What Is the Relationship Between Efficacy of Seed Treatment with Insecticides Against Dalbulus maidis (Delong and Wolcott) (Hemiptera: Cicadellidae) Healthy and Infected with Spiroplasm in the Corn Stunt Control?
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Maize Seeds, Insecticides, and Experiments
2.3. Statistical Analysis
Analysis of Mortality of Healthy and Infective Leafhoppers
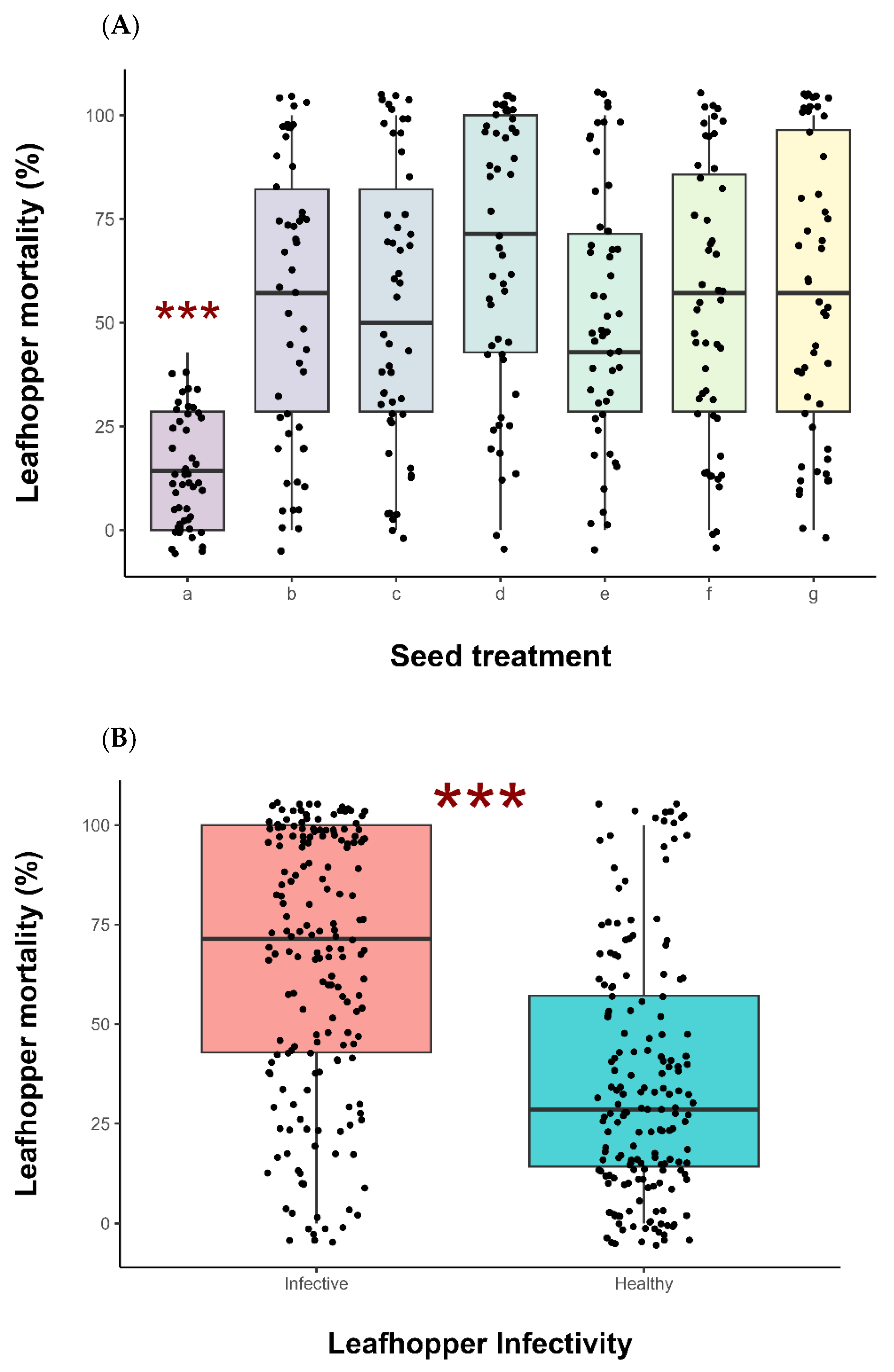
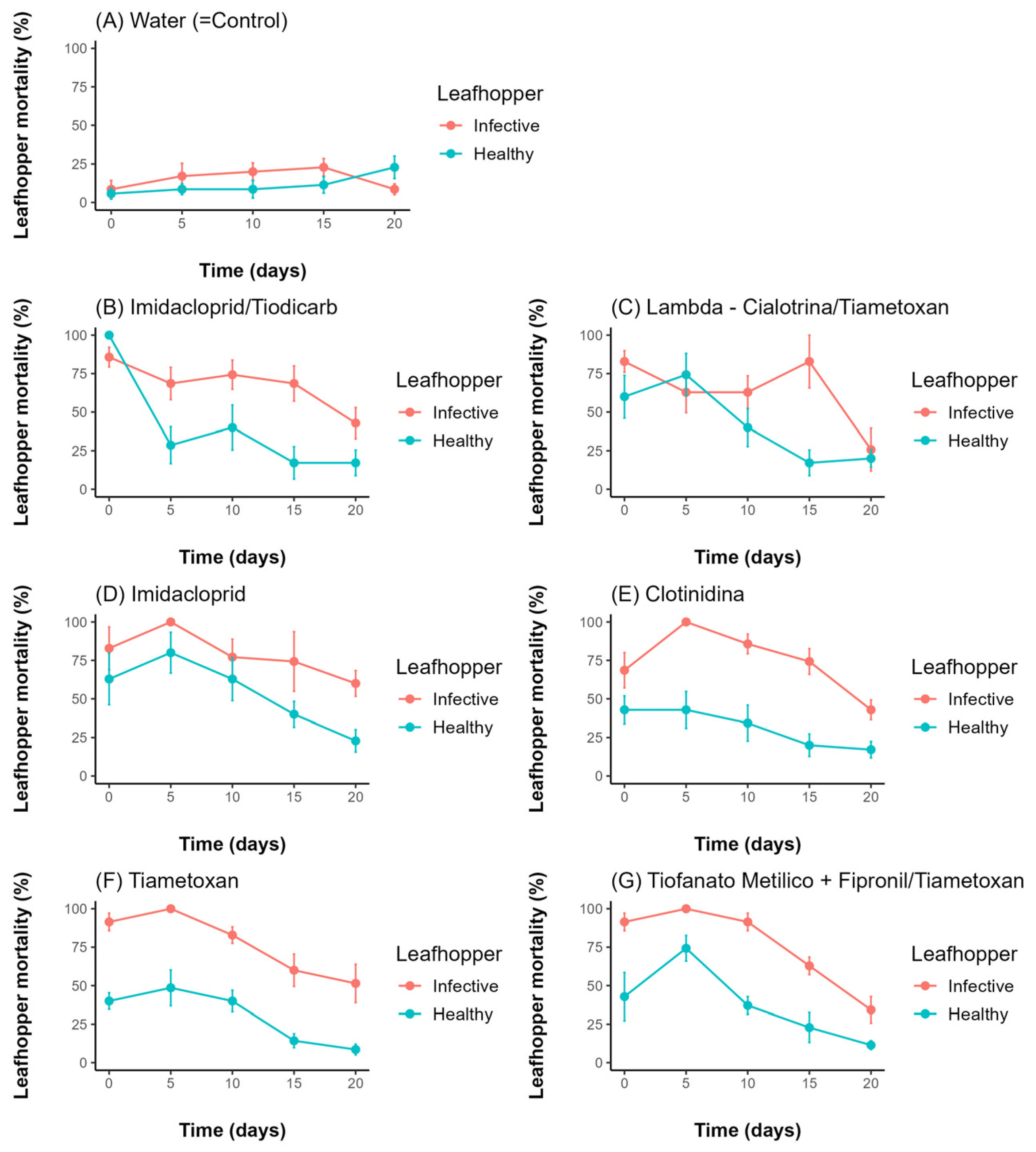
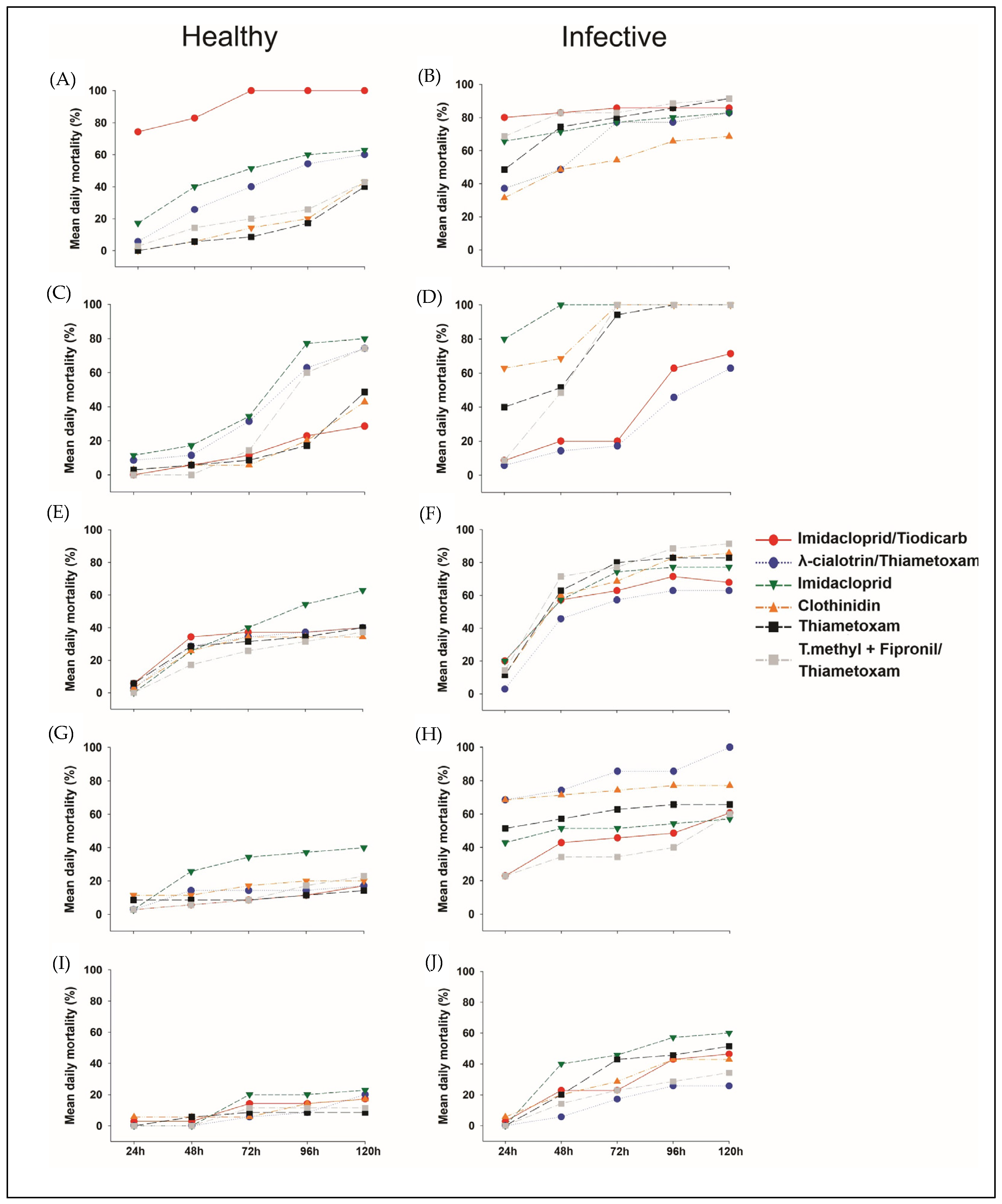
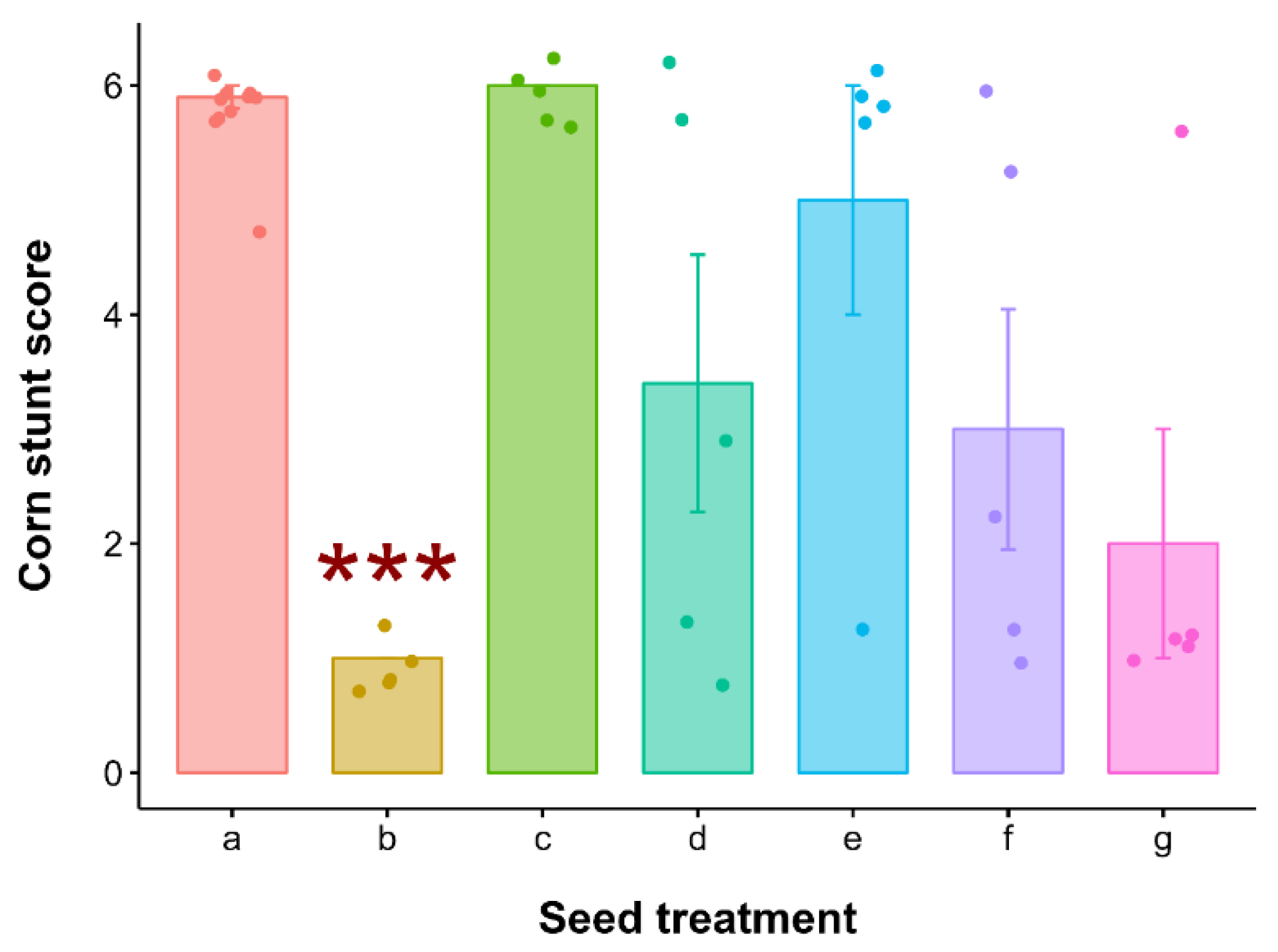
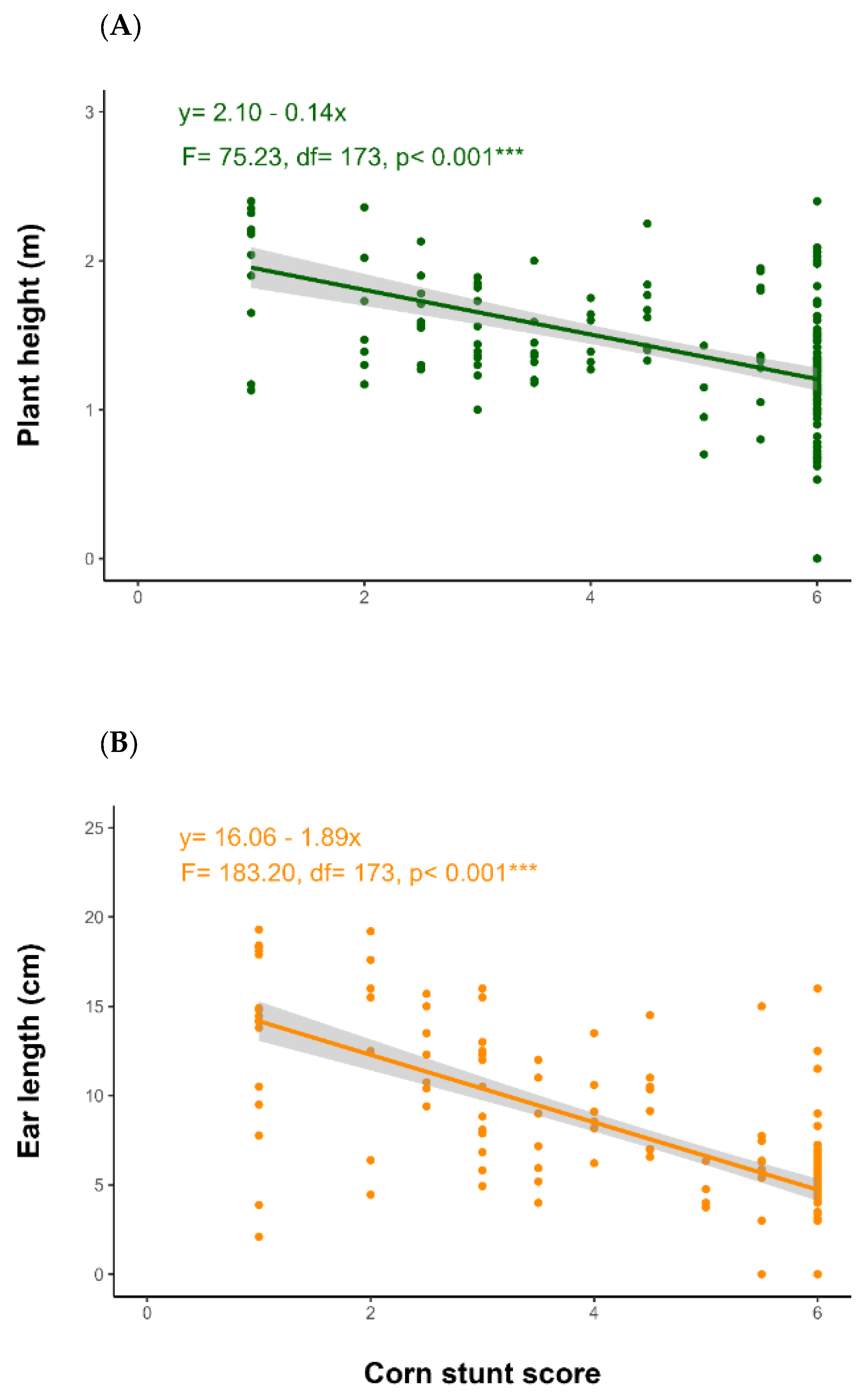
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Virla, E.G. Dalbulus maidis (Hemiptera: Cicadellidae), vetor del “achaparramiento o raquitismo” del maíz. Aspectos biológicos más relevantes, com especial referência a los conocimientos generados em Argentina. Miscelanea 2024, 152, 50. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Sabato, E.O.; Barros, B.A. Natural infectivity of Dalbulus maidis populations and incidence of corn stunt and virus diseases in maize over time. Trop. Plant Path. 2023, 48, 575–580. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Lopes, J.R.S. Técnicas para criação da cigarrinha-do-milho e inoculação de molicutes e vírus em planta. In Doenças em Milho: Molicutes, Vírus, Vetores, Mancha por Phaeosphaeria. Brasília, DF: Embrapa Informação Tecnológica; de Oliveira, E., de Oliveira, C.M., Eds.; Embrapa Milho e Sorgo: Sete Lagoas, Brazil, 2004; pp. 89–116. [Google Scholar]
- Madden, L.V.; Nault, L.R.; Heady, S.E.; Styer, W.E. Effect of maize stunting mollicutes on survival and fecundity of Dalbulus leafhopper vectors. Ann. App. Biol. 1984, 105, 431–441. [Google Scholar] [CrossRef]
- Jones, T.L.; Medina, R.F. Corn Stunt Disease: An Ideal Insect–Microbial–Plant Pathosystem for Comprehensive Studies of Vector-Borne Plant. Dis. Corn. Plants. 2020, 9, 747–763. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Frizzas, M.R. Eight decades of Dalbulus maidis (DeLong & Wolcott) (Hemiptera, Cicadellidae) in Brazil: What we know and what we need to know. Neot. Entomol. 2021, 51, 1–17. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Sabato, E.O. (Eds.) Diseases in Maize: Insect Vectors, Mollicutes and Viruses; Embrapa: Brasília, Brazil, 2017; 278p. [Google Scholar]
- Ebbert, M.A.; Lowell, R. Nault. Survival in Dalbulus leafhopper vectors improves after exposure to maize stunting pathogens. Entomol. Exp. App. 2001, 100, 311–324. [Google Scholar] [CrossRef]
- Ebbert, M.A.; Nault, L.R. Improved Overwintering Ability in Dalbulus maidis (Homoptera: Cicadellidae) Vectors Infected with (Mycoplasmatales: Spiroplasmataceae). Environ. Entomol. 1994, 23, 634–644. [Google Scholar] [CrossRef]
- Christensen, M.N.; Axelsen, K.B.; Nicholaisen, M.; Schulz, A. Phytoplasmas and their interactions with hosts. Trends in Plant Sci. 2005, 10, 526–535. [Google Scholar] [CrossRef]
- Gonzales, V.; Gámez, R. Algunos factores que afectam la transmisión del virus del rayado fino del maíz por Dalbulus maidis (DeLong & Wolcott). Turrialba 1974, 24, 51–57. [Google Scholar]
- Alivizatos, A.S.; Markham, P.G. Multiplication of corn stunt spiroplasma in Dalbulus maidis and transmission in vitro, following injection. Ann. Appl. Biol. 1986, 108, 545–554. Available online: https://onlinelibrary.wiley.com/doi/pdf/10.1111/j.1744-7348.1986.tb01993.x (accessed on 26 May 2024).
- Oliveira, C.M.D.; Oliveira, E.D.; Canuto, M.; Cruz, I. Eficiência de inseticidas em tratamento de sementes de milho no controle da cigarrinha Dalbulus maidis (Hemiptera: Cicadellidae) em viveiro telado. Cienc. Rural 2008, 38, 231–235. [Google Scholar] [CrossRef]
- Agrofit: Sistema de Agrotóxicos Fitossanitários; MAPA: Brasília, DF, Brazil, 2024. Available online: http://agrofit.agricultura.gov.br/agrofit_cons/principal_agrofit_cons (accessed on 12 May 2024).
- Meneses, A.R.; Querino, R.B.; Oliveira, C.M.; Maia, A.H.N.; Silva, P.R.R. Seasonal and vertical distribution of Dalbulus maidis (Hemiptera: Cicadellidae) in Brazilian corn fields. Fla. Entomol. 2016, 99, 750–754. [Google Scholar] [CrossRef]
- Virla, E.G.; Coll Araoz, M.V.; Luft-Albarracin, E. Estimation of direct damage to maize seedlings by the corn leafhopper, Dalbulus maidis (Hemiptera: Cicadellidae), under different watering regimes. Bull. Entomol. Res. 2021, 111, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, S.; Hanway, J.J. How a Corn Plantdevelops; Special Report; Iowa State University of Science and Technology, Cooperative Extension Service: Ames, IA, USA, 1989; Volume 48, 24p. Available online: https://publications.iowa.gov/id/eprint/18027 (accessed on 25 June 2025).
- Silva, R.G.; Galvão, J.C.C.; Miranda, G.V.; Oliveira, E. Controle genético da resistência aos enfezamentos do milho. Pesqui. Agropecu. Bras. 2003, 38, 921–928. [Google Scholar] [CrossRef]
- Saghai-Maroof, M.A.; Soliman, K.M.; Jorgensen, R.A.; Allard, R. Ribosomal DNA spacer-length polymorphisms in barley: Mendelian inheritance, chromosomal location, and population dynamics. Proc. Natl. Acad. Sci. USA 1984, 81, 8014–8018. [Google Scholar] [CrossRef]
- Sousa, S.M.; Barros, B.A. Detecção molecular de molicutes em milho In Doenças em Milho: Insetos-Vetores Molicutes e Virus; Oliveira, C.M., Sabato, E.O., Eds.; Embrapa: Brasília, Brazil, 2017; pp. 25–34. [Google Scholar]
- Barros, T.S.L.; Davis, R.E.; Resende, R.O.; Dally, E.L. Design of a polymerase chain reaction for specific detection of corn stunt spiroplasma. Plant Dis. 2001, 85, 475–480. [Google Scholar] [CrossRef]
- R Development Core Team R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Gandrud, C. Reproducible Research with R and RStudio, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2020; 276p. [Google Scholar]
- Machado, E.P.; Souza, E.V.; Dias, G.S.; Sacilotto, M.G.; Omoto, C. Is insecticide resistance a factor contributing to the increasing problems with Dalbulus maidis (Hemiptera: Cicadellidae) in Brazil? Pest Manag. Sci. 2024, 80, 5120–5130. [Google Scholar] [CrossRef]
- Alves, A.P.; Parody, B.; Barbosa, C.M.; de Oliveira, C.M.; Sachs, C.; Sabato, E.d.O.; Gava, F.; Daniel, H.; de Oliveira, I.R.; Foresti, J.; et al. Guia de Boas Práticas para o Manejo dos Enfezamentos e da Cigarrinha-do-Milho. Folhetos Embrapa Cerrados. 2020. 34p. Available online: https://www.embrapa.br/busca-de-publicacoes/-/publicacao/1129511/guia-de-boas-praticas-para-o-manejo-dos-enfezamentos-e-da-cigarrinha-do-milho (accessed on 30 July 2024).
- Cota, L.V.; Oliveira, I.R.; Silva, D.D.; Mendes, S.M.; Costa, R.V.; Souza, I.R.P.; Silva, A.F. Manejo da Cigarrinha e Enfezamentos na Cultura do Milho. Cartilha Embrapa Milho e Sorgo. 2021. 16p. Available online: https://www.infoteca.cnptia.embrapa.br/infoteca/bitstream/doc/1130346/1/Cartilha-Manejo-cigarrinha-enfezamentos-milho.pdf (accessed on 25 July 2024).
- Oliveira, C.M.; Oliveira, E.; Canuto, M.; Cruz, I. Chemical control of corn leafhopper and incidence of corn stunting diseases caused by mollicutes. Pesqui. Agropecu. Bras. 2007, 42, 297–303. (In Portuguese) [Google Scholar] [CrossRef]
- Neves, T.N.C.; Josemar, F.; Silva, P.R.; Alves, E.; Rocha, R.; Oliveira, C.; Picanço, M.C.; Pereira, E.J.G. Insecticide seed treatment against corn leafhopper: Helping protect grain yield in critical plant growth stages. Pest Manag. Sci. 2021, 78, 1482–1491. [Google Scholar] [CrossRef]
- Xavier, J.M.; Tonello, A.P. Treatments to control the corn scarlet (Daubulus maidis): A literature review. J. Exact Sci. 2023, 39, 36–41. [Google Scholar]
- Martins, G.; Toscano, C.; Tomquelski, G.; Maruyama, W.I. Eficiência de inseticidas no controle de Dalbulus maidis (Hemiptera: Cicadellidae) na cultura do milho. Rev. Caatinga 2008, 21, 196–200. [Google Scholar]
- Ruegger, D.G. Efeito de Inseticidas Sobre Duas Populações da Cigarrinha-do-Milho, Dalbulus maidis (DeLong & Wolcott) (Hemiptera: Cicadellidae). (2019). Trabalho de Conclusão de Curso—Escola Superior de Agricultura Luiz de Queiroz, Universidade de São Paulo, Piracicaba, 2019. Available online: https://bdta.abcd.usp.br/item/003171602 (accessed on 23 July 2024).
- Ozbek, E.; Miller, S.A.; Meulia, T.; Hogenhout, S.A. Infection and replication of Spiroplasma Kunkelii (Class: Mollicutes) in midgut and Malpighian tubules of the leafhopper Dalbulus maidis. J. Invert. Path. 2003, 82, 167–175. [Google Scholar] [CrossRef]
- Kwon, M.O.; Wayadande, A.C.; Fletcher, J. Spiroplasma citri movement into the intestines and salivary glands of its leafhopper vector, Circulifer tenellus. Phytopathology 2007, 89, 1144–1151. [Google Scholar] [CrossRef]
- Jeschke, P.; Nauen, R.; Schindler, M.; Elbert, A. Overview of the Status and Global Strategy for Neonicotinoid. J. Agric. Food Chem. 2011, 59, 2897–2908. [Google Scholar] [CrossRef]
- Bonmatin, J.M.; Giorio, C.; Girolami, V.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; Long, E.; Marzaro, M.; Mitchell, E.A.; et al. Environmental fate and exposure; neonicotinoids and fipronil. Environ. Sci. Pollut. Res. Int. 2015, 22, 35–67. [Google Scholar] [CrossRef]
- Alford, A.; Krupke, C.H. Correction: Translocation of the neonicotinoid seed treatment clothianidin in maize. PLoS ONE 2017, 12, e0186527. [Google Scholar] [CrossRef]
- Omoto, C. Modo de ação de inseticidas e resistência de insetos a inseticida. In Bases e Técnicas no Manejo de Insetos; Guedes, J.C., Costa, I.D., Castiglioni, E., Eds.; UFSM/CCR/DFS: Santa Maria, CA, USA, 2000; pp. 31–49. [Google Scholar]
- Bhirud, K.M.; Pitre, H.N. Bioactivity of systemic insecticides in corn: Relationship to leafhopper vector control and corn stunt disease incidence. J. Econ. Entomol. 1972, 65, 1134. [Google Scholar] [CrossRef]
- Douglas, M.R.; Tooker, J.F. Large-scale deployment of seed treatments has driven rapid increase in use of neonicotinoid insecticides and preemptive pest management in 15 U.S. field crops. Environ. Sci. Technol. 2015, 49, 5088–5097. [Google Scholar] [CrossRef]
- Hruska, A.J.; Peralta, M.G. Maize response to corn leafhopper (Homoptera: Cicadellidae) infestation and achaparramiento disease. J. Econ. Entomol. 1997, 90, 604–610. [Google Scholar]
- Virla, E.G.; Díaz, C.; Carpane, P.; Laguna, I. Estimación preliminar de la disminución en la producción de maíz causada por el Corn Stunt Spiroplasma (CSS) en Tucumán, Argentina. Bol. Sanid. Veg. Plag. 2004, 30, 257–267. [Google Scholar]
- Sabato, E.O. Corn stunting diseases. In Diseases in Maize: Insect Vetors, Mollicutes and Viruses; Oliveira, C.M., Sabato, E.O., Eds.; Embrapa Informação Tecnológica: Brasília, Brazil; Embrapa Milho e Sorgo: SeteLagoas, Brazil, 2017; pp. 11–23. [Google Scholar]
- Lopes, B.R.; Faria, M.; Oliveira, C.M. Susceptibility of Dalbulus maidis to-pathogenic fungi: Unveiling the protective role of brochosomes and self-cleaning behavior. J. Pest Sci. 2025, 98, 759–768. [Google Scholar] [CrossRef]
- Jeger, M.J. The epidemiology of plant virus disease: Towards a new synthesis. Plants 2020, 9, 1768. [Google Scholar] [CrossRef]
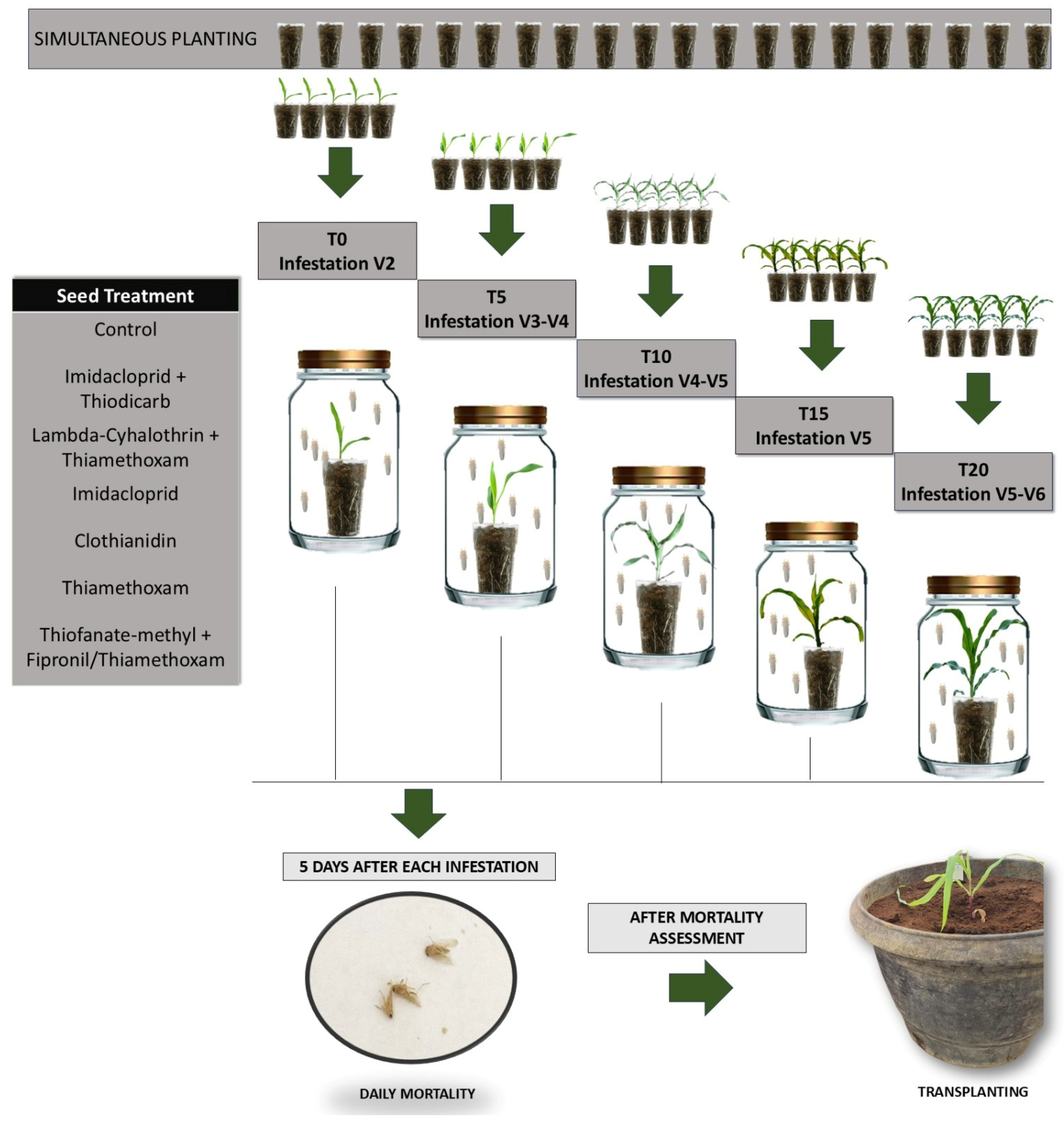
| Trade Name (1) | Active Ingredient | Chemical Group | Concentration (2) | Dose (3) |
|---|---|---|---|---|
| Control | Water | ---- | ----- | ----- |
| Nuprid Star FS | Imidacloprid + tiodicarb | Neonicotinoid + carbamate | 150 + 450 | 1.75 |
| Cruiser Opti | Lambda Cyhalothrin + thiamethoxam | Pyrethroid + neonicotinoid | 37.5 + 210 | 1 |
| Ouro fino | Imidacloprid | Neonicotinoid | 350 | 0.08 |
| Poncho | Clothianidin | Neonicotinoid | 660 | 0.1 |
| Cruiser 600 FS | Tiametoxam | Neonicotinoid | 600 | 0.5 |
| Standak Top*+ Impar BR | Thiophanate methyl + fipronil/thiamethoxam | Pyrethroid + neonicotinoid | 225 + 250/350 | 1/0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Redoan, A.C.M.; Marques, V.M.; Pereira, P.S.; de Oliveira, I.R.; Silva-Araújo, D.D.; Cota, L.V.; Fadini, M.A.M.; Oliveira, C.M.; Rafael, D.D.; Mendes, S. What Is the Relationship Between Efficacy of Seed Treatment with Insecticides Against Dalbulus maidis (Delong and Wolcott) (Hemiptera: Cicadellidae) Healthy and Infected with Spiroplasm in the Corn Stunt Control? Insects 2025, 16, 713. https://doi.org/10.3390/insects16070713
Redoan ACM, Marques VM, Pereira PS, de Oliveira IR, Silva-Araújo DD, Cota LV, Fadini MAM, Oliveira CM, Rafael DD, Mendes S. What Is the Relationship Between Efficacy of Seed Treatment with Insecticides Against Dalbulus maidis (Delong and Wolcott) (Hemiptera: Cicadellidae) Healthy and Infected with Spiroplasm in the Corn Stunt Control? Insects. 2025; 16(7):713. https://doi.org/10.3390/insects16070713
Chicago/Turabian StyleRedoan, Ana Carolina M., Vinicius M. Marques, Poliana S. Pereira, Ivênio R. de Oliveira, Dagma D. Silva-Araújo, Luciano V. Cota, Marcos Antonio M. Fadini, Charles M. Oliveira, Diego D. Rafael, and Simone Mendes. 2025. "What Is the Relationship Between Efficacy of Seed Treatment with Insecticides Against Dalbulus maidis (Delong and Wolcott) (Hemiptera: Cicadellidae) Healthy and Infected with Spiroplasm in the Corn Stunt Control?" Insects 16, no. 7: 713. https://doi.org/10.3390/insects16070713
APA StyleRedoan, A. C. M., Marques, V. M., Pereira, P. S., de Oliveira, I. R., Silva-Araújo, D. D., Cota, L. V., Fadini, M. A. M., Oliveira, C. M., Rafael, D. D., & Mendes, S. (2025). What Is the Relationship Between Efficacy of Seed Treatment with Insecticides Against Dalbulus maidis (Delong and Wolcott) (Hemiptera: Cicadellidae) Healthy and Infected with Spiroplasm in the Corn Stunt Control? Insects, 16(7), 713. https://doi.org/10.3390/insects16070713







