1. Introduction
Trichogramma wasps, serving as critical egg parasitoids in global lepidopteran pest management, have achieved remarkable ecological success through decades of field implementation [
1,
2]. In Northeast China, the release of
T. dendrolimi protects over 4 million hectares of cornfields annually against the Asian corn borer (
Ostrinia furnacalis), covering 35% of regional maize cultivation [
2]. While extensive applied research has focused on ecological optimization and breeding technology enhancements, a fundamental disconnect persists between empirical biocontrol practices and mechanistic understanding due to a critical knowledge gap perpetuated by the lack of robust genetic tools in this parasitoid wasp.
RNA interference (RNAi) technology has become an important tool for genetic function research in various insects [
3]. However, its efficiency is constrained by delivery methods [
4], commonly dsRNA delivery methods including feeding, microinjection, and soaking; each presents distinct advantages and limitations shaped by developmental biology constraints. New delivery methods are being developed, e.g., through nanocarriers [
5,
6]. Feeding-based RNAi, while offering simplicity and minimal invasiveness for insects, is inherently restricted to feeding-active stages and exhibits delayed efficacy, rendering it ineffective during non-feeding phases such as embryogenesis or pupal development [
7]. Microinjection, the most widely utilized method, enables precise dsRNA delivery across all ontogenetic stages from embryos to adults, achieving high phenotypic penetrance [
8]. However, this technique demands specialized expertise and induces significant mechanical stress, with high mortality rates due to injection trauma [
9]. Soaking protocols, involving the immersion of permeable developmental stages (e.g., larvae/pupae) in dsRNA solutions, provide a technically accessible alternative but require higher dsRNA concentrations than microinjection to attain comparable silencing efficiency [
10]. This methodological triad underscores a critical trade-off in parasitoid RNAi research: balancing operational feasibility against biological precision and organismal viability.
The application of RNAi in insects exhibits profound taxonomic divergence, with Coleopteran insects demonstrating robust systemic silencing compared to Lepidoptera and Diptera [
11]. Within Hymenopteran parasitoids, RNAi advancements have been predominantly confined to larger-bodied larval and pupal parasitoids. For instance, silencing the
doublesex gene in early male larvae of
Nasonia vitripennis using microinjection impacts the growth and differentiation of reproductive tissue [
12]. In the fruit fly parasitoid
Asobara japonica, the microinjection of dsRNA targeting the
ebony gene successfully alters the body color [
13]. However, these methods face significant technical challenges in minute egg parasitoids like
Trichogramma, which are typically smaller than 1 mm. Most of the current RNAi research in
Trichogramma has focused on
T. dendrolimi. Microinjection combined with nanocarriers enables
VgR silencing in prepupae, disrupting ovarian development [
14], while pupal-stage injections achieve
Ferhch knockdown, causing wing deformities [
15]. Moreover, RNAi targeting
chitinase 10,
v-ATPase A, and
v-ATPase B genes has been achieved through microinjection at the pupa stage, while feeding larvae and adults with dsRNA failed to silence these genes, underscoring the ineffectiveness of feeding as a delivery method for RNAi in
T. dendrolimi [
16]. Despite these advances, the application of RNAi in these small parasitoids is still hindered by several limitations. Microinjection, though effective, causes mechanical damage to the tiny individuals, resulting in high mortality rates [
17]. More importantly, existing technologies are not adaptable to
T. ostriniae (body size <0.5 mm) reared on eggs of rice meal moth
Corcyra cephalonica or Asian corn borer
O. furnacalis, as their size differences and host specificity make traditional approaches difficult to apply across species.
This study focuses on T. dendrolimi and T. ostriniae as dual models, targeting two phenotypically clear, stage-specific genes: white (responsible for eye pigment deposition) and laccase 2 (responsible for epidermal tanning). By comparing the delivery efficiency of microinjection and soaking at different developmental stages (prepupal/pupal) and between species, a standardized operational framework for cross-host and cross-species RNAi delivery is established for the first time. The innovation of this research lies in the construction of the first RNAi technology system applicable to multiple Trichogramma species, providing a key tool for analyzing the molecular mechanisms of host adaptability in Trichogramma. This technological framework not only advances genetic function research in Trichogramma but also offers new ideas and methods for further studying the molecular interactions between Trichogramma and its hosts.
2. Materials and Methods
2.1. Insect Rearing
T. dendrolimi and T. ostriniae were originally collected from the parasitized eggs of O. furnacalis in the corn fields in Yitong, Jilin Province, China (125°11′ E, 43°3′ N) in 2015 and were identified through the morphological characteristics of the male genital capsule and rDNA-ITS2 sequence analysis. T. dendrolimi and T. ostriniae were reared and maintained on the eggs of C. cephalonica under laboratory conditions at 25 ± 1 °C, 75 ± 5% relative humidity, and a photoperiod of 16L:8D. After being continuously reared for several generations on C. cephalonica eggs, T. dendrolimi and T. ostriniae colonies were reared on Antheraea pernyi eggs and O. furnacalis eggs, respectively, for subsequent RNAi experiments.
2.2. Identification and Analysis of White and Laccase 2 Genes
To identify
white and
laccase 2 genes in
T. dendrolimi and
T. ostriniae, BLASTP v2.16.0 search (e-value < 1 × 10
−5, bit score > 100, identity > 70% and query coverage > 70%) was performed against the genomes of
T. dendrolimi and
T. ostriniae, using query sequences from
Drosophila melanogaster (
white: NP476787.1;
laccase 2: NP724412.1),
Bombyx mori (
white: NP001037034.1;
laccase 2: NP001103395.1), and
Apis mellifera (
white: NP001403446.1;
laccase 2: XP006562317.1) retrieved from the NCBI database (
http://www.ncbi.nlm.nih.gov/, accessed on 1 April 2023). Multiple sequence alignment of the
white or
laccase 2 gene sequence of
T. dendrolimi and
T. ostriniae with the sequence of
D. melanogaster,
B. mori, and
A. mellifera was performed to check the sequence similarity (
Figures S1 and S2).
2.3. Temporal Expression Analysis of White and Laccase 2 Genes
To evaluate the temporal expression of white (accession number: PV568319.1) and laccase 2 (accession number: PV568320.1) genes, samples from different developmental stages were collected for total RNA extraction. For T. dendrolimi, the fresh A. pernyi eggs were inoculated with newly emerged T. dendrolimi (<24 h old) for 24 h at a ratio of wasps to host eggs of 1:10. The samples were collected at different developmental stages, including egg (1st day post-parasitism), larva (3rd day post-parasitism), prepupa (5th-6th day post-parasitism), pupa (7th–11th day post-parasitism), and newly emerged adult wasps. For T. ostriniae, fresh corn borer egg masses were parasitized with newly emerged T. ostriniae (<24 h old) for 24 h at a ratio of wasps to host eggs of 1:6, and samples were collected at different developmental stages, including egg (4 h after parasitism), larva (2nd day post-parasitism), prepupa (4th–5th day post-parasitism), pupa (6th–9th day post-parasitism), and newly emerged adult wasps. For each biological replicate, approximately 1 g of host egg samples containing different developmental stages of T. dendrolimi or T. ostriniae was collected. In total, three biological replicates were performed for each developmental stage. The samples were rapidly frozen in liquid nitrogen and stored at −80 °C.
Primers for RT-qPCR were designed using the IDT PrimerQuest™ Tool (
https://eu.idtdna.com/pages/tools/primerquest) (accessed on 17 October 2024) [
18], based on the sequences of the
white,
laccase 2, and
GAPDH genes from both species. The primers are listed in
Table 1 and were designed to avoid overlap with synthesized dsRNA regions. Total RNA was extracted using the EASYspin plus Cell/Tissue Total RNA Isolation Kit (Aidlab Biotechnologies Co., Ltd., Beijing, China), and cDNA synthesis was performed using the TRUEscript RT MasterMix (OneStep gDNA Removal) (Aidlab Biotechnologies Co., Ltd., Beijing, China). RT-qPCR was conducted on a qTOWER
3G system (Analytik Jena, Jena, TH, Germany) using 2× SGExcel FastSYBR Mixture (Sangon, Shanghai, China) under the following conditions: 95 °C for 5 min, followed by 40 cycles of 95 °C for 5 s and 60 °C for 20 s. Relative expression levels were calculated using the 2
−ΔΔCt method, with
GAPDH as the reference gene.
2.4. Preparation of dsRNAs
Primers for RNAi fragments targeting the
white and
laccase 2 genes, with product sizes ranging from 600 to 800 bp, were designed using SnapDragon-sRNA DesignTool (
https://www.flyrnai.org/cgi-bin/RNAi_find_primers.pl) (accessed on 10 October 2024.), based on regions of high sequence similarity between these genes in
T. dendrolimi and
T. ostriniae (
Figures S9 and S10). The primers are listed in
Table 2. The T7 promoter sequence (5′-TAATACGACTCACTATAGGG-3′) was added to the 5′ end of all primers. PCR amplification of the dsRNA synthesis templates was performed using Taq PCR Master Mix (Sangon, Shanghai, China), and the products were purified using the Wizard
® SV Gel and PCR Clean-Up System (Promega, Madison, WI, USA) and synthesized using the T7 RNA Transcription Kit (ZSYJ, Shanghai, China).
2.5. RNA Interference Assay via Soaking and Microinjection Delivery Method
To systematically evaluate RNA interference efficacy across developmental stages and species, we implemented complementary soaking and microinjection protocols for
T. dendrolimi (reared in
A. pernyi eggs) and
T. ostriniae (reared in
O. furnacalis eggs). For the soaking method, synchronized prepupae and pupae were dissected under stereomicroscopy (
Figures S3 and S4) at species-specific post-parasitism timepoints (
T. dendrolimi: prepupae at 6 days, pupae at 7 days;
T. ostriniae: prepupae at 4 days, pupae at 6 days) and transferred to Parafilm-based artificial hosts with hemispherical chambers (
Figure S5) [
19]. Initial survival assays involved 48 h of soaking with 1 μL ddH
2O or 500 ng/μL ds
GFP, followed by excess solution removal via filter paper. For each biological replicate, 30 individuals were placed within a single artificial host egg (3 eggs per replicate, each containing 30 wasps), with three independent replicates performed per treatment group (total
n = 270 per stage/species). Adult emergence was monitored to quantify survival rates. Subsequent silencing optimization comprised two experimental designs: (1) time-course analysis—prepupae of
T. dendrolimi (6th day post-parasitism) and
T. ostriniae (4th day post-parasitism) soaked in the ds
white solution (500 ng/μL and 2000 ng/μL) and pupae of
T. dendrolimi (7th day post-parasitism) and
T. ostriniae (6th day post-parasitism) in the ds
laccase 2 solution (500 ng/μL and 2000 ng/μL) for 12, 24, and 48 h; and (2) dose–response profiling—prepupae of
T. dendrolimi (6th day post-parasitism) and
T. ostriniae (4th day post-parasitism) exposed to the ds
white solution (100, 300, 500, 1000, 1500, and 2000 ng/μL) and pupae of
T. dendrolimi (7th day post-parasitism) and
T. ostriniae (6th day post-parasitism) to the ds
laccase 2 solution (100, 300, 500, 1000, 1500, and 2000 ng/μL) for 48 h, with ds
GFP (500 ng/μL) controls. After soaking for 48 h, samples were pooled (30 individuals/replicate, 3 replicates) to check the gene silencing efficiency via RT-qPCR validation.
Parallel microinjection experiments utilized the same developmental stages as soaking treatments to ensure direct methodological comparability:
T. dendrolimi prepupae (6th day post-parasitism) and pupae (7th day) and
T. ostriniae prepupae (4th day) and pupae (6th day). Synchronized individuals were immobilized on 2.5% agarose plates, and a PV850 microinjector (WPI) delivered 1 μL ddH
2O or 500 ng/μL ds
GFP into the thoraco-abdominal junction at 10.0 PSI to assess baseline survival (total n = 270 per stage/species) (
Figure S6). For gene silencing, prepupae were injected with the ds
white solution (100, 300, 500, 1000, 1500, and 2000 ng/μL) and pupae were injected with the ds
laccase 2 solution (100, 300, 500, 1000, 1500, and 2000 ng/μL), with identical ds
GFP controls. After 48 h of the treatment, samples were collected (30 individuals/replicate, 3 replicates), and the silencing efficiency was assessed via RT-qPCR. Developmental stage alignment and concentration gradients mirrored soaking protocols to enable direct methodological comparison.
2.6. Phenotypic Evaluation
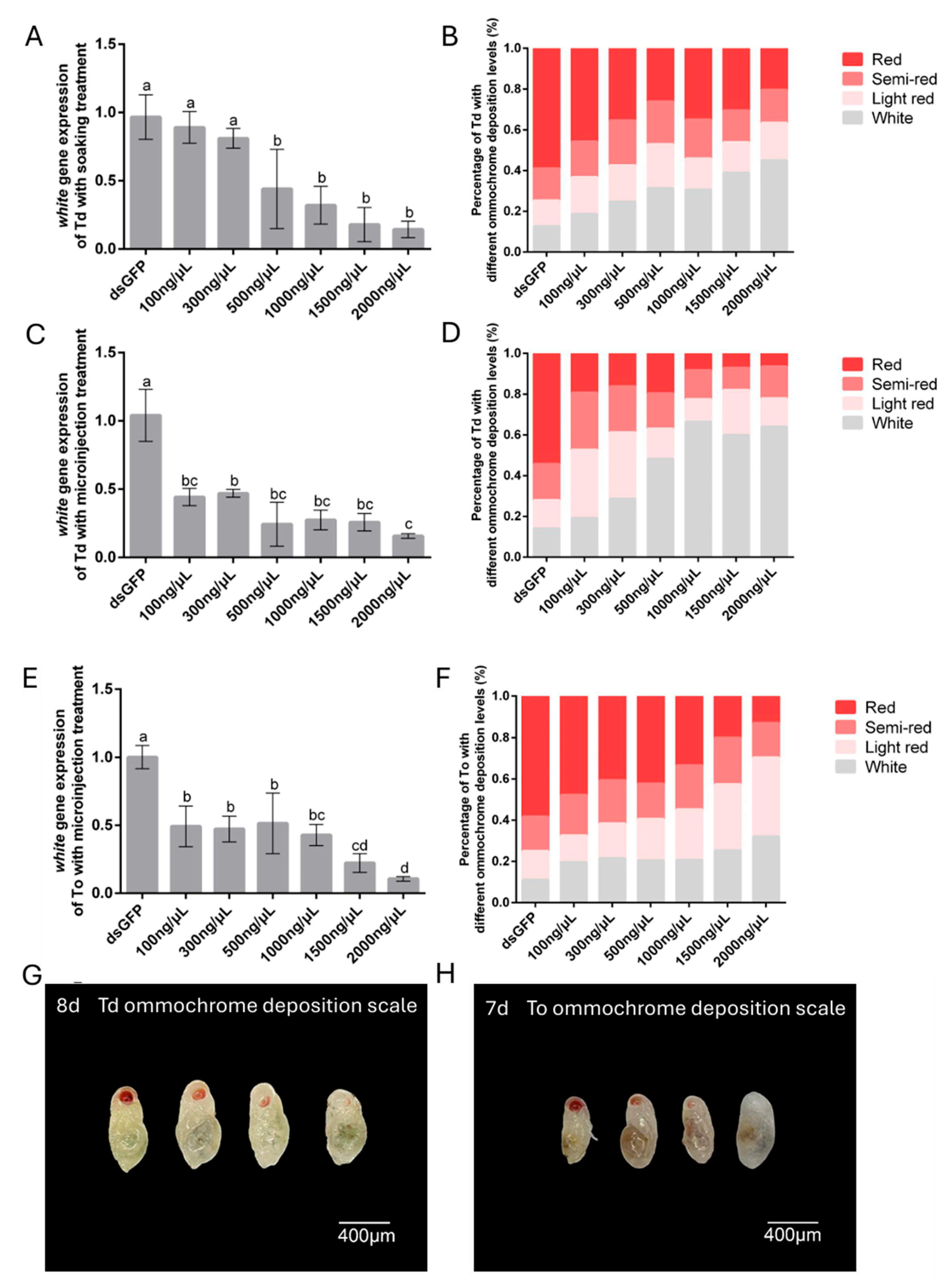
Following 48 h post-treatment intervals (soaking or microinjection with dswhite solution), eye pigmentation phenotypes were assessed using a VHX-7000 digital microscope (Keyence, Osaka, Japan) at 200× magnification. To standardize the quantification of white gene silencing efficacy, a four-tier chromatic classification system based on ommochrome deposition levels was established: Class I (fully pigmented, red), Class II (partial pigmentation, semi-red), Class III (low pigmentation, light red), and Class IV (unpigmented, white). All evaluations were performed on immobilized individuals under consistent illumination parameters (LED ring light, 5600K color temperature) to minimize optical artifacts.
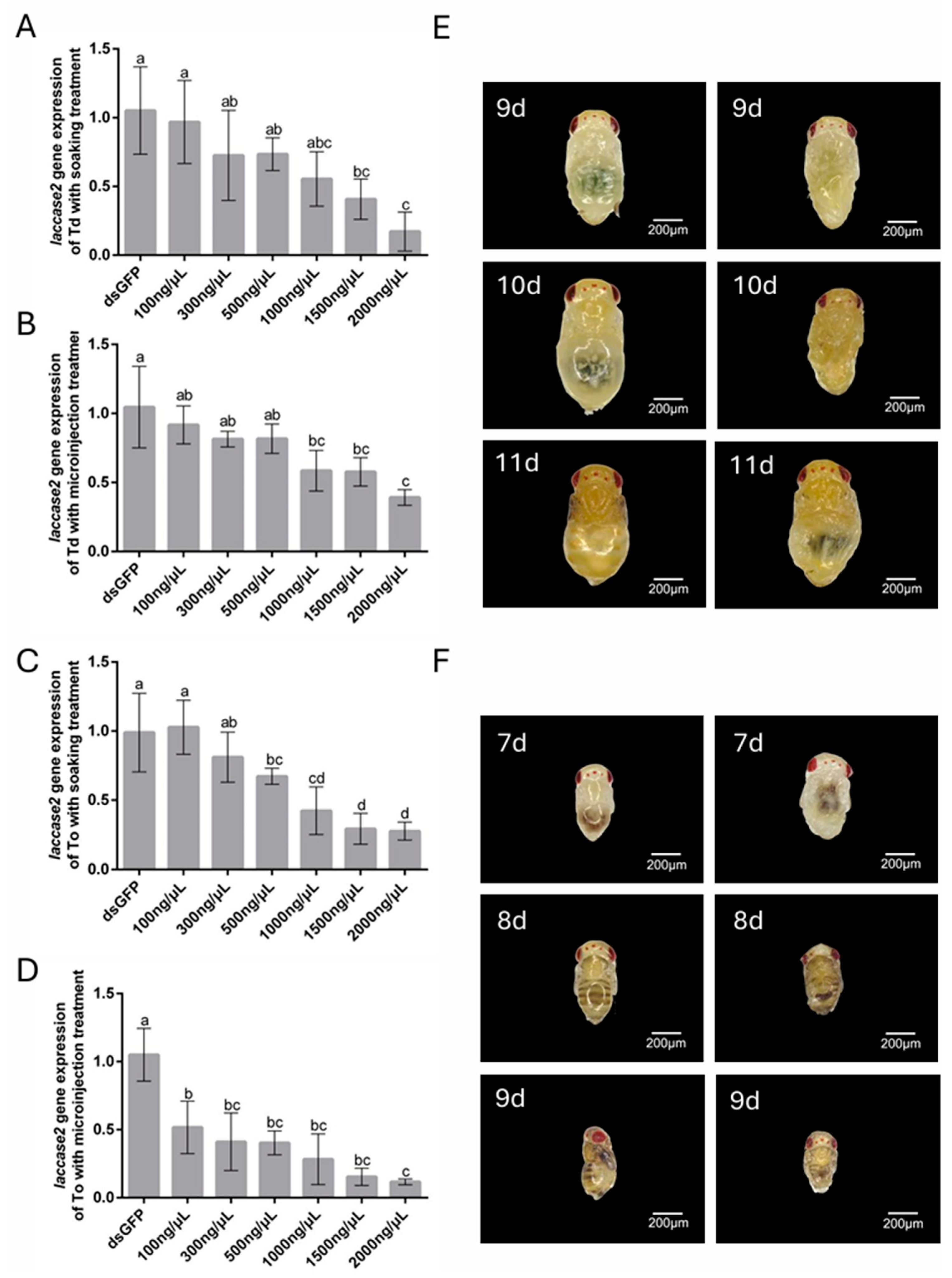
Following 48 h post-treatment intervals (soaking or microinjection with dslaccase 2 solution), deformations to the cuticle and body segments, as well as a lack of pigmentation, were recorded using a Keyence VHX-7000 digital microscope at 200× magnification. Trichogramma wasps were scored as having a knockdown phenotype if we observed one or more of the traits described above.
2.7. Statistical Analysis
Statistical analyses were performed using GraphPad Prism 6.0 (GraphPad Software, San Diego, CA, USA). Two-tailed Student’s t-test was used to analyze differences between two samples, and one-way ANOVA was performed for multiple group comparisons.
4. Discussion
The establishment of RNAi systems in Trichogramma wasps has long been constrained by their minute size and host-rearing dependencies. While prior studies focused exclusively on T. dendrolimi, our work bridges a critical gap by successfully implementing RNAi in T. ostriniae, a superior biocontrol agent used to control O. furnacalis traditionally hindered by its reliance on C. cephalonica eggs and sub-0.5 mm body size. By tailoring delivery methods to species-specific physiological constraints, we demonstrate that soaking remained effective for pupal-stage gene silencing in both T. dendrolimi and T. ostriniae, whereas microinjection enables robust gene silencing in T. ostriniae prepupae—a breakthrough that expands functional genomics tools to non-model Trichogramma species. This advancement not only resolves the historical neglect of T. ostriniae in molecular studies but also provides a framework for adapting RNAi protocols to microhymenopteran parasitoids with diverse host preferences.
The selection of dsRNA delivery methods in
Trichogramma wasps hinges on balancing technical feasibility, species-specific physiological constraints, and silencing efficiency. Microinjection, the predominant RNAi method in parasitoid wasps [
20,
21], offers precise tissue-targeted delivery with controlled dsRNA concentration but requires specialized equipment (e.g., microprocessor-controlled injectors) and incurs operational complexity compared to non-invasive methods. While studies report dsRNA injection amounts ranging widely from 500 ng/μL to 6 μg/μL in other parasitoids [
22,
23], our results demonstrate that
Trichogramma species achieve significant gene silencing at lower concentrations. For instance, the microinjection of 100 ng/μL dsRNA induced 50.8% transcript reduction in the
laccase 2 gene in
T. ostriniae and 57.46% and 49% transcript reduction in the
white gene in
T. dendrolimi and
T. ostriniae, respectively, highlighting exceptional dsRNA bioavailability in these microhymenopterans. Intriguingly, increasing dsRNA concentrations to 200–1500 ng/μL were not effective in enhancing silencing efficacy, consistent with the hypothesis of a saturation threshold in the RNAi machinery [
10], which underscores the efficiency of low-dose microinjection in
Trichogramma.
In contrast, the non-invasive soaking method offers operational simplicity by facilitating passive dsRNA uptake through cuticular or spiracular pathways during the pupal stage [
24,
25], eliminating the mechanical trauma associated with microinjection. However, its efficacy is strongly species-dependent due to variations in osmotic tolerance. For
T. dendrolimi, which is industrially reared in
A. pernyi eggs, soaking at 2000 ng/μL achieved >80% survival and silencing efficiency for both
white and
laccase 2 genes, aligning well with large-scale experimental requirements. Conversely, the smaller-bodied
T. ostriniae (0.3 mm) exhibited severe sensitivity to this passive absorption mechanism, with prepupal survival dropping below 20% under identical conditions, likely due to osmotic stress. Notably, at the elevated concentration of 2000 ng/μL, soaking matched microinjection in silencing efficacy across both species, demonstrating its potential as a viable alternative where species-specific physiological barriers are absent.
Temporal dynamics further modulate RNAi efficacy during soaking, necessitating a balance between silencing optimization and developmental viability. In
T. dendrolimi, soaking with 2000 ng/μL ds
white for 24 h reduced
white and
laccase 2 transcripts by 51.95% and 61.66%, respectively. Extending the soaking period to 48 h further enhanced
white silencing to 83.23%, though
laccase 2 efficiency slightly declined to 84.29%, accompanied by an increase in mortality. This divergence suggests gene-specific susceptibility to prolonged dsRNA exposure, potentially linked to differential expression kinetics or pathway saturation. Similar trends were observed in
Microplitis mediator, where
MmedOR49 silencing efficiency rose from 30% (24 h) to 50% (48 h) under equivalent conditions [
20], underscoring the universality of time-dependent RNAi efficacy in parasitoids. The progressive improvement in silencing with extended soaking likely stems from cumulative dsRNA absorption through cuticular or spiracular pathways, enabling the sustained saturation of RNAi machinery components. This contrasts starkly with microinjection, where a single bolus dose degrades or dilutes over time, requiring precise alignment with transcriptional peaks [
3].
RNAi efficiency also exhibits substantial variability among genes within the same insect species, even when uniform delivery methods are employed [
11]. For instance, in
Aphidius ervi, dsRNA targeting
AeSPH1 induced a significant reduction in its transcript levels, whereas dsRNA targeting
AeSPN1 failed to alter
AeSPN1 expression under identical experimental conditions [
26]. Similarly, in
T. dendrolimi, the silencing efficacy of the
white gene consistently surpassed that of the
laccase 2 gene across both soaking and injection delivery methods. These observations underscore the intrinsic gene-specific sensitivity to RNAi, which may arise from factors such as transcript abundance, dsRNA accessibility, or sequence-specific secondary structures [
27,
28]. This phenomenon is not unique to hymenopterans. In coleopterans, broad-scale screens of dsRNAs targeting 290 genes in
Diabrotica virgifera virgifera revealed marked differences in efficacy, with the LC
50 values of the 17 most effective dsRNAs varying by nearly 100-fold [
29]. Similarly, in the Colorado potato beetle (
Leptinotarsa decemlineata), transcript suppression levels ranged from approximately 60% to 93% depending on the target gene [
30]. Such variability emphasizes the necessity of empirical gene screening and mechanistic studies to optimize RNAi applications [
3,
11,
31].
The successful implementation of RNAi in both T. dendrolimi and T. ostriniae demonstrates that the optimized framework in this study holds significant potential for broader application across the Trichogramma genus. Crucially, the observed interspecies divergence between these species underscores that protocol transferability fundamentally depends on accommodating species-specific physiological constraints. The comparative analysis reveals that optimal dsRNA delivery method selection is dictated by developmental tolerance, where robust species like T. dendrolimi achieve efficient silencing via non-invasive soaking during pupal stages, while T. ostriniae prepupae necessitate microinjection to maintain viability. Beyond delivery routes, key operational parameters require species-tailored optimization; although 2000 ng/µL dsRNA proved effective here, optimal concentrations for silencing efficiency and minimal toxicity may vary across species targets or developmental stages and must be empirically determined through dose–response testing. Similarly, treatment parameters including soaking duration and microinjection volume demand calibration based on biological factors like body size and cuticle permeability. This dual emphasis on physiological adaptation and parametric calibration establishes a replicable blueprint for extending RNAi methodologies to previously intractable Trichogramma species.
Our study advances RNAi methodology in Trichogramma wasps by overcoming species-specific limitations and elucidating the intricate interplay between delivery methods, the temporal dynamics of gene silencing, and gene-specific susceptibility to RNAi. Specifically, by systematically addressing the technical bottlenecks in dsRNA delivery that have long hindered functional genomics research in minute-bodied parasitoids, this work establishes a robust framework for precise genetic manipulation. These advancements not only establish an efficient RNAi system tailored to Trichogramma biology but also directly enable targeted genetic interventions to enhance traits critical for biocontrol efficacy, providing a crucial technical support for pest control and management.