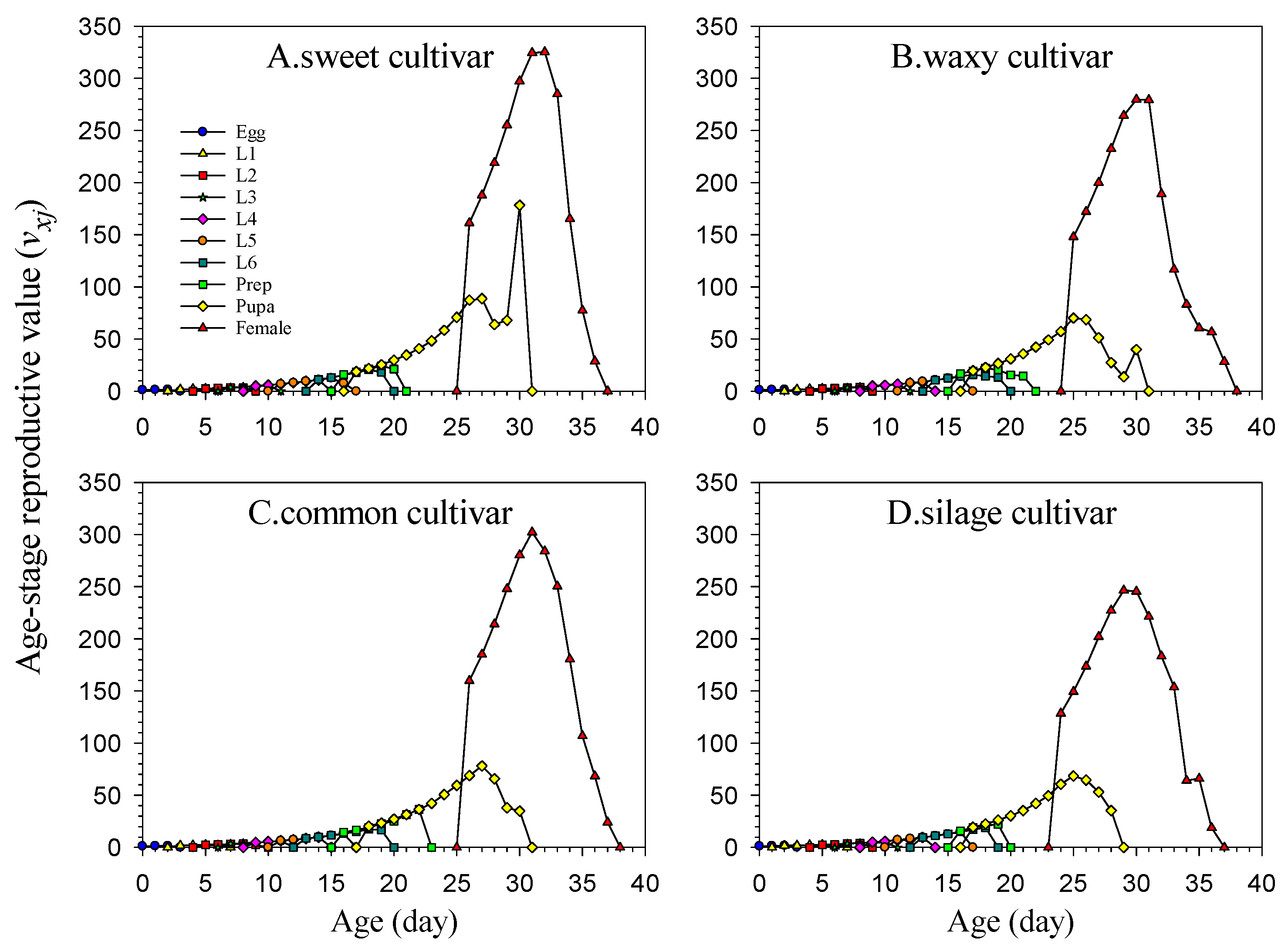
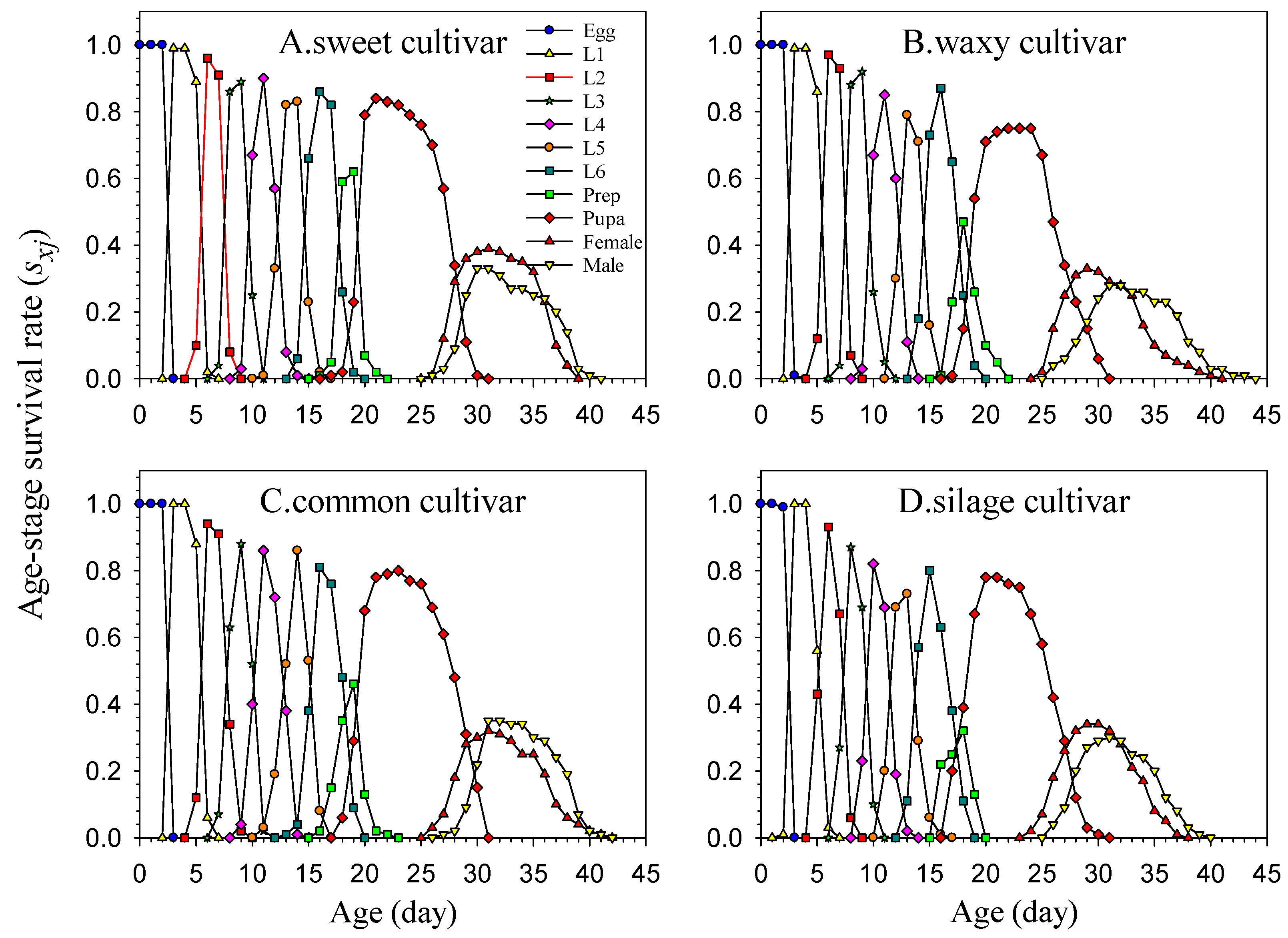
The age-stage, two-sex life table provides a comprehensive and accurate description of the performance of insect populations under specific experimental conditions [
23]. Under laboratory conditions, the age-stage, two-sex life table method was used to analyze the FAW population that feeds on various maize cultivars and has been reared for multiple generations. The F3 generation population’s growth and development indexes (including developmental duration, pupation rate, eclosion rate, and fecundity in the adult stage) on various maize cultivars will be assessed and compared to determine their relative fitness. These research findings offer a basis for making informed decisions on planting various maize cultivars and supply fundamental knowledge for comprehensive FAW management.
2. Materials and Methods
2.1. Insect
The experimental insects were raised in an artificial climate box at a temperature of 25 ± 1 °C, relative humidity of 65 ± 5%, photoperiod of 16 L: 8 D, and light intensity of 12,000 lux (No. PRX-450C; Ningbo Jiangnan Instrument Factory, Ningbo, China).
Newly hatched larvae were introduced into a plastic insect-rearing box with a calligraphy brush and fed artificial diet (
Table A1 for formula component and quantity) [
24]. The first- to third-instar larvae were fed in groups, while the fourth- to sixth-instar larvae were individually fed in round plastic boxes (25 mL, bottom diameter 3 cm, top diameter 4 cm, height 3.5 cm). After the larvae pupated, the pupae were collected in a round plastic box covered with sand until emergence, and newly emerged adults were placed in an insect cage (35 cm × 35 cm × 35 cm) for mating. Adults were provisioned with a 15% honey solution, and eggs were collected and placed in a ziplock bag for incubation and hatching.
Insect-rearing boxes, each containing different cultivar maize leaves (sweet, waxy, ordinary, or silage), were provided to newly hatched FAW larvae that had been fed an artificial diet. The larvae of the F1 and F2 generations were reared in groups in the insect box before the third instar and individually reared in a plastic box after the third instar. The FAWs of generation F3 were reared individually during the larval and pupal stages, and a bisexual life table was created. They were observed every 24 h, the insect boxes were cleaned, and leaves were replaced.
2.2. Host Plants
The tested maize seeds were sweet cultivar (cv. Jinchaotian), waxy cultivar (cv. Jinxiannuo 6), silage cultivar (cv. Tongli 8), and common cultivar (cv. Dafeng 30). Four different cultivars of maize seeds were planted in plastic pots (50 cm × 35 cm × 25 cm), and substrate nutrient soil was used for planting. The sowing depth was 5 cm, and the planting density was 50 plants per pot. The maize plants were cultivated under natural outdoor lighting conditions. Irrigation was performed at 2- to 3-day intervals. After the maize had grown to the 5-leaf stage, young leaves were cut for feeding and testing. None of the host plants for the test were exposed to any pesticides.
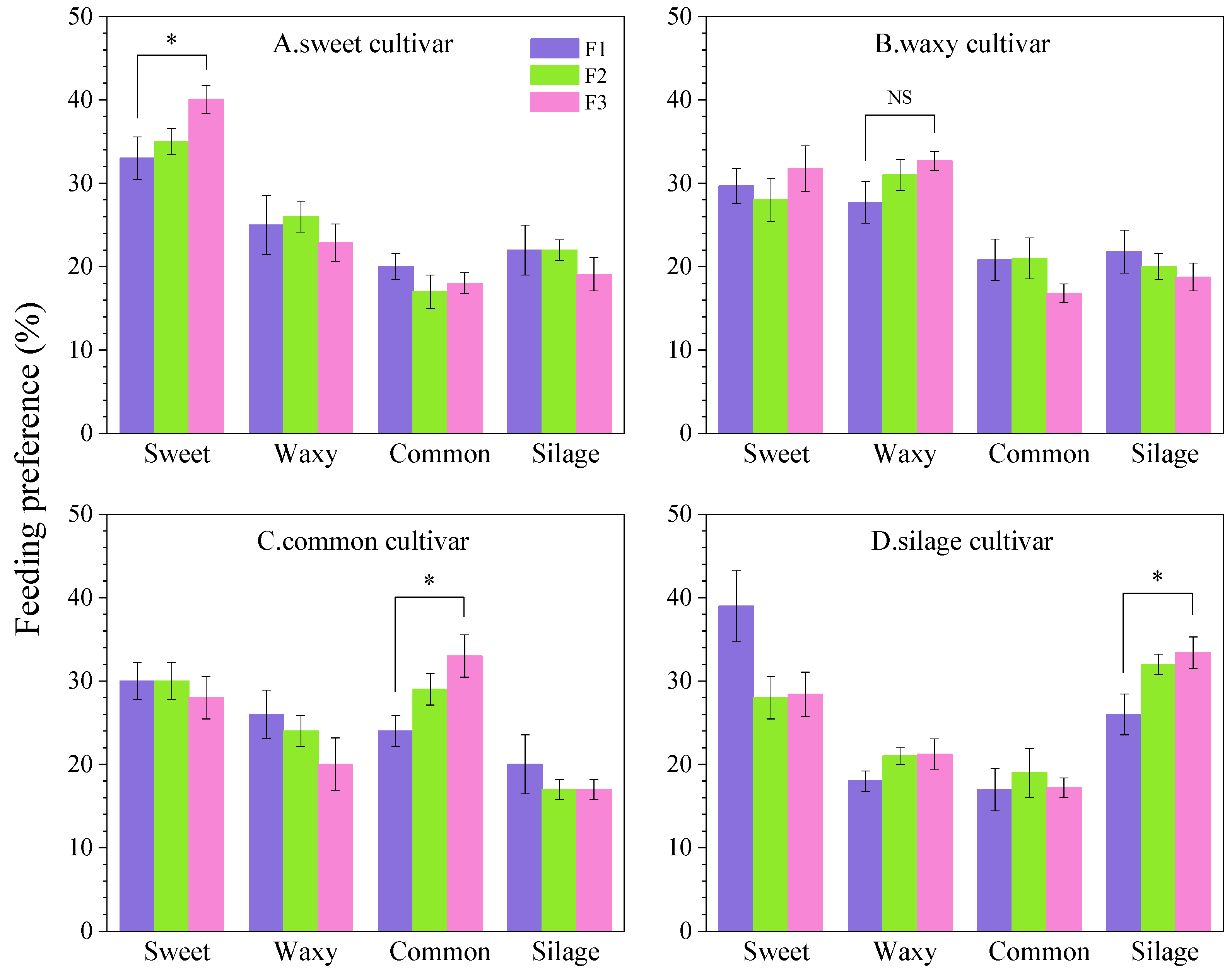
2.3. Determination of Spodoptera frugiperda Oviposition Preference
An oviposition preference assay was conducted using a cage experiment to evaluate the oviposition preferences of female fall armyworm adults on four maize varieties. Maize plants of the four varieties at the three-leaf stage with similar plant heights and leaf sizes were randomly arranged at the four corners of a mesh cage (40 cm × 40 cm × 40 cm, 200-mesh). A plastic Petri dish filled with cotton moistened with a 15% honey solution was placed at the center of the cage. Eight newly emerged adult pairs (1:1 sex ratio) were introduced into the cage and allowed to mate freely. The number of eggs deposited on each variety was recorded 48 h post-introduction, with data collected continuously for 5 days across three experimental replicates. Plants were watered daily, and the honey solution was replenished as needed. The formula for calculating the egg attachment rate was as follows: (number of eggs on a single maize variety/total number of eggs across the four maize varieties) × 100%. The entire experiment was conducted in a climate-controlled greenhouse at a temperature of 25 ± 1 °C, relative humidity of 65 ± 5%, and a photoperiod of 16 L: 8 D.
2.4. Determination of Spodoptera frugiperda Feeding Preference
Feeding preference was assessed using the leaf disk assay. Plastic Petri dishes (15 cm diameter) lined with filter paper were divided into four equal sections, each containing maize leaf segments of equal weight that were moistened to maintain hydration. Ten second-instar larvae starved for 6 h prior to the assay were placed at the center of each dish. The experiment included ten replicates per treatment. The number of larvae feeding on each variety was recorded after 24 h to calculate the feeding selection rates. The experimental conditions were identical to those described in the
Section 2.1.
2.5. Construction of Life Tables of Spodoptera frugiperda
Using the laboratory population life table method, we evaluated the effects of continuous multi-generation rearing on different maize cultivars on the growth, development, and reproduction of FAWs. F3 generation eggs that were continuously reared for multiple generations with different maize cultivars were used as the test insects. After hatching, the leaves of the four different maize cultivars were used to feed the larvae. Newly hatched larvae were placed in plastic boxes for individual rearing. In the life table study, a total of 100 newly hatched larvae were used for each maize cultivar, each larva as a replicate. Maize leaves were replaced every day, and larval survival, instar, pupation, eclosion, and other parameters were recorded daily. The pupal weight was measured on the second day after pupation. After emergence, adults of the same day (one female and one male) were placed in a transparent plastic cup (250 mL, with a bottom diameter of 5 cm, a top diameter of 7.5 cm, and a height of 7 cm) for 1:1 pairing. The plastic cup was sealed with double-layer degreasing gauze for female adults to lay eggs. A cotton ball soaked with 15% honey water was hung in the plastic cup for adults to replenish nutrition and was replaced daily. On the second day after mating, egg laying by the adults in the plastic cup was observed and recorded. The eggs were collected daily until the adults died, and the lifespans of male and female adults were recorded. The entire test was conducted in an artificial climate box with a temperature of (25 ± 1) °C, relative humidity of (65 ± 5) %, and photoperiod of 16 L: 8 D.
2.6. Life Table Data Analysis
The data for the experiment were collected and analyzed using the TWOSEX-MS Chart 2022 software [
25] (available at
http://lifetablechi.com/software/, accessed on 9 January 2023). The age-stage-specific survival rate (
sxj), age-specific survival rate (
lx), age-stage-specific fecundity (
fxj), age-specific fecundity (
mx), age-specific maternity (
lxmx), net reproductive rate (
R0), intrinsic rate of increase (
r), finite rate of increase (
λ), mean generation time (
T), age-stage-specific life expectancy (
exj), age-stage-specific reproductive value (
vxj), and other analytical data were processed and plotted [
26,
27]. All definitions, equations, and references are listed in
Table A2.
The mean and standard errors of each parameter were calculated by using the bootstrap method supported by the TWOSEX-MS Chart software (with 100,000 bootstraps), and then the paired bootstrap test was used to conduct significance analysis of differences in the data [
28].
2.7. Data Processing and Mapping
The original data source was recorded and organized using Excel 2022, and then the original data source was analyzed using the TWOSEX-MS Chart software and plotted using SigmaPlot 15.0 (SigmaPlot Software, San Rafael, CA, USA). Normality of feeding and oviposition preferences were measured using the Kolmogorov–Smirnov test in SPSS v.27.0, then one-way analysis of variance (significance level set at 0.05) was performed, followed by Duncan test comparisons to determine significant differences. Percentage data were converted to the square root of the arcsine before one-way analysis of variance (ANOVA) to meet the requirements of ANOVA.
4. Discussion
The results of this study revealed that F1 generation fall armyworm populations reared on the four maize cultivars exhibited larval and adult preferences for sweet maize. Relevant studies have shown that substrate characteristics, such as sweetness and hardness, influenced oviposition choices, with a positive correlation observed between oviposition preference and glucosinolate content in host plants, which may also explain the fall armyworm’s preference for sweet maize [
29,
30]. With successive generations, the preference hierarchy of the FAW for the four maize cultivars changed significantly and consistently as larvae and adults gradually increased their feeding and oviposition activities on their original host plants. This indicated that the FAW had learning behavior, and feeding experience could induce preference-driven host selection, aligning with Hopkins’ host selection principle (HHSP). Female onion flies (
Delia antiqua) exhibit a strong preference and deposit more eggs on their original host plant [
13]. Conversely, when female tobacco hornworms (
Manduca sexta L.) completed a single oviposition event on one of two host plants (
Datura wrightii or
Nicotiana attenuata), they immediately selected the previously experienced plant foliage when re-exposed to both options, demonstrating that a single oviposition event can drive subsequent oviposition preference changes [
31].
Host plants play an important role in the growth, development, and reproduction of insects, and suitable hosts can improve the growth rate, survival rate, and fecundity of insect offspring [
32]. This experiment studied the effects of feeding on different maize cultivars on the growth, development, and reproduction of successive generations of FAW until the F3 generation. These results showed significant effects on the developmental duration of each insect stage, pupal weight, survival rate, and oviposition of the FAW population reared on the different maize cultivars. FAWs feeding on six rice cultivars resulted in significant differences in larval development duration, pupal duration, pupation rate, adult lifespan, and egg production [
33]. These results showed that the larval stage and adult lifespan of FAWs reared on sweet maize were both longer than those reared on waxy maize [
34]. Similarly, Zhang et al. indicated that special maize cultivars are more suitable for the growth and development of FAWs than ordinary maize cultivars [
35]. Compared to common maize cultivars, these populations have stronger adaptability to waxy maize, which is consistent with the results of our study. However, the larval stage and adult lifespan of FAWs feeding on waxy maize are longer than those on common maize, which is contrary to the results of this study, and might be caused by differences in cultivars or rearing patterns [
10,
36]. Generally, when an insect on a certain host plant has higher fitness, it will have higher rates of development, survival, and reproduction. Although the growth and development speed are faster when the FAW feeds on wheat (
Triticum aestivum L.) than on maize, its food utilization efficiency and population reproduction ability were all lower [
37], which is similar to our results showing that the larval development duration of the silage maize population was the shortest, but the survival, pupation, and eclosion rates were the lowest.
The state of the pupa reflects the adaptability of the larva to a particular host or environment, and the weight of the pupae reflects the insect’s appetite for the host plant [
38]. The pupal weight and fecundity of female lepidopteran adults are positively correlated with their adaptive potential [
35]. The results of our study showed that the pupal weight of the FAW differed significantly among the four maize cultivars (sweet maize > common maize > waxy maize > silage maize). Meanwhile, the larval survival rate, pupal weight, and egg production of female adults of FAWs fed sweet maize were significantly higher than those fed waxy maize [
34]. Additionally, the pupal weight and egg production of FAWs fed on common maize were both higher than those of waxy maize [
10]. These results are consistent with those of our study. The female pupal weight on special maize cultivars was higher than that of common maize cultivars [
35]; however, in our study, the pupal weight of waxy maize was lower than that of common maize, which may be related to the nutritional quality or resistance among different maize cultivars. Other relevant studies have shown that the change in pupal weight may be related to the amino acid content among different cultivars. Cultivars with strong resistance generally have higher glutamic acid content and lower tyrosine content, and the resulting pupal weight is lighter [
39].
The biological parameters
R0,
r,
λ, and
T indicate the growth, development, reproduction, and survival changes in insects and the population growth ability in a specific environment [
40]. There were significant differences in the population parameters of FAWs among different maize cultivars. Our results showed that the
R0,
r, and
λ values were highest on sweet maize. Similarly, the intrinsic rate of increase and net reproductive rate of FAWs feeding on sweet maize were also higher than those on waxy maize [
34]. In contrast, Zhang et al. reported that the net reproductive rate and intrinsic rate of growth were waxy maize > sweet maize > common maize, which was different from the results of our study [
35]. This may be caused by different generations of FAW on the same host.
FAWs feeding on plants with secondary substances that are different from hosts for multiple generations will cause changes in the activities of some enzymes in the insects and have an impact on the growth and development of larvae [
41]. When the FAW feeds on
Vicia villosa Roth, as the number of generations increases, its adaptability gradually decreases until it cannot complete a subsequent generation or maintain its population at the fourth generation [
22]. When returned to the original host maize, the offspring displayed reduced performance and loss of adaptation to their host. Although Bt cotton had a negative impact on
Spodoptera exigua Hübner for three continuous generations, the survival rate and fecundity of adults increased significantly, and the lipase and trypsin activities of the third generation were significantly lower than those of the first generation; however, the activities of carboxylesterase and acetylcholinesterase were significantly higher than those of the first generation [
42]. This indicates that the enhancement of its adaptability may be closely related to the enhancement of detoxifying enzyme activities rather than digestive enzymes. Therefore, further verification is needed in the next research. Similarly, the pupal weight, survival rate, fecundity, relative growth rate, and relative digestion rate of the third generation were all significantly higher than those of the first generation when the larvae of
Helicoverpa armigera Hübner fed on high-gossypol cultivars [
21]. The developmental duration was prolonged with an increase in successive rearing generations when the guava fruit fly (
Bactrocera correcta Bezzi) was continuously reared indoors on an artificial diet [
43]. After being fed rape pollen, the F1 and F2 generations of
Micraspis discolor showed a lower survival rate and female ratio, but the F3 and F4 generations had higher survival rates, female ratios, and weights, suggesting that
M. discolor gradually adapted to the pollen [
44].
Developmental duration is critically important for insect survival, as prolonged exposure to natural environments elevates the risk of biotic (e.g., pathogens and natural enemies) and abiotic (e.g., natural disasters and adverse environmental conditions) stressors, which may pose critical threats to their viability. Because the life table of the F1 generation of FAWs has already been studied in our previous experiments, we measured the parameters of the F3 generation in our study. By comparing our results with those of the F1 generation, it can be seen that the developmental duration of the F3 generation of FAWs was shortened significantly [
45]. This indicates that the FAW can adapt to diverse plant species through evolution, shortened developmental duration, and mitigated adverse factors affecting its development, thereby enhancing its adaptability to different ecological regions.
In conclusion, since FAWs are a type of polyphagous pest that can damage different crops and cultivars within a certain area, at the same time, long-term cultivation of a single plant or cultivar may also aggravate the damage situation of FAWs. Therefore, it is necessary to implement habitat regulation technology such as intercropping instead of a single crop or variety in the field, or plant protective row plants at the edge of the field, to reduce the possibility of multiple generations of FAWs completing their development on the same plant. These strategies will reduce the loss of crop production due to the presence of FAWs.