Combination of Chitosan and Essential Oils for Tomatoes Protection Against the Insect Pest Spodoptera littoralis (Lepidoptera: Noctuidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Essential Oils Purchase and Chemical Characterisation
2.2. Chitosan and Essential Oils-Enriched Chitosan Solutions
2.3. Insects Rearing
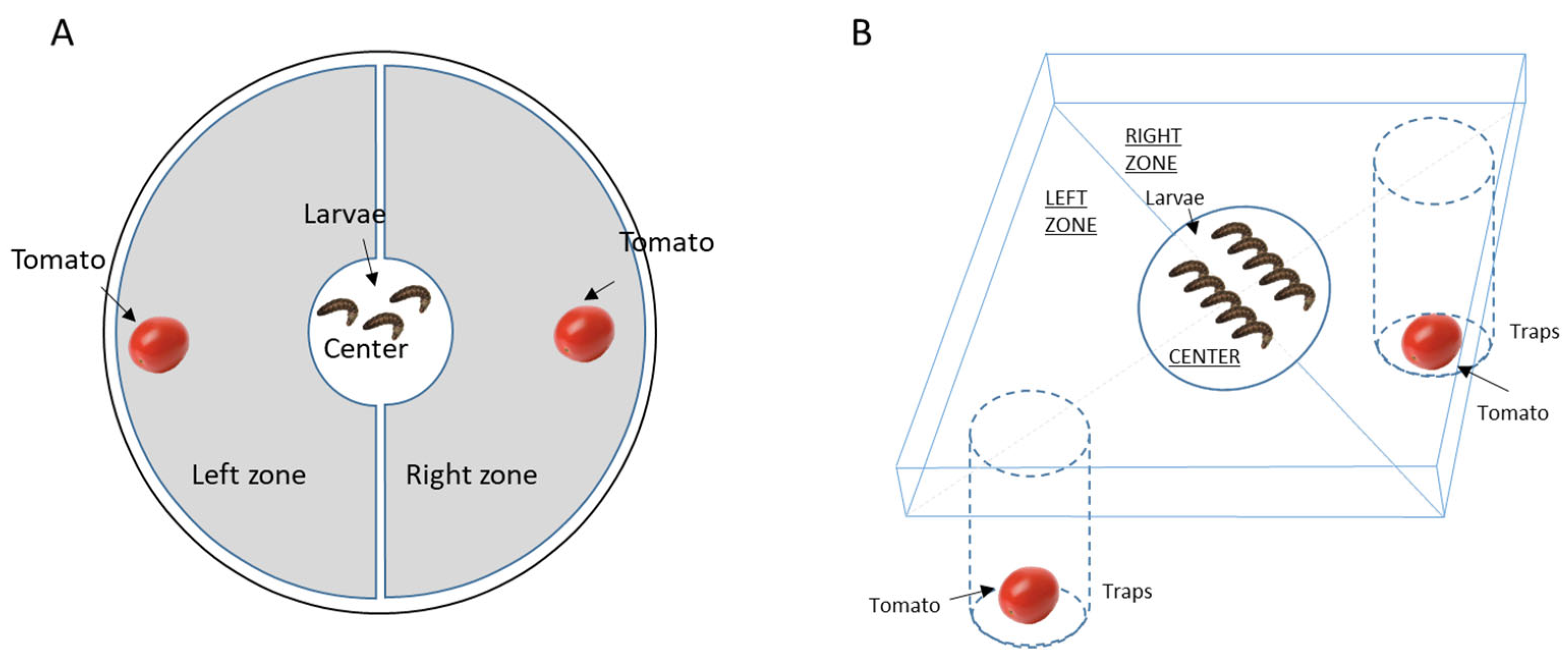
2.4. Two-Choice Behavioural Test
2.5. Tomato Consumption by Larvae
2.6. Behavioural Tests with Larvae of Different Ages and Weights
2.7. Statistical Analyses
3. Results
3.1. Essential Oil (EO) Compositions
3.2. Two-Choice Behavioural Test
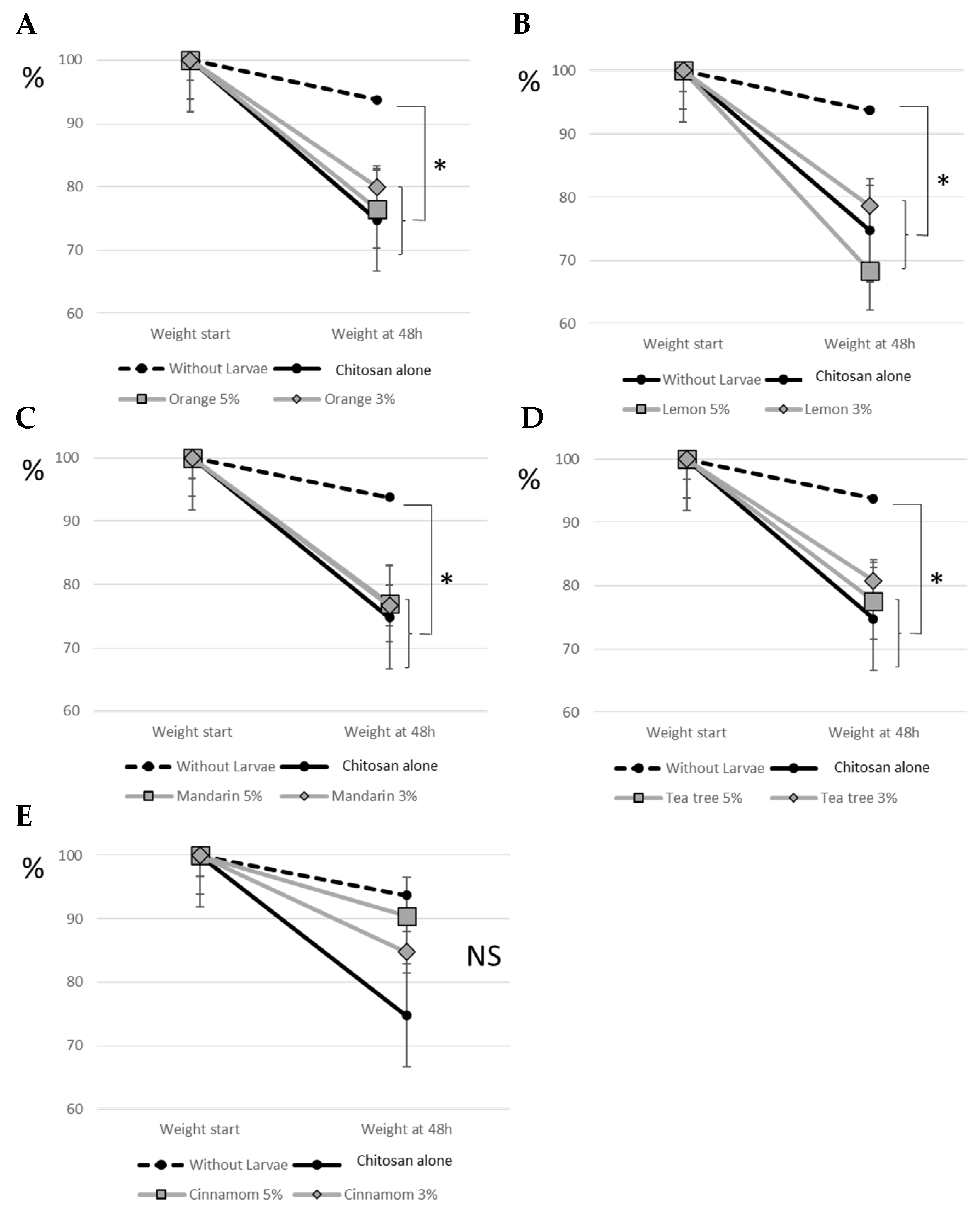
3.3. Tomato Consumption by Larvae
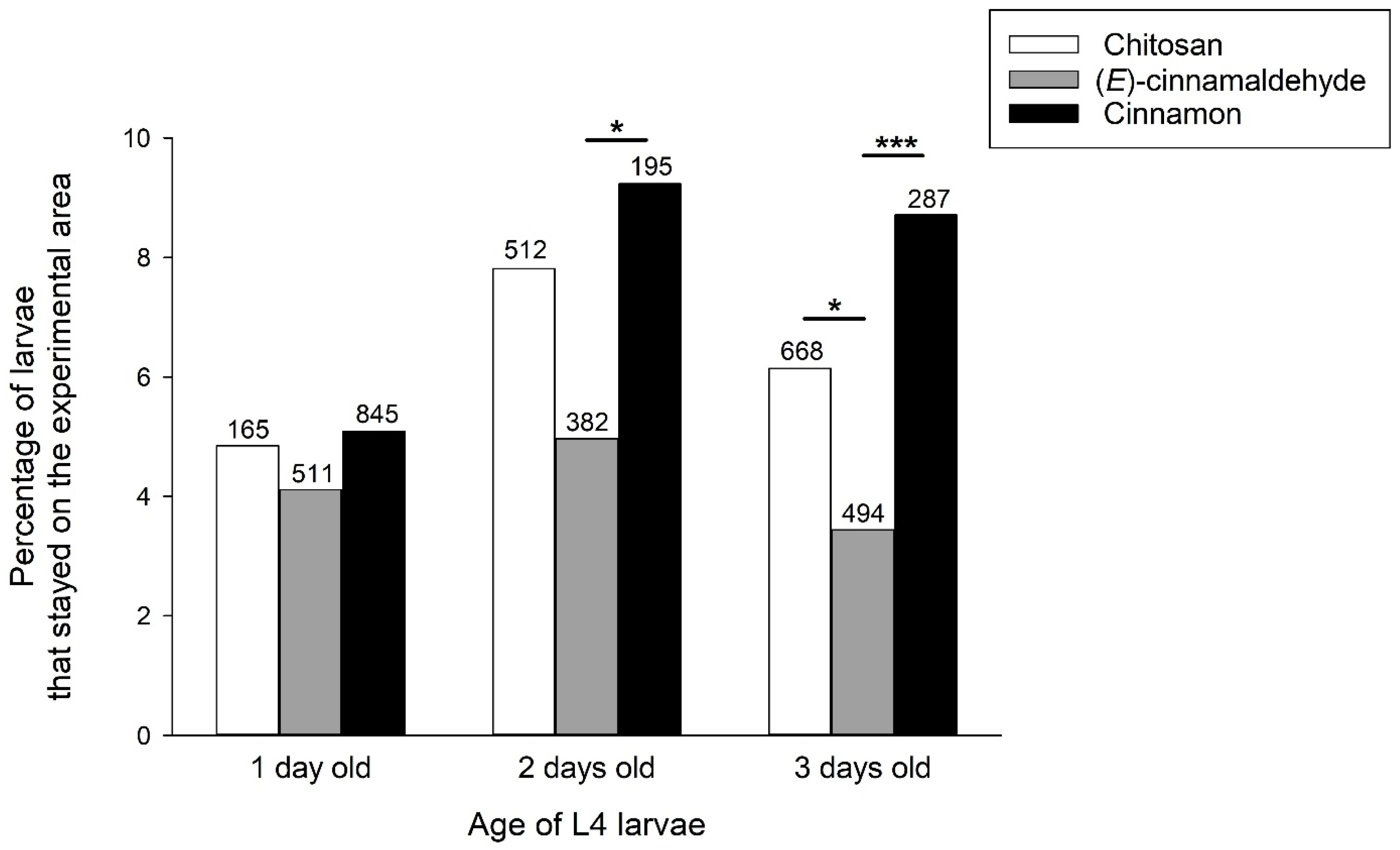
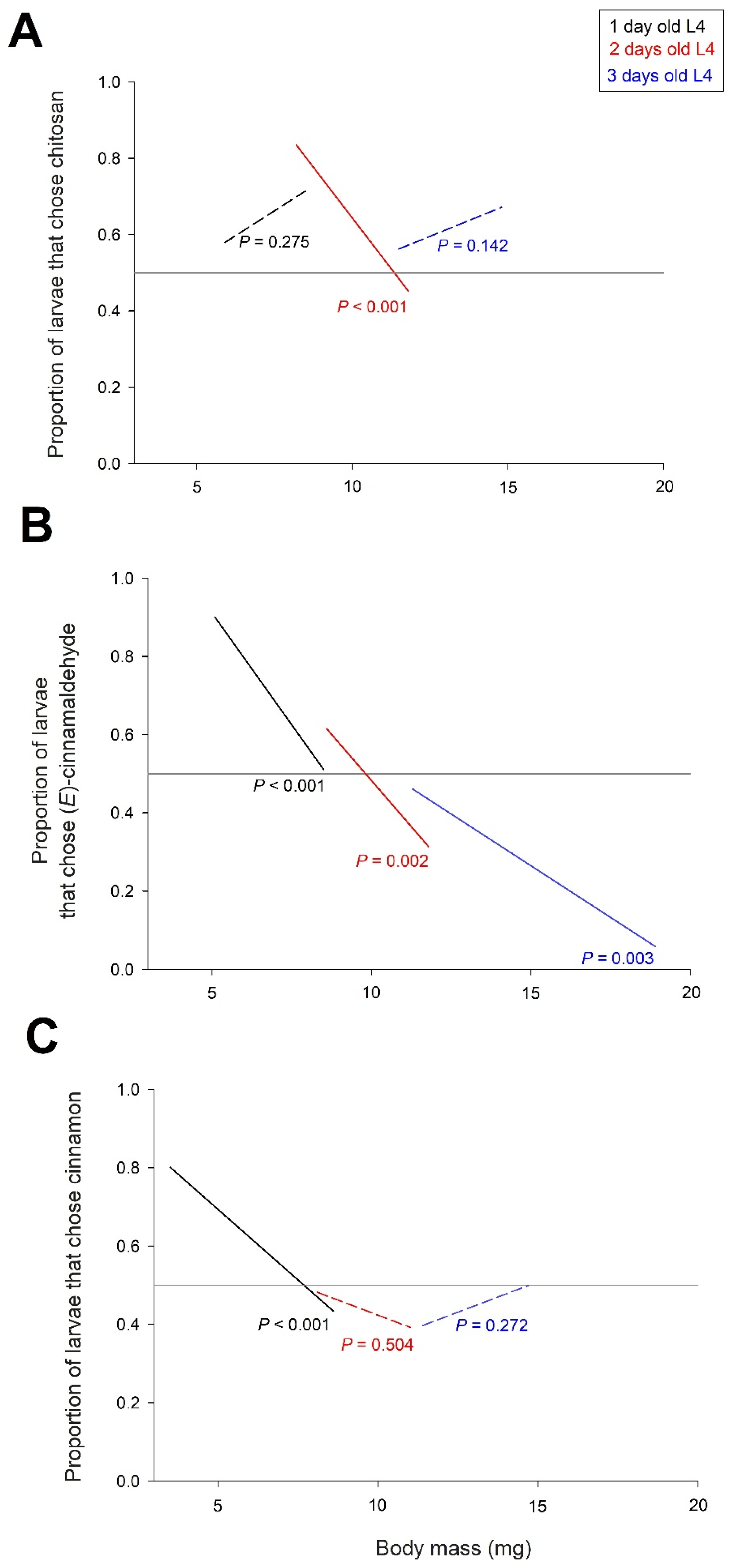
3.4. Behavioural Tests with Larvae of Different Ages and Weights
4. Discussion
4.1. The Repellent Effect of Cinnamon EO
4.2. Lack of Overall Effect of the Compound (E)-Cinnamaldehyde
4.3. Age- and Weight-Dependent Effects of Cinnamon EO and (E)-Cinnamaldehyde
4.4. Time-Dependent Effect of Cinnamon EO and (E)-Cinnamaldehyde
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. Available online: https://www.fao.org/land-water/databases-and-software/crop-information/tomato/en/ (accessed on 10 June 2025).
- Naika, S.; de Jeude, J.; de Goffau, M.; Hilmi, M.; van Dam, B. Cultivation of Tomato: Production, Processing and Marketing; Agromisa Foundation; CTA: Wageningen, The Netherlands, 2005. [Google Scholar]
- Adenuga, A.H.; Muhammad-Lawal, A.; Rotimi, O.A. Economics and Technical Efficiency of Dry Season Tomato Production in Selected Areas in Kwara State, Nigeria. Agris-Line Pap. Econ. Inform. 2013, 5, 11–20. [Google Scholar]
- Al-Dairi, M.; Pathare, P.B.; Al-Yahyai, R.; Opara, U.L. Mechanical damage of fresh produce in postharvest transportation: Current status and future prospects. Trends Food Sci. Technol. 2022, 124, 195–207. [Google Scholar] [CrossRef]
- Moradinezhad, F.; Ranjbar, A. Advances in Postharvest Diseases Management of Fruits and Vegetables: A Review. Horticulturae 2023, 9, 1099. [Google Scholar] [CrossRef]
- Khan, A.A.; Qadar, G.; Abro, A.A.; Awais, M. Tomato yield losses due to attack of insects/pests in Pakkhal Valley of District Mansehra Khyber Pakhtunkhwa, Pakistan. Sarhad J. Agric. 2023, 39, 21–28. [Google Scholar] [CrossRef]
- FAO. Available online: https://www.fao.org/3/I8167EN/i8167en.pdf (accessed on 10 June 2025).
- FAO. Available online: https://www.fao.org/fileadmin/user_upload/sap/docs/Post-harvest%20losses%20along%20value%20and%20supply%20chains%20in%20the%20Pacific%20Island%20Countries.pdf (accessed on 10 June 2025).
- Goka, M.G.; Dufrechou, M.; Picouet, P.; Soncy, K.; Ameyapoh, Y. Determinants of Postharvest Losses in Tomato Production in The Savannah Region of Togo. Eur. J. Agric. Food Sci. 2021, 3, 40–45. [Google Scholar] [CrossRef]
- Abenaim, L.; Conti, B. Chitosan as a Control Tool for Insect Pest Management: A Review. Insects 2023, 14, 949. [Google Scholar] [CrossRef]
- Farina, P.; Ascrizzi, R.; Bedini, S.; Castagna, A.; Flamini, G.; Macaluso, M.; Mannucci, A.; Pieracci, Y.; Ranieri, A.; Sciampagna, M.C.; et al. Chitosan and Essential Oils Combined for Beef Meat Protection against the Oviposition of Calliphora vomitoria, Water Loss, Lipid Peroxidation, and Colour Changes. Foods 2022, 11, 3994. [Google Scholar] [CrossRef]
- Ascrizzi, R.; Flamini, G.; Bedini, S.; Tani, C.; Giannotti, P.; Lombardi, T.; Conti, B.; Fraternale, D. Ferulago campestris Essential Oil as Active Ingredient in Chitosan Seed-Coating: Chemical Analyses, Allelopathic Effects, and Protective Activity against the Common Bean Pest Acanthoscelides obtectus. Agronomy 2021, 11, 1578. [Google Scholar] [CrossRef]
- CABI. Spodoptera littoralis (cotton leafworm). Compendium 2022, 51070. [Google Scholar] [CrossRef]
- EFSA Panel on Plant Health (PLH). Scientific Opinion on the pest categorisation of Spodoptera littoralis. EFSA J. 2015, 13, 3987. [CrossRef]
- Parichanon, P.; Ascrizzi, R.; Tani, C.; Sanmartin, C.; Taglieri, I.; Macaluso, M.; Flamini, G.; Pieracci, Y.; Venturi, F.; Conti, B. The protective combined effect of chitosan and essential oil coatings on cheese and cured meat against the oviposition of Piophila casei. Food Biosci. Curr. Opin. Green Sustain. Chem. 2023, 56, 103132. [Google Scholar] [CrossRef]
- Soltani, A.; Haouel-Hamdi, S.; Ajmi, I.S.; Djebbi, T.; Ben Abada, M.; Yangui, I.; Chouachi, N.; Hassine, K.; Majdoub, H.; Messaoud, C.; et al. Insights for the control of dried-fruit beetle Carpophilus hemipterus (Nitidulidae) using rosemary essential oil loaded in chitosan nanoparticles. Int. J. Environ. Health Res. 2023, 33, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Djebbi, T.; Ascrizzi, R.; Bedini, S.; Farina, P.; Sanmartin, C.; Mediouni Ben Jemâa, J.; Bozzini, M.F.; Flamini, G.; Conti, B. Physicochemical and repellent properties of chitosan films loaded with essential oils for producing an active packaging effective against the food pest Sitophilus oryzae. J. Stored Prod. Res. 2024, 106, 102297. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy; Allured Pub. Corp.: Carol Stream, IL, USA, 2007. [Google Scholar]
- Peng, Y.; Li, Y. Combined effects of two kinds of essential oils on physical, mechanical and structural properties of chitosan films. Food Hydrocoll. 2014, 36, 287–293. [Google Scholar] [CrossRef]
- Casariego, A.; Souza, B.W.S.; Vicente, A.A.; Teixeira, J.A.; Cruz, L.; Díaz, R. Chitosan coating surface properties as affected by plasticizer, surfactant and polymer concentrations in relation to the surface properties of tomato and carrot. Food Hydrocoll. 2008, 22, 1452–1459. [Google Scholar] [CrossRef]
- Hinks, C.F.; Byers, J.R. Biosystematics of the genus Euxoa (Lepidoptera: Noctuidae): V. Rearing procedures, and life cycles of 36 species. Can. Entomol. 1976, 108, 1345–1357. [Google Scholar] [CrossRef]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients, 6th ed.; CRC Press: Boca Raton, FL, USA, 2009; ISBN 9781420090772. [Google Scholar]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent activity of essential oils: A review. Bioresour. Technol. 2010, 101, 372–378. [Google Scholar] [CrossRef]
- Narayanankutty, A.; Kunnath, K.; Alfarhan, A.; Rajagopal, R.; Ramesh, V. Chemical Composition of Cinnamomum verum Leaf and Flower Essential Oils and Analysis of Their Antibacterial, Insecticidal, and Larvicidal Properties. Molecules 2021, 26, 6303. [Google Scholar] [CrossRef]
- Szelényi, M.O.; Erdei, A.L.; Jósvai, J.K.; Radványi, D.; Sümegi, B.; Vétek, G.; Molnár, B.P.; Kárpáti, Z. Essential Oil Headspace Volatiles Prevent Invasive Box Tree Moth (Cydalima perspectalis) Oviposition-Insights from Electrophysiology and Behaviour. Insects 2020, 11, 465. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, T.; Jiang, Y.; Chen, D.; Zuo, W.; Guo, J.; Jin, D. Behavioral Response, Fumigation Activity, and Contact Activity of Plant Essential Oils Against Tobacco Beetle (Lasioderma serricorne (F.). Front Chem. 2022, 10, 880608. [Google Scholar] [CrossRef]
- Farag, S.M.; Moustafa, M.A.M.; Fónagy, A.; Kamel, O.M.H.M.; Abdel-Haleem, D.R. Chemical composition of four essential oils and their adulticidal, repellence, and field oviposition deterrence activities against Culex pipiens L. (Diptera: Culicidae). Parasitol. Res. 2024, 123, 110. [Google Scholar] [CrossRef]
- Fouad, H.A.; da Camara, C.A.G. Chemical composition and bioactivity of peel oils from Citrus aurantiifolia and Citrus reticulata and enantiomers of their major constituent against Sitophilus zeamais (Coleoptera: Curculionidae). J. Stored Prod. Res. 2017, 73, 30–36. [Google Scholar] [CrossRef]
- Deletre, E.; Chandre, F.; Williams, L.; Duménil, C.; Menut, C.; Martin, T. Electrophysiological and behavioral characterization of bioactive compounds of the Thymus vulgaris, Cymbopogon winterianus, Cuminum cyminum and Cinnamomum zeylanicum essential oils against Anopheles gambiae and prospects for their use as bednet treatments. Parasites Vectors 2015, 8, 316. [Google Scholar] [CrossRef] [PubMed]
- Badeke, E.; Haverkamp, A.; Hansson, B.S.; Sachse, S. A challenge for a male noctuid moth? Discerning the female sex pheromone against the background of plant volatiles. Front. Physiol. 2016, 7, 143. [Google Scholar] [CrossRef] [PubMed]
- Hansson, B.S.; Stensmyr, M.C. Evolution of insect olfaction. Neuron 2011, 72, 698–711. [Google Scholar] [CrossRef] [PubMed]
- Conchou, L.; Lucas, P.; Deisig, N.; Demondion, E.; Renou, M. Effects of Multi-Component Backgrounds of Volatile Plant Compounds on Moth Pheromone Perception. Insects 2021, 12, 409. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority); Barthélémy, E.; Bolognesi, C.; Castle, L.; Crebelli, R.; Di Consiglio, E.; Franz, R.; Grob, K.; Hellwig, N.; Lambré, C.; et al. Principles that Could be Applicable to the Safety Assessment of the Use of Mixtures of Natural Origin to Manufacture Food Contact Materials; EN-8409; EFSA Supporting Publication: Parma, Italy, 2023; 32p. [Google Scholar] [CrossRef]
- Malbert-Colas, A.; Drozdz, T.; Massot, M.; Bagni, T.; Chertemps, T.; Maria, A.; Maïbèche, M.; Siaussat, D. Effects of low concentrations of deltamethrin are dependent on developmental stages and sexes in the pest moth Spodoptera littoralis. Environ. Sci. Pollut. Res. 2020, 27, 41893–41901. [Google Scholar] [CrossRef]
- Bagni, T.; Siaussat, D.; Chertemps, T.; Montagné, N.; Maria, A.; Fuentes, A.; Couzi, P.; Massot, M. The effect of developmental temperature on olfaction in a moth revealed by its interaction with body mass. Communications Biology 2024, 7, 1133. [Google Scholar] [CrossRef]
- Pineda, M.; Alves, E.L.d.A.; Antunes, J.A.; Carvalho, V.d.C.; Haddi, K. Low concentrations of eucalyptus essential oil induce age, sex, and mating status-dependent stimulatory responses in Drosophila suzukii. Agriculture 2023, 13, 404. [Google Scholar] [CrossRef]
- Guesmi, F.; Amari, R.; Ajmi, I.S.; Athmouni, K.; Hfaiedh, N.; Allagui, M.S.; Ben Jemâa, J.M.; Landoulsi, A. Promising bioinsecticidal effect of Tunisian Anethum graveolens L. (dill) (Umbelliferae) essential oil against confused flour beetle, Tribolium confusum Jaquelin du Val. 1863 (Coleoptera: Tenebrionidae). J. Stored Prod. Res. 2024, 106, 102273. [Google Scholar] [CrossRef]
- Revadi, S.V.; Giannuzzi, V.A.; Rossi, V.; Hunger, G.M.; Conchou, L.; Rondoni, G.; Conti, E.; Anderson, P.; Walker, W.B.; Jacquin-Joly, E.; et al. Stage-specific expression of an odorant receptor underlies olfactory behavioral plasticity in Spodoptera littoralis larvae. BMC Biol. 2021, 19, 231. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Isman, M.; Tak, J.H. Insecticidal Activity of 28 Essential Oils and a Commercial Product Containing Cinnamomum cassia Bark Essential Oil against Sitophilus zeamais Motschulsky. Insects 2020, 11, 474. [Google Scholar] [CrossRef] [PubMed]


| Compounds | l.r.i. 1 | Relative Abundance (%) 2 | ||||
|---|---|---|---|---|---|---|
| Cinnamomum Cassia | Citrus limon (L.) Osbeck | Citrus reticulata Blanco | Citrus sinensis (L.) Osbeck | Melaleuca alternifolia Cheel | ||
| α-thujene | 931 | - 3 | 0.2 | 0.5 | - | 0.3 |
| α-pinene | 941 | - | 1.4 | 1.5 | 0.4 | 1.0 |
| benzaldehyde | 959 | 0.4 | - | - | - | - |
| sabinene | 976 | - | 1.1 | 0.1 | 0.2 | 0.2 |
| β-pinene | 982 | - | 10.1 | 1.4 | - | 0.3 |
| myrcene | 993 | - | 1.0 | 0.9 | 1.2 | - |
| octanal | 1001 | - | - | - | 0.2 | - |
| α-terpinene | 1018 | - | 0.2 | 0.2 | - | 0.9 |
| p-cymene | 1027 | - | 0.8 | 0.4 | - | 9.6 |
| limonene | 1032 | - | 64.2 | 71.6 | 93.7 | 0.5 |
| 1,8-cineole | 1034 | - | - | - | - | 2.5 |
| salicylaldehyde | 1047 | 0.2 | - | - | - | - |
| γ-terpinene | 1062 | - | 10.5 | 19.1 | - | 3.5 |
| terpinolene | 1088 | - | 0.3 | 0.4 | - | 0.8 |
| trans-sabinene hydrate | 1095 | - | - | - | - | 0.3 |
| linalool | 1101 | - | - | - | 0.4 | - |
| phenylethyl alcohol | 1112 | 0.2 | - | - | - | - |
| trans-p-mentha-2,8-dien-1-ol | 1121 | - | - | - | 0.4 | - |
| cis-p-mentha-2,8-dien-1-ol | 1122 | - | - | - | 0.5 | 0.2 |
| cis-p-menth-2-en-1-ol | 1124 | - | - | - | - | 0.3 |
| cis-limonene oxide | 1134 | - | - | - | 0.4 | - |
| trans-p-menth-2-en-1-ol | 1140 | - | - | - | - | 0.3 |
| trans-limonene oxide | 1141 | - | - | - | 0.4 | - |
| hydrocinnamaldehyde | 1161 | 0.3 | - | - | - | - |
| borneol | 1165 | 0.1 | - | - | - | - |
| 4-terpineol | 1178 | - | - | - | - | 42.7 |
| p-cymen-8-ol | 1183 | - | - | - | - | 0.4 |
| α-terpineol | 1189 | - | - | 0.1 | - | 4.4 |
| decanal | 1204 | - | - | - | 0.5 | - |
| (Z)-cinnamaldehyde | 1217 | 0.2 | - | - | - | - |
| trans-carveol | 1218 | - | - | - | 0.6 | - |
| cis-carveol | 1229 | - | - | - | 0.2 | - |
| neral | 1240 | - | 1.6 | - | - | - |
| carvone | 1244 | - | - | - | 0.9 | - |
| (E)-ascaridol glycol | 1266 | - | - | - | - | 2.3 |
| (E)-cinnamaldehyde | 1268 | 70.1 | - | - | - | - |
| geranial | 1273 | - | 3.3 | - | - | - |
| neryl acetate | 1366 | - | 1.1 | - | - | - |
| α-copaene | 1376 | 1.2 | - | - | - | 0.2 |
| geranyl acetate | 1385 | - | 1.5 | - | - | - |
| methyl N-methyl anthranilate | 1406 | - | - | 2.6 | - | - |
| α-gurjunene | 1410 | - | - | - | - | 0.7 |
| β-caryophyllene | 1420 | 0.3 | 0.2 | 0.2 | - | 0.6 |
| trans-α-bergamotene | 1438 | - | 0.4 | - | - | - |
| α-guaiene | 1439 | - | - | - | - | 0.2 |
| (E)-cinnamyl acetate | 1444 | 2.1 | - | - | - | - |
| aromadendrene | 1445 | - | - | - | - | 2.6 |
| alloaromadendrene | 1461 | 0.3 | - | - | - | 1.3 |
| trans-cadina-1(6),4-diene | 1470 | - | - | - | - | 0.5 |
| γ-muurolene | 1477 | 0.4 | - | - | - | - |
| ar-curcumene | 1483 | 0.2 | - | - | - | - |
| β-selinene | 1485 | - | - | - | - | 0.2 |
| δ-selinene | 1490 | - | - | - | - | 0.4 |
| valencene | 1492 | 0.2 | - | - | - | - |
| viridiflorene | 1496 | - | - | - | - | 3.3 |
| α-muurolene | 1498 | 0.3 | - | - | - | 0.4 |
| (E,E)-α-farnesene | 1507 | - | - | 0.2 | - | - |
| β-bisabolene | 1509 | 0.3 | 2.1 | - | - | - |
| trans-γ-cadinene | 1513 | 0.3 | - | - | - | - |
| (E)-o-methoxy cinnamaldehyde | 1525 | 15.2 | - | - | - | - |
| δ-cadinene | 1526 | 0.8 | - | - | - | 5.0 |
| cadina-1,4-diene | 1534 | - | - | - | - | 0.5 |
| α-calacorene | 1546 | 0.3 | - | - | - | - |
| germacrene B | 1554 | - | - | - | - | 0.3 |
| (E)-nerolidol | 1565 | 0.3 | - | - | - | - |
| palustrol | 1568 | - | - | - | - | 0.3 |
| spathulenol | 1576 | 0.4 | - | - | - | 3.1 |
| caryophyllene oxide | 1581 | 0.5 | - | - | - | - |
| globulol | 1583 | - | - | - | - | 1.8 |
| viridiflorol | 1590 | - | - | - | - | 0.7 |
| guaiol | 1595 | - | - | - | - | 0.7 |
| rosifoliol | 1602 | - | - | - | - | 0.6 |
| tetradecanal | 1614 | 0.3 | - | - | - | - |
| 1-epi-cubenol | 1628 | - | - | - | - | 1.0 |
| isospathulenol | 1639 | - | - | - | - | 0.7 |
| epi-α-cadinol | 1641 | - | - | - | - | 0.7 |
| α-bisabolol | 1683 | 0.3 | - | - | - | - |
| α-sinensal | 1752 | - | - | 0.7 | - | - |
| benzyl benzoate | 1764 | 0.3 | - | - | - | - |
| phenylethyl benzoate | 1860 | 0.2 | - | - | - | - |
| rimuene | 1930 | 0.4 | - | - | - | - |
| pimaradiene | 1941 | 0.3 | - | - | - | - |
| m-camphorene | 1960 | - | - | 0.2 | - | - |
| 13-epi-manoyl oxide | 2010 | 0.1 | - | - | - | - |
| ----------------------------- | ||||||
| Monoterpene hydrocarbons | - | 89.8 | 96.1 | 95.5 | 17.1 | |
| Oxygenated monoterpenes | 0.1 | 7.5 | 0.1 | 3.8 | 53.3 | |
| Sesquiterpene hydrocarbons | 4.6 | 2.7 | 0.3 | - | 16.2 | |
| Oxygenated sesquiterpenes | 1.5 | - | 0.7 | - | 9.6 | |
| Diterpene hydrocarbons | 0.7 | - | 0.2 | - | - | |
| Oxygenated diterpenes | 0.1 | - | - | - | - | |
| Phenylpropanoids | 87.9 | - | - | - | - | |
| Others | 1.6 | - | 2.6 | 0.7 | - | |
| Total identified (%) | 96.5 | 100.0 | 100.0 | 100.0 | 96.2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drozdz, T.; Couzi, P.; Massot, M.; Conti, B.; Ascrizzi, R.; Siaussat, D. Combination of Chitosan and Essential Oils for Tomatoes Protection Against the Insect Pest Spodoptera littoralis (Lepidoptera: Noctuidae). Insects 2025, 16, 718. https://doi.org/10.3390/insects16070718
Drozdz T, Couzi P, Massot M, Conti B, Ascrizzi R, Siaussat D. Combination of Chitosan and Essential Oils for Tomatoes Protection Against the Insect Pest Spodoptera littoralis (Lepidoptera: Noctuidae). Insects. 2025; 16(7):718. https://doi.org/10.3390/insects16070718
Chicago/Turabian StyleDrozdz, Thomas, Philippe Couzi, Manuel Massot, Barbara Conti, Roberta Ascrizzi, and David Siaussat. 2025. "Combination of Chitosan and Essential Oils for Tomatoes Protection Against the Insect Pest Spodoptera littoralis (Lepidoptera: Noctuidae)" Insects 16, no. 7: 718. https://doi.org/10.3390/insects16070718
APA StyleDrozdz, T., Couzi, P., Massot, M., Conti, B., Ascrizzi, R., & Siaussat, D. (2025). Combination of Chitosan and Essential Oils for Tomatoes Protection Against the Insect Pest Spodoptera littoralis (Lepidoptera: Noctuidae). Insects, 16(7), 718. https://doi.org/10.3390/insects16070718








