1. Introduction
In the context of the legal investigation of a victim’s death, forensic entomology relies on the examination of insects and other arthropods for the estimation of postmortem interval [
1]. Two principal methodologies are often used in estimating the PMI: ecological succession and Accumulated Degree Days or Hours (ADD/ADH). The ecological succession approach uses predictable changes in insect species that occur within an ecosystem over the decomposition period. This allows forensic entomologists to establish a chronological sequence of insect colonization based on the geographical and seasonal location of a cadaver [
2]. The Accumulated Degree Days (ADD) and Accumulated Degree Hours (ADH) methods use insects’ physiological responses to temperature changes to quantitatively measure heat accumulated units, and this helps in terms of making more precise PMI estimates. Moreover, integrating ecological succession patterns with quantitative ADD/ADH calculations can enhance the accuracy of PMI assessments [
3,
4,
5].
Developmental data serve as a dependable tool for estimating the PMI. This method leverages information obtained from studies on carrion insect species. A crucial aspect of this approach is determining the minimum developmental temperature, often called the base temperature, which is important in calculating the Accumulated Degree Days/Hours (ADD/ADH). The variability of the base temperature can sometimes be substantial, particularly among entomologists, and often depends on the specific species under investigation and the geographic region of discovery [
6]. Previous studies on blow flies have demonstrated that temperature affects the rate of insect development. Higher temperatures (to a limit) accelerate the development of immature stages, shortening the insect life cycle, while cooler temperatures prolong it [
7].
Research has indicated that adult blow flies had lower survival rates when exposed to high temperatures. Studies on
P. regina and
L. sericata show that survival significantly declines above 37 °C, with total death at 44 °C [
8]. Likewise, at consistent temperatures of 30 °C,
Calliphora varifrons do not properly emerge [
9]. Although maggot mass formation and heat generation have been documented in research for many years, our knowledge of maggot masses, especially the entire aggregation’s physiological ecology, remains limited. Understanding the potential survival mechanisms of larvae within these aggregations is of great interest for a more precise PMI estimation.
This study aims to analyze the temperature distribution within larval aggregations to determine the optimal mass size, investigate its thermal impact on survival and developmental rates, determine lethal temperatures, and examine the maggot mass effect on the developmental processes of major forensic blowflies.
2. Materials and Methods
2.1. Rearing of Blow Fly Colonies
Calliphora vicina and Lucilia sericata (Diptera: Calliphoridae) were purchased online from BestBait.com (Marblehead, OH, USA; URL accessed on 2 June 2023), and the species were confirmed by the authors. Five hundred larvae of each species were obtained in containers filled with pine shavings, and upon arrival, the larvae were placed in separate 20 cm × 20 cm × 20 cm net cages for adult emergence. The emerged adults were maintained at a room temperature of 23.3 °C (±1 °C) with ca. 70% RH under a 16:8 (L:D) photoperiod and provided granulated sugar and water ad libitum. Raw beef liver was offered as a protein source for oviposition. The infested liver was collected in Petri dishes and distributed in bulk into 1.26 L (ca. 8 × 10 × 16 cm) plastic boxes containing pine shavings. These boxes were placed in incubators at 25 °C (±0.1 °C) to ensure controlled developmental conditions.
2.2. Experimental Design
In this study, we designed a split-plot design where three growth chambers served as experimental units, with two different temperatures (25 °C and 30 °C). The temperature was assigned to each chamber as the whole-plot treatment. Within each growth chamber, there was a total of four containers containing larval masses and substrate, serving as the split-plot. Each container was assigned a treatment factor for different larval density levels in the split-plot. Larvae were divided into groups based on a specific number of individuals, respectively, 50, 200, 1000, and 2000. This experiment was repeated three times.
2.3. Choice of Larvae
To collect eggs for the experiment, an 18 mL cup containing diced liver was placed inside the adult fly cage to serve as an oviposition substrate. Adults deposited their eggs in crevices and spaces within the liver. The collected eggs were then distributed in bulk into different rearing boxes (ca. 8 × 10 × 16 cm), where additional liver was provided as a food source for the larvae. The boxes were placed in rearing chambers (SMY04-1 DigiTherm® CirKinetics Incubators; TriTech Research, Inc., Los Angeles, CA, USA) maintained at 25 °C. On the third and fourth days, respectively, larvae of L. sericata and C. vicina were randomly selected for experimentation. Larvae were distributed in their respective experimental boxes: 50 (control), 200 (small), medium (1000), and large (2000). At each density, 10% of larvae were randomly selected to record a baseline to evaluate the weight gained. The balance was tared each time before a new larva was placed on it. To ensure a correct weight result, the larva was left on a Metler analytical balance for about 10 s (until the signal of stable balance was observed). After completing the process, the larvae were introduced back to their corresponding experimental box. Three days after setting the experiments for temperature data, larval weight was individually recorded at 10% of their respective density.
2.4. Temperature Data
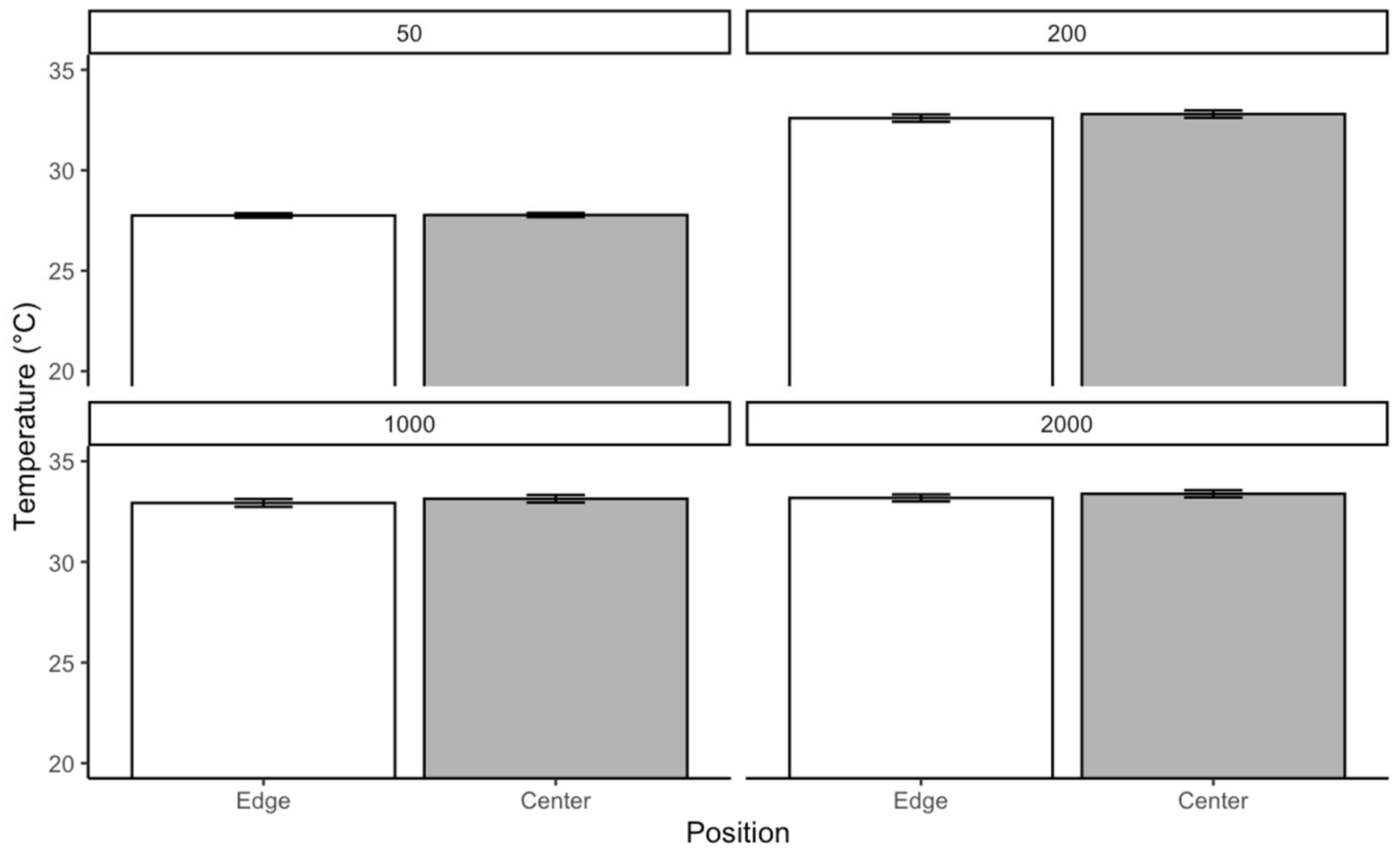
Each box in the rearing chamber contains a disc containing liver at a density of ca. 6 g/50 larvae. A petri dish containing pine shavings was placed in the middle of the box. The petri dishes were used to favor localized mass with a gradient of aggregation. The pine shavings were changed daily to avoid accumulating waste and CO2. At each larval density, temperature data were recorded for 4 days. From the edges to the center of the mass, temperature was recorded at 4 points using a ThermoPro digital thermometer. Data were collected three times a day within a 6-h interval starting at 09:00 h.
2.5. Developmental Time
We measured the minimum developmental time by recording the earliest time the first individuals completed development from egg to adult under controlled maggot mass conditions (all larvae aggregated into a single mass). Blow fly eggs were initially laid at a room temperature of 23.3 °C, and the experiments were conducted in rearing chambers set at 25 °C and 30 °C. Pupation occurred in pine shavings. Development was monitored daily, and the minimum developmental time was determined as the earliest recorded adult emergence. Environmental conditions were controlled to minimize external influences on development.
2.6. Lethal Temperatures
We used a sous vide container filled with water to conduct our lethal temperature assays. This experiment involved three blow fly species: L. sericata, C. vicina, and C. vomitoria. A single larva from each species was placed in a 0.5 mL vial, secured with a steel wire, and suspended within a sinkable plastic shell. Each trial included four vials as replicates.
For each species, two conditions were tested: preconditioned and regular treatments. In the preconditioned treatment, the water was preheated to 32 °C for 15 or 30 min before proceeding with the lethal temperature assessment. The sous vide container was restarted following preconditioning to determine the temperature threshold leading to larval mortality. The standard treatment served as a control, where larvae were directly subjected to the lethal temperature test without prior preconditioning.
2.7. Incubators
The four incubators used in our study were previously described [
10,
11]. The incubators were customized SMY04-1 DigiTherm
® CirKinetics Incubators (TriTech Research, Inc., Los Angeles, CA, USA). These incubators feature microprocessor-controlled temperature regulation, internal lighting, and a recirculating air system to help maintain humidity levels. Unlike conventional growth chambers that rely on coolant and condensers, these units use a thermoelectric heat pump for temperature control. Several modifications were made to enhance functionality, including installing a data port, vertical lighting to ensure uniform illumination across shelves, and an additional internal fan.
According to the manufacturer’s specifications, the incubators maintain an operational temperature range of 10–60 °C with a precision of ±0.1 °C, which is notably more accurate than standard growth chambers. Temperature fluctuations inside growth chambers can differ significantly from programmed settings, whereas internal thermocouple assessments in a replicated study confirmed that temperatures remained within 0.1 °C of the setpoint across all shelves.
2.8. Statistical Analysis
All analyses were performed using GraphPad Prism 10.4 for model fitting and visualization, while R version 4.4.3 (R Core Team, 2025) within RStudio 2024.12.1.563 was used for statistical computations. Statistical significance was evaluated at p < 0.05 for all tests unless otherwise noted.
For developmental time, pupation success, and emergence rates, Analysis of Variance (ANOVA) was used to assess the significance of chamber temperature, larval density, and their interaction. Data were tested to ensure the validity of assumptions for ANOVA. When ANOVA results were significant, post hoc Tukey tests were conducted to compare specific density groups.
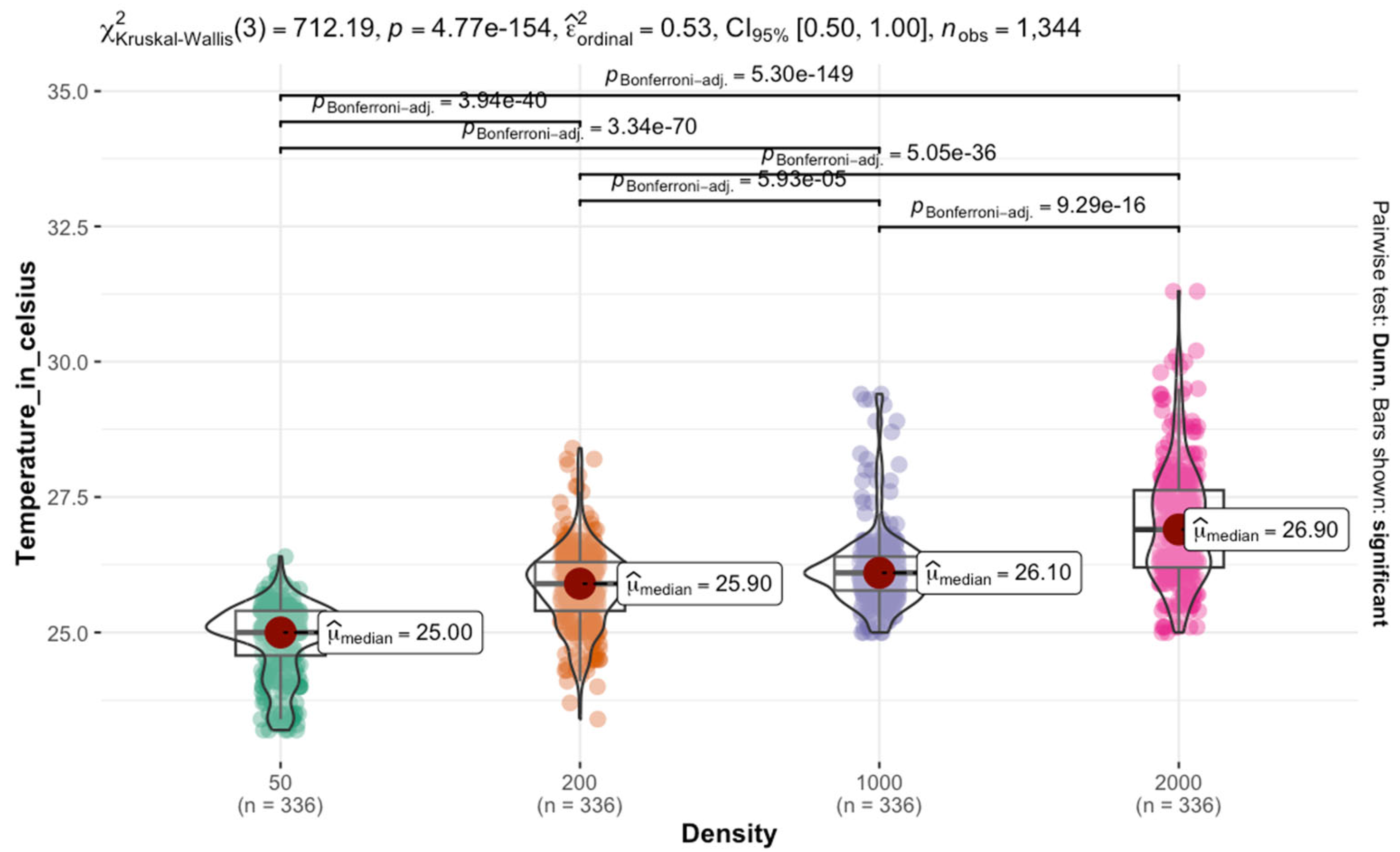
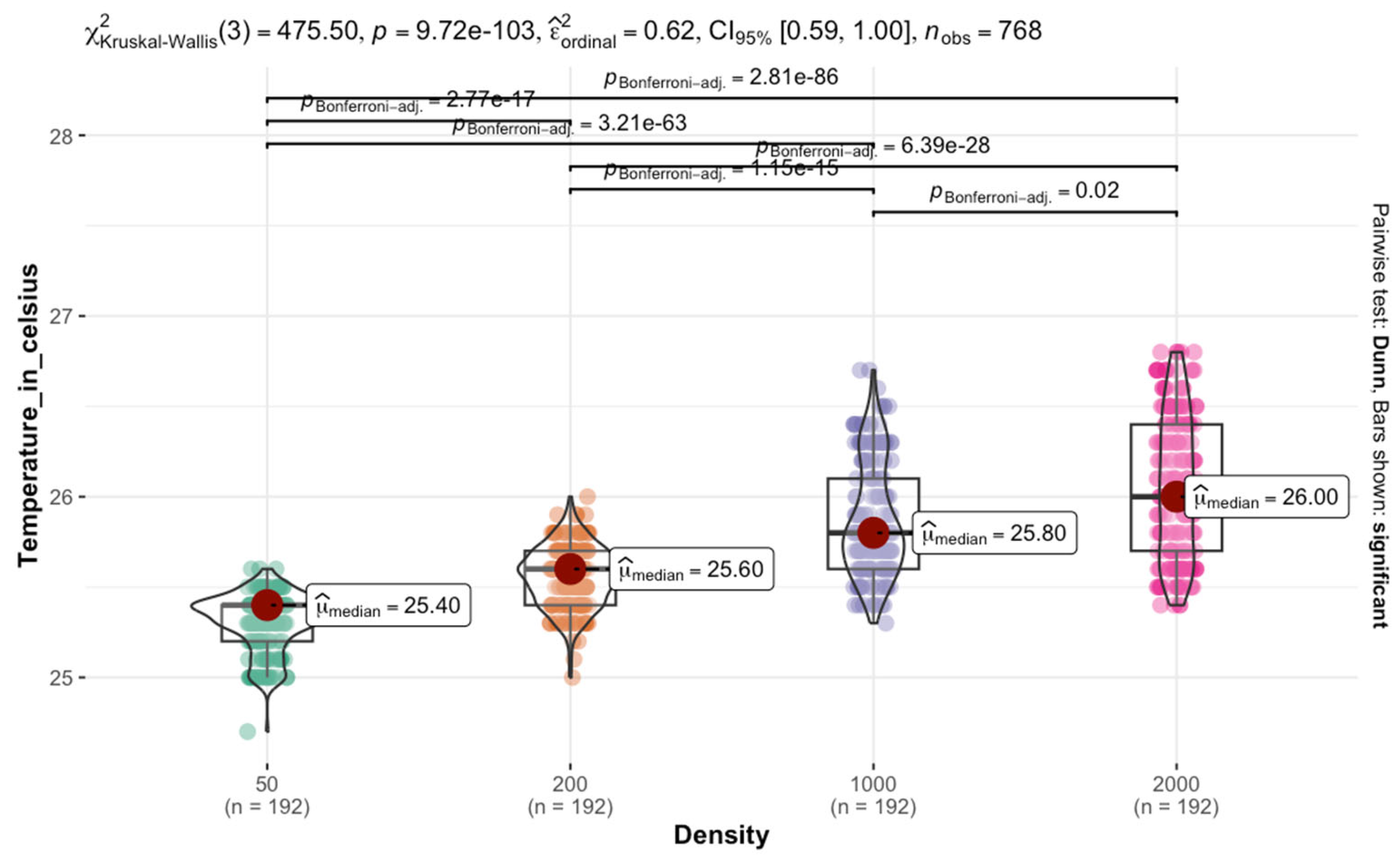
Temperature retention within larval aggregations was analyzed using non-parametric Kruskal–Wallis tests due to non-normal data distributions, followed by Dunn’s tests with Bonferroni correction for multiple comparisons (adjusted p < 0.05 considered significant). Weight gain analysis employed a split-plot mixed-effects model to examine fixed effects of density and temperature and their interaction, with random effects accounting for experimental blocks. Model assumptions were verified through residual diagnostics. Significant main effects and interactions were interpreted at p < 0.05.
For lethal temperature thresholds, we used two-sample t-tests to compare preconditioning treatments, with Welch’s correction applied when variances were unequal. All p-values were two-tailed with α = 0.05 determining significance.
Effect sizes were reported for significant findings, including partial η2 for ANOVA models and Cohen’s d for t-tests, to complement p-value interpretations. Data were presented as mean ± SEM.
4. Discussion
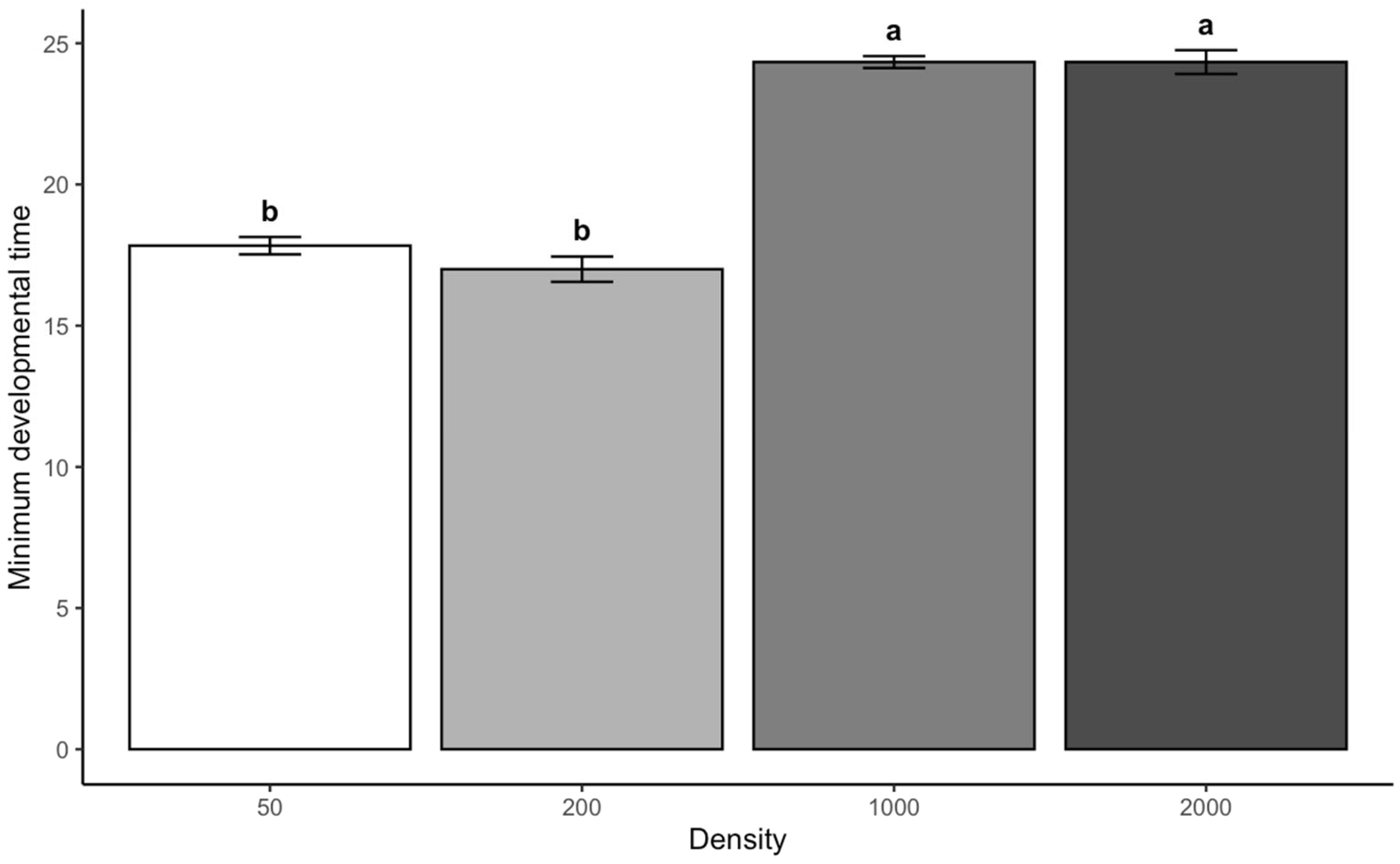
This study shows that larval density significantly determines the minimal developing period for
L. sericata and
C. vicina. Because of competition and the physiological stress brought on by crowding, the effect of ambient temperature may differ between species. Following the non-linear dynamics suggested, our findings shared a similar framework of density-dependent competition in necrophagous Diptera [
12,
13]. For both species studied, the increase in larval density resulted in a significant extension of developmental time, especially at high larvae densities. Meanwhile, the developmental times at low-density larvae were not significantly different for either species. This pattern suggests the existence of a density threshold between 200 and 1000 larvae where resource competition transitions from negligible to biologically significant. We can refer to this phenomenon as the “critical mass effect.” This term describes the density threshold at which larvae transition from “solo” dynamics to “group” dynamics. Below this threshold, their development and resource use remain largely unaffected by their neighbors. However, once the threshold is exceeded, emergent, non-linear effects start to appear.
The absence of developmental delays at lower densities indicates that resource availability remains sufficient to support optimal growth rates when competition is minimal. It is well-documented that blow fly larvae exhibit density-dependent feeding plasticity to mitigate intraspecific competition [
14]. Larval growth rates remain stable below certain density thresholds due to efficient food partitioning mechanisms.
The threshold at which density effects become significant was between 200 and 1000 larvae. This transition likely reflects multiple interacting factors, including hypoxia-induced metabolic suppression [
15,
16], proteolytic enzyme inhibition from waste accumulation [
17,
18], and pathogenic microbial proliferation in crowded environments [
19,
20]. These mechanisms collectively create a density-dependent bottleneck effect on development that becomes physiologically consequential beyond a critical larval mass.
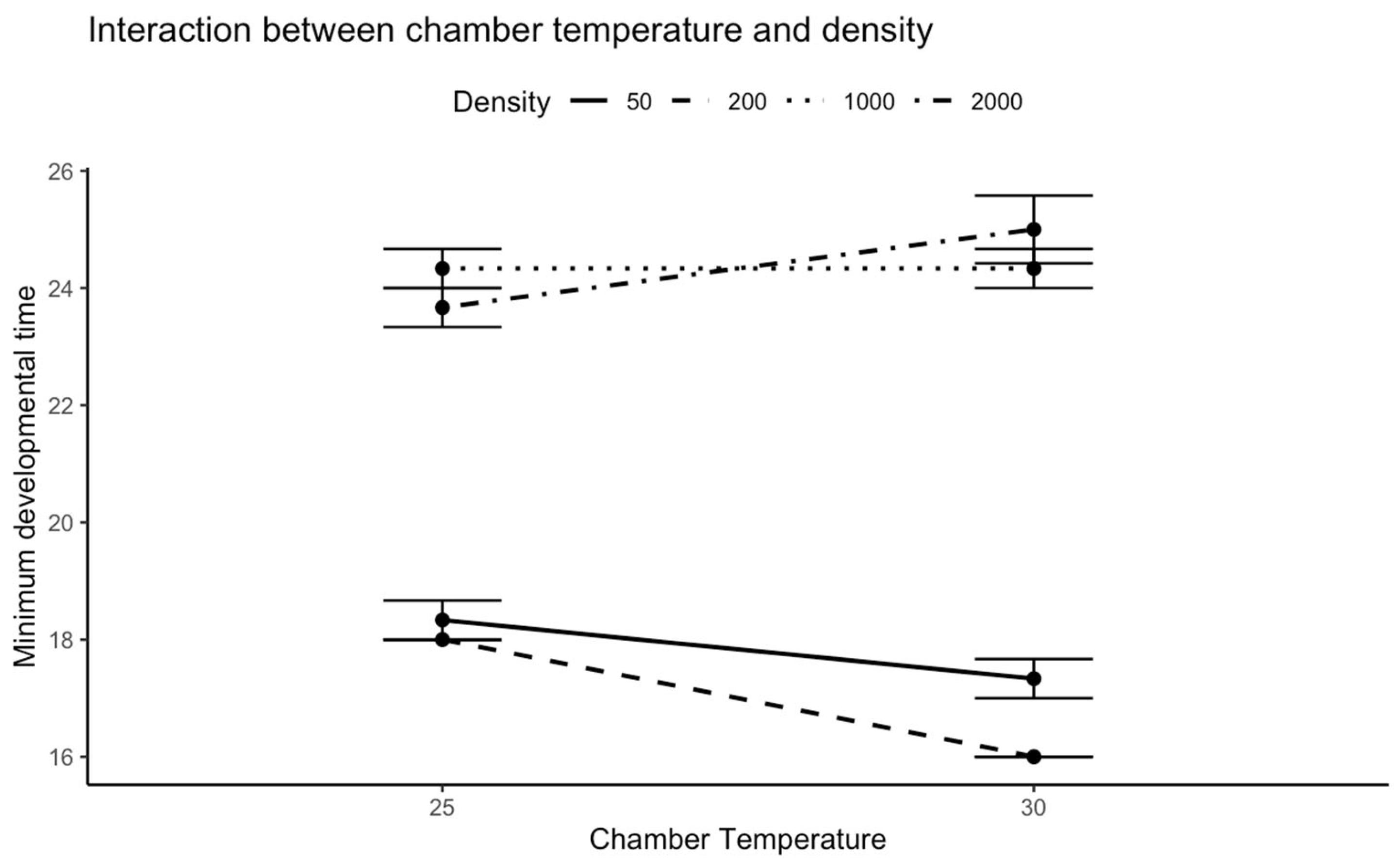
For
C. vicina, the significant temperature-density interaction demonstrates that thermal effects on development are contingent upon larval density. The shortest developmental time was observed at 30 °C with a density of 200 larvae, indicating that higher temperatures may accelerate development under moderate densities by increasing metabolic rates [
5,
21]. However, the extended developmental period at the maximum density at 30 °C might suggest that at extremely high densities, the advantages of higher temperatures are offset by the combined effects of thermal stress and competition.
Lucillia sericata, on the other hand, showed no discernible relationship between density and temperature. This suggests that developmental time is primarily dictated by density rather than thermal conditions within the tested temperature range. The lack of temperature effects at tested ranges suggests
L. sericata possesses more remarkable metabolic plasticity, especially its anaerobic capabilities under crowding stress [
22]. This species-specific difference could be attributed to varying thermal tolerance mechanisms or differences in metabolic plasticity between the two species.
Lucillia sericata prefers warm, sunny conditions and exhibits a developmental minimum between 7.5 °C and 10.0 °C, with optimal larval heat emission and growth occurring between 22 °C and 25 °C [
11,
23].
Calliphora vicina, in contrast, favors cooler conditions, with a lower developmental threshold around 1.0 °C and active development beginning at about 13–16 °C, and it develops most efficiently at moderate temperatures near 20–25 °C [
24,
25].
Studies on blow fly development have shown that while temperature generally modulates insect growth rates, the extent of this effect can vary significantly among species depending on their ecological adaptations [
26,
27]. The significant growth delay at high densities points to hypoxic conditions limitation as a primary mechanism [
28].
Additionally, accumulating metabolic waste products, such as ammonia and CO
2, may exacerbate physiological stress [
29,
30]. These factors match the broader framework of density-dependent competition in necrophagous Diptera as larval aggregations face trade-offs between thermoregulatory benefits and resource depletion. While temperatures exceeding 40 °C for maggots in anoxic conditions were documented as critical, our study recorded a maximum of 35 °C in aerated containers. This suggests that thermal stress and anoxic conditions alone may not fully explain the observed developmental delays under our experimental conditions [
31].
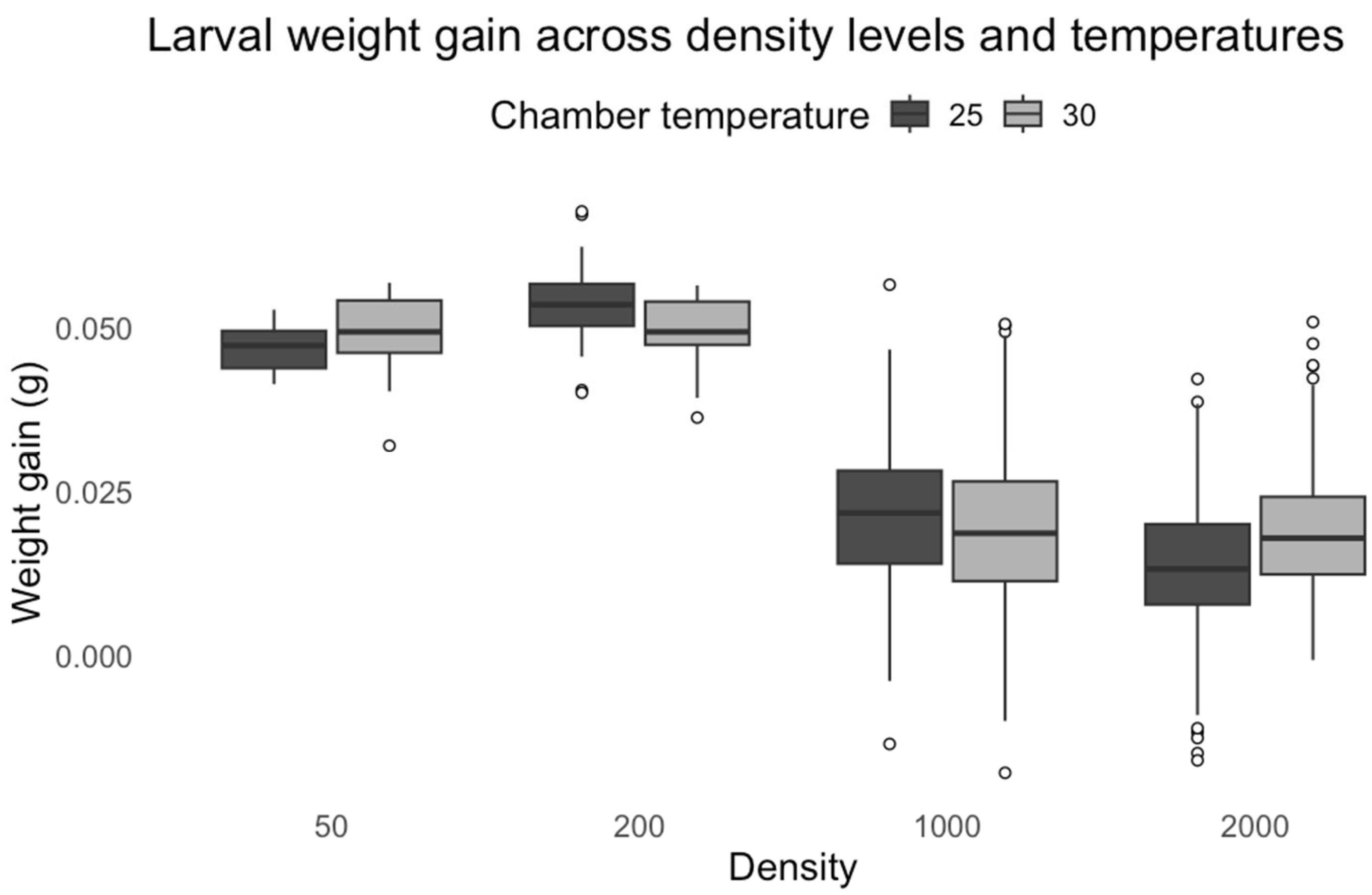
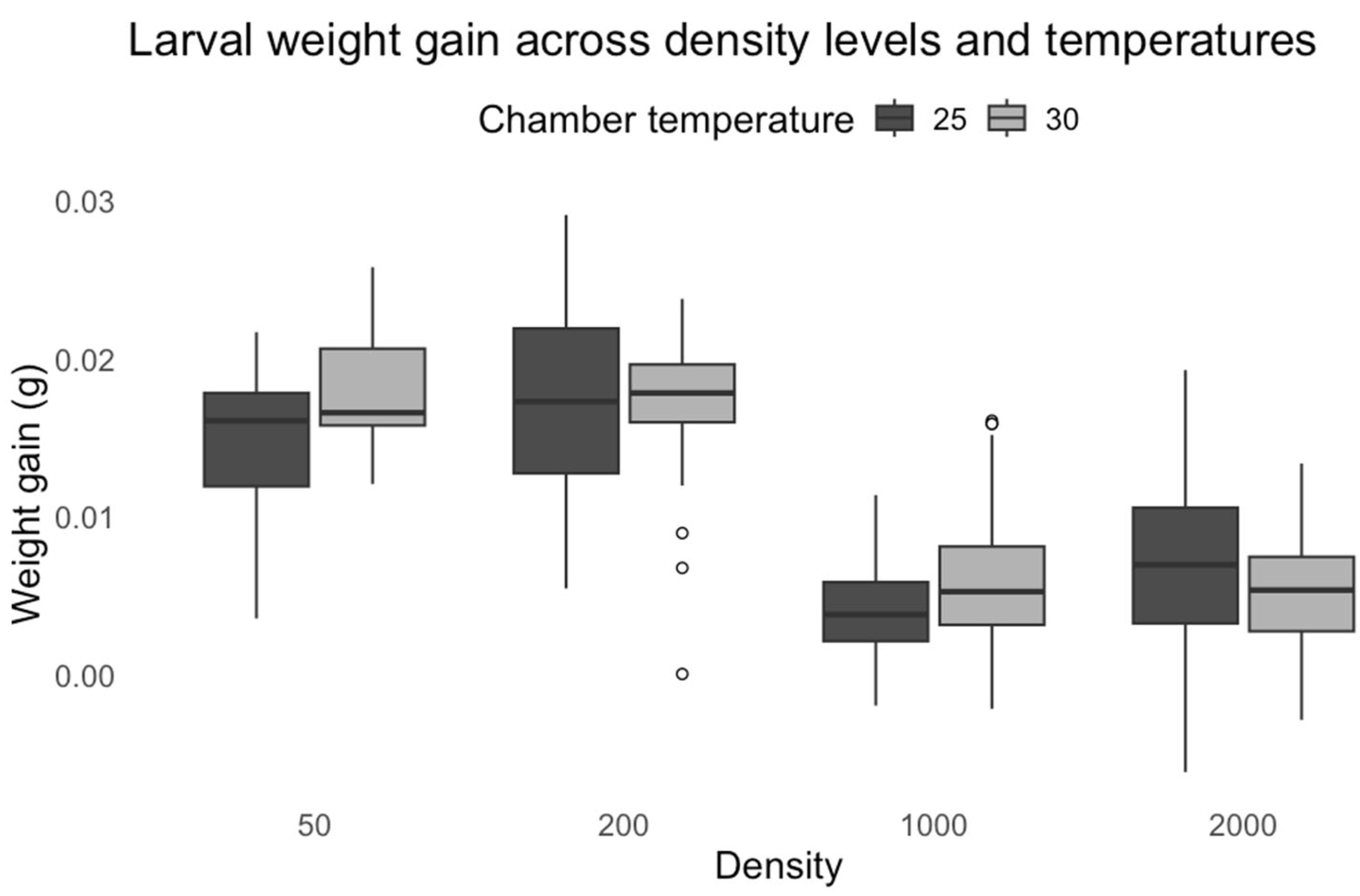
While developmental time provides crucial insights into growth dynamics, larval weight gain offers a complementary metric for assessing resource allocation and physiological stress under competitive conditions.
Our results demonstrate that larval weight in
C. vicina showed significant density-dependent patterns, with increased weight gain at low densities but marked reductions at higher densities. Moderate crowding initially enhances growth through cooperative feeding behaviors before reaching a competitive threshold [
32]. The temperature-density interaction suggests that thermal conditions modulate competitive outcomes, potentially through temperature-dependent effects on feeding rates and metabolic efficiency. Elevated temperatures increase metabolic demand while accelerating food depletion rates in crowded conditions [
33]. The growth response of
L. sericata differed from that of
C. vicina, with both temperature and density demonstrating significant effects on larval weight gain. The increased weight observed at 30 °C proved metabolic acceleration as temperatures enhance enzymatic activity and nutrient assimilation rates. However, the negative interaction between temperature and density reveals important constraints on this thermal advantage. The expected temperature-mediated growth enhancement was attenuated at small densities, possibly due to reduced cooperative feeding behaviors that normally optimize resource utilization in moderately crowded conditions. Similarly, at high densities, intensified competition likely offsets the metabolic benefits of warmer temperatures. Our findings extend this model by demonstrating that the thermal modulation of competition follows density-dependent thresholds, with the most pronounced effects occurring at intermediate densities where resource competition becomes significant but before complete resource depletion occurs. The species-specific responses to temperature and density interactions highlight the complexity of larval development under varying environmental conditions. The ability of
L. sericata to gain weight at higher temperatures suggests more remarkable metabolic plasticity, allowing larvae to adjust their physiology to optimize growth. In contrast,
C. vicina appears more constrained by density effects, likely due to differences in competitive behavior or inability to adapt to higher temperatures. These findings reinforce the importance of considering intrinsic and extrinsic factors in modeling larval growth and development in forensic and ecological studies.
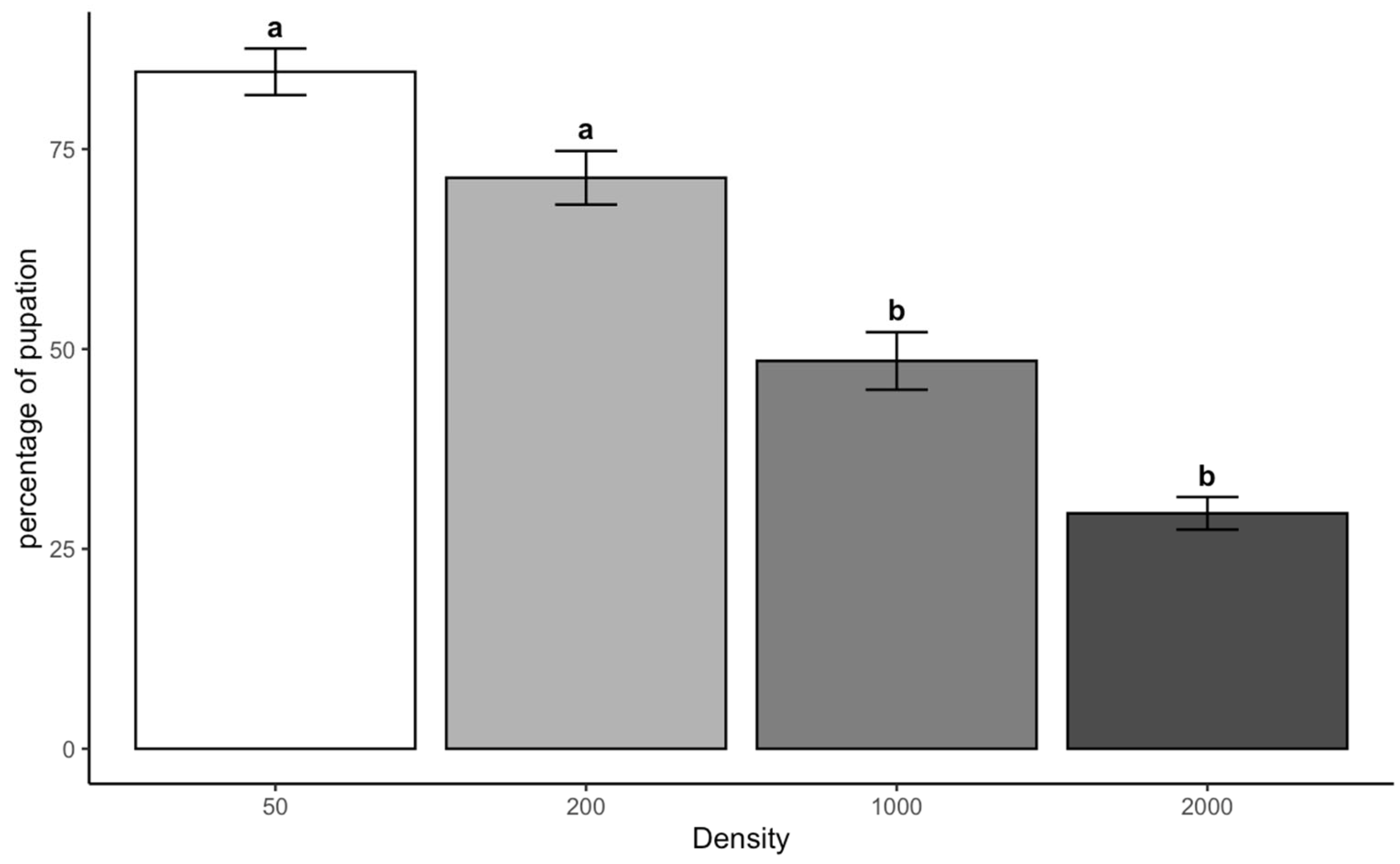
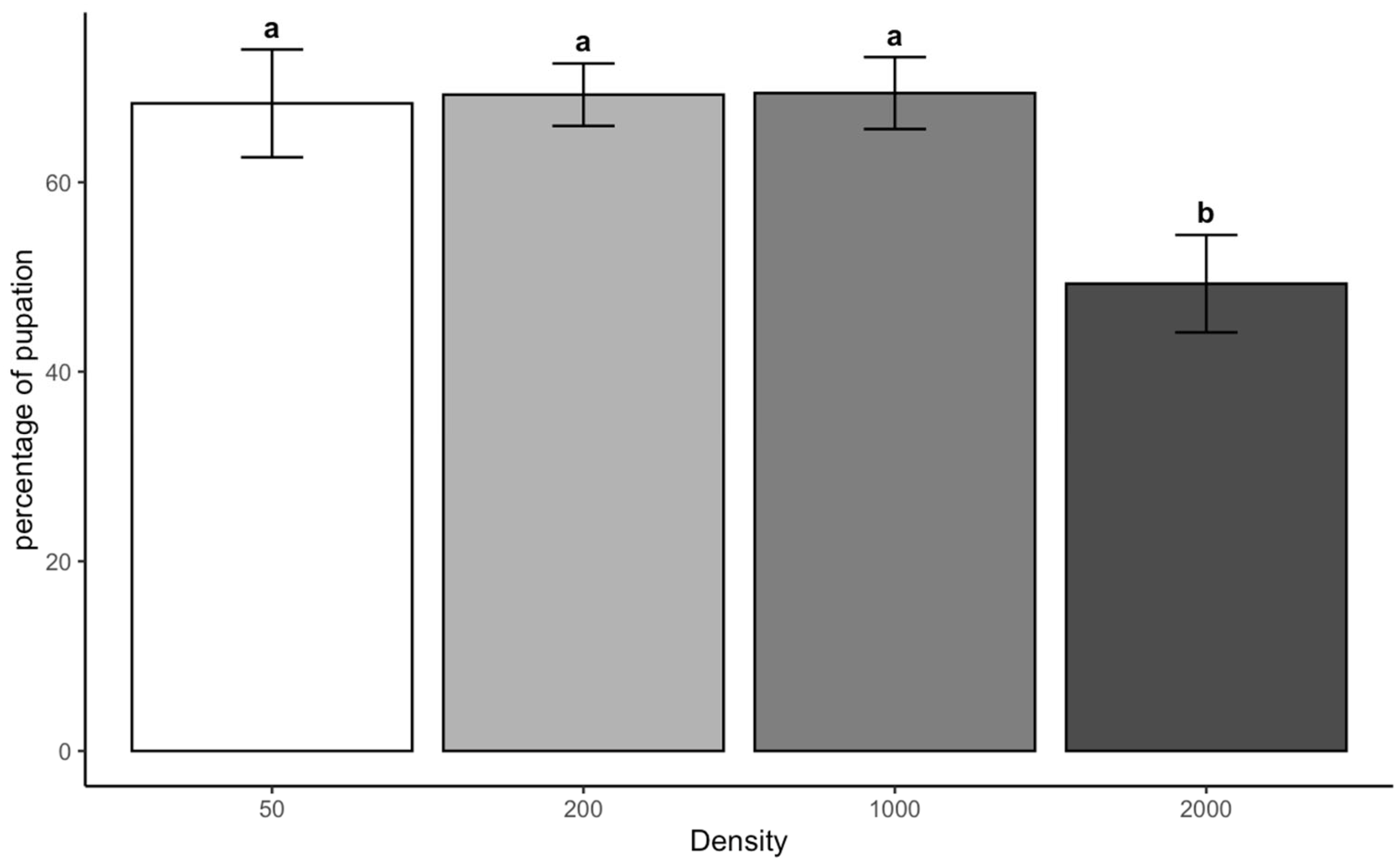
The density-dependent emergence patterns observed in both species correspond with the Allee effect threshold model [
34,
35]. However, we observed a 72% reduction in emergence success at 2000 larvae compared to low-density groups. We could, therefore, identify critical larval mass densities where cooperative feeding transitions to detrimental competition in necrophagous systems [
36].
The interspecific variation in thermal tolerance between
C. vicina and
L. sericata reflects fundamental differences in physiological adaptation.
C. vicina showed 58% lower emergence at 30 °C compared to 25 °C, consistent with its known thermal sensitivity near the upper physiological limit [
19]. This species’ poor performance under combined high-density and temperature conditions demonstrates how ambient conditions can compound thermal stress. In contrast,
L. sericata maintained more stable emergence rates across temperatures, confirming its broader thermal tolerance range.
The significant interaction between density and temperature challenges conventional developmental models used in forensic applications [
37]. These results emphasize how crowding effects can alter temperature-dependent development patterns [
38]. Such complex interactions must be accounted for in PMI estimation, as field conditions rarely involve single stressor scenarios [
39].
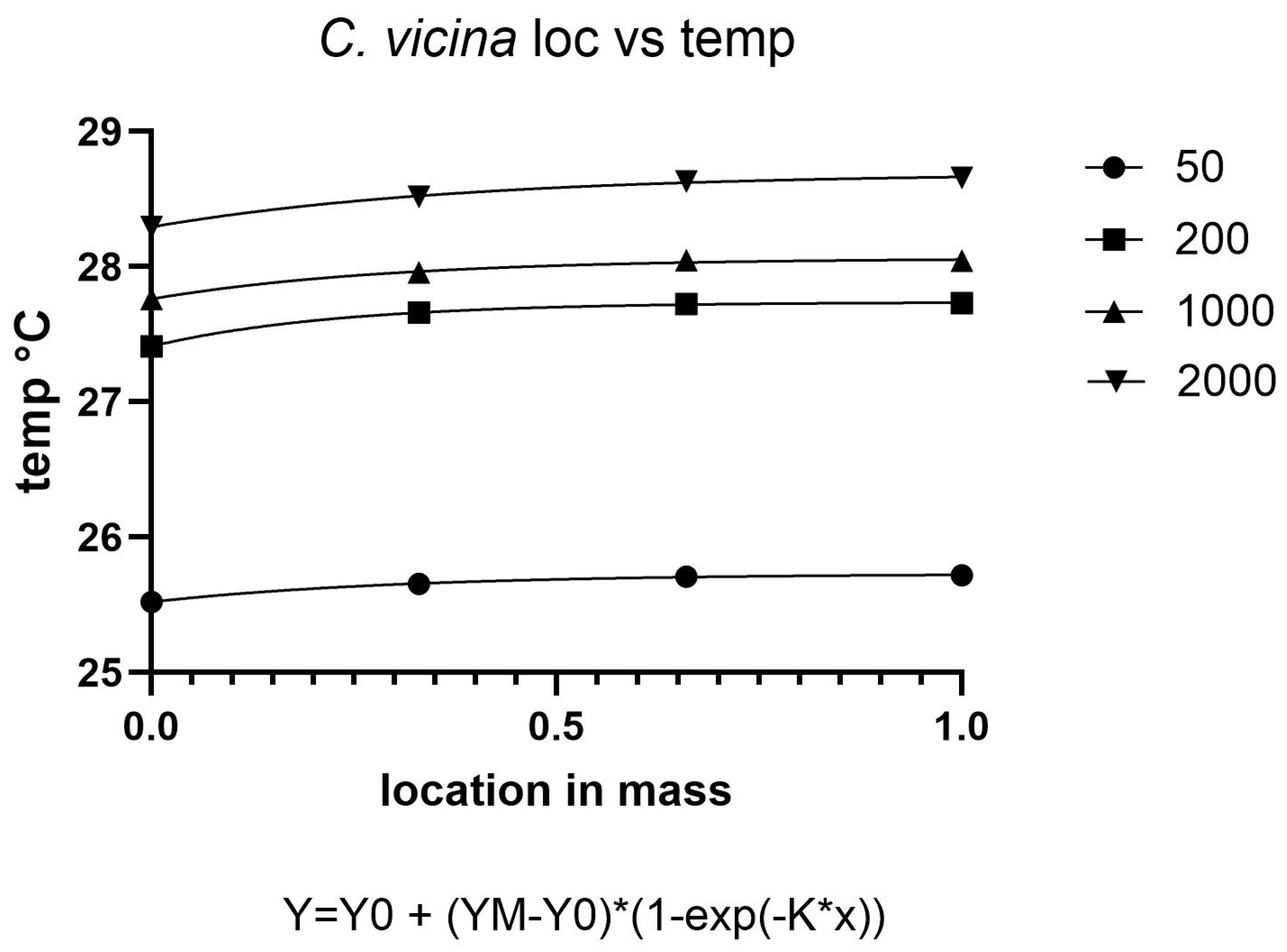
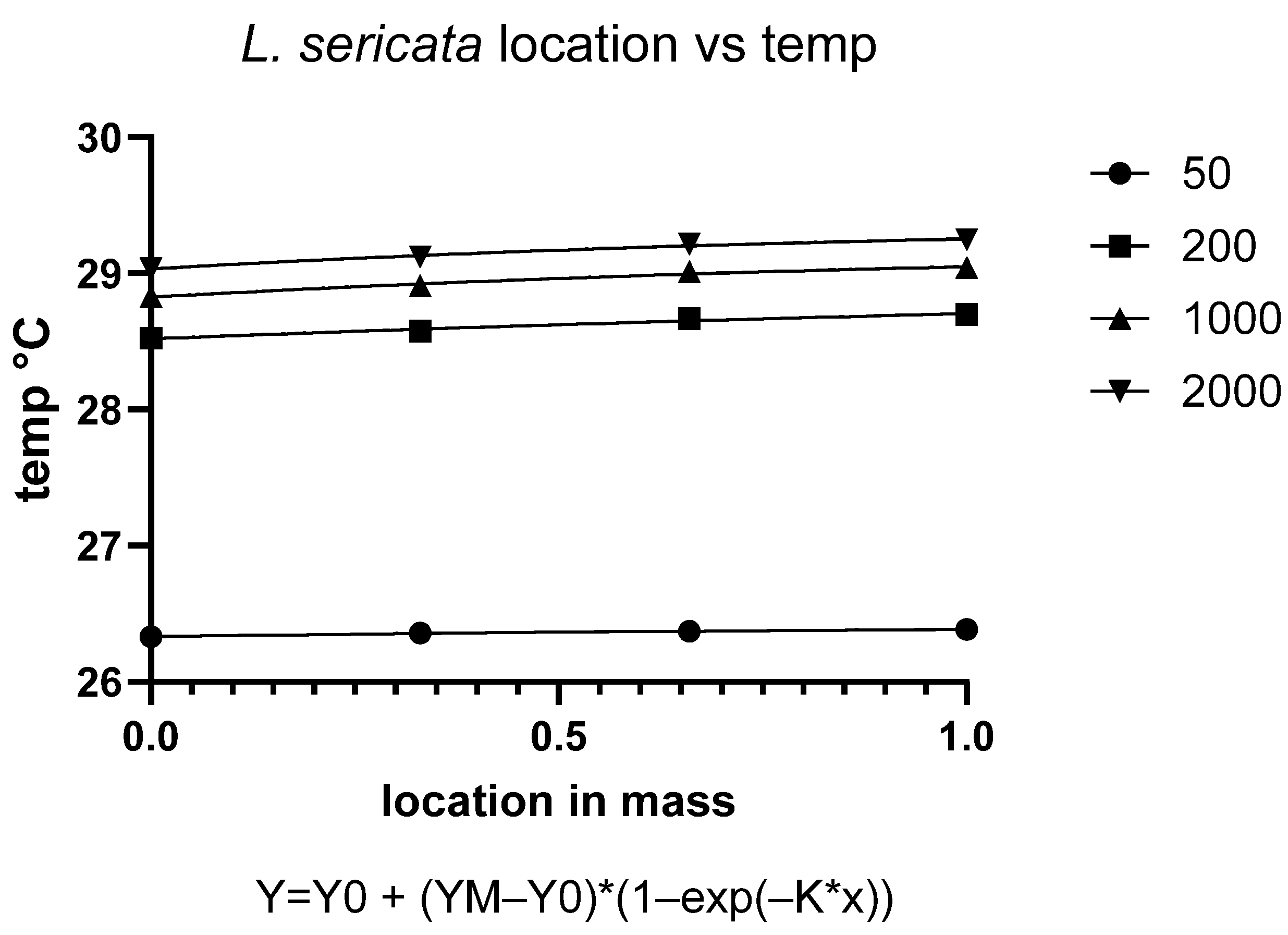
The metabolic activity of blow fly larvae generates substantial heat within aggregations, creating localized temperature increases that significantly impact developmental physiology. Our findings demonstrate a clear positive relationship between density and temperature elevation in both
C. vicina and
L. sericata, confirming established principles of larval thermoregulation [
18]. This phenomenon results from the combined effects of collective metabolic output and limited heat dissipation in dense aggregations, as documented in forensic entomology studies [
12].
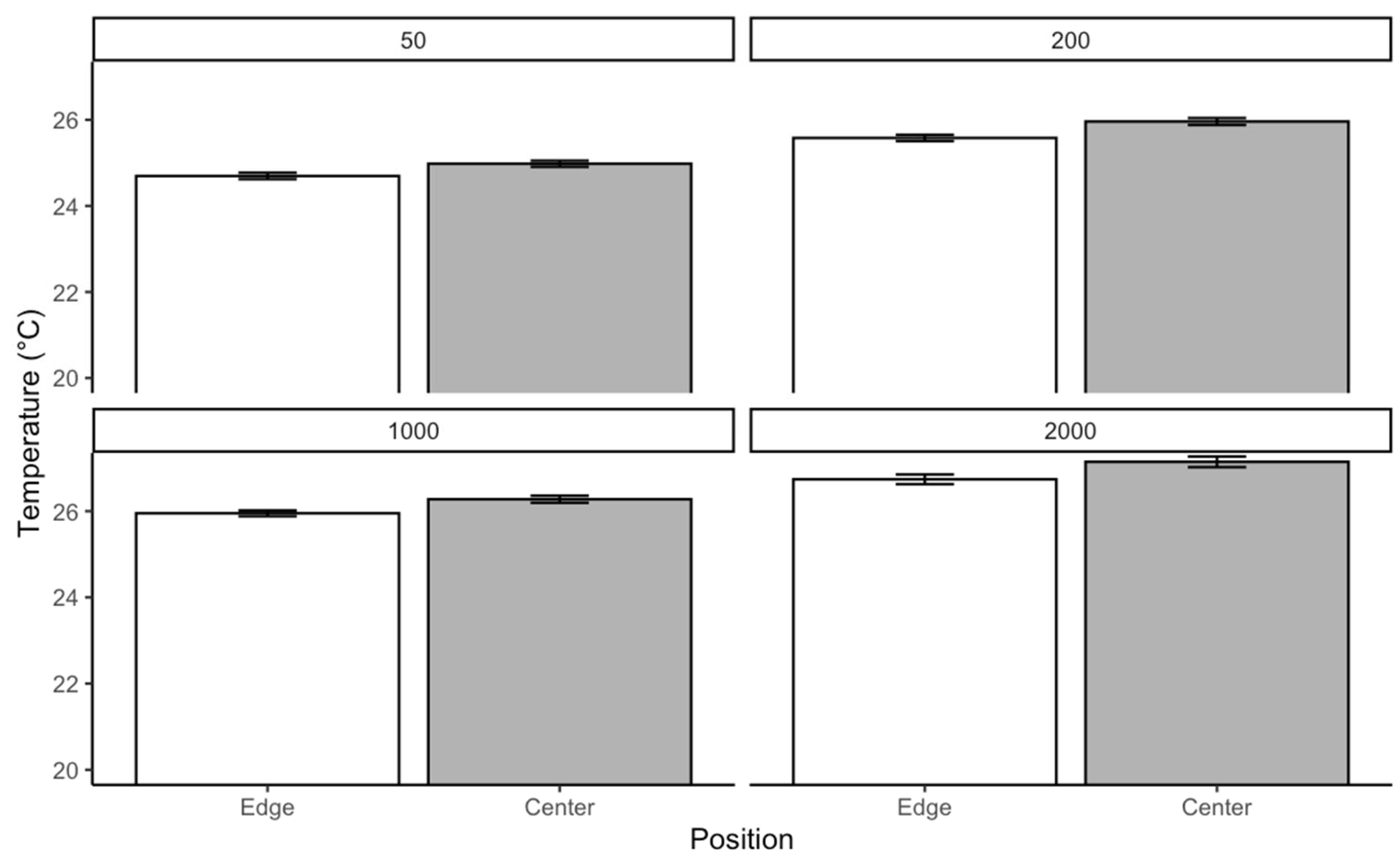
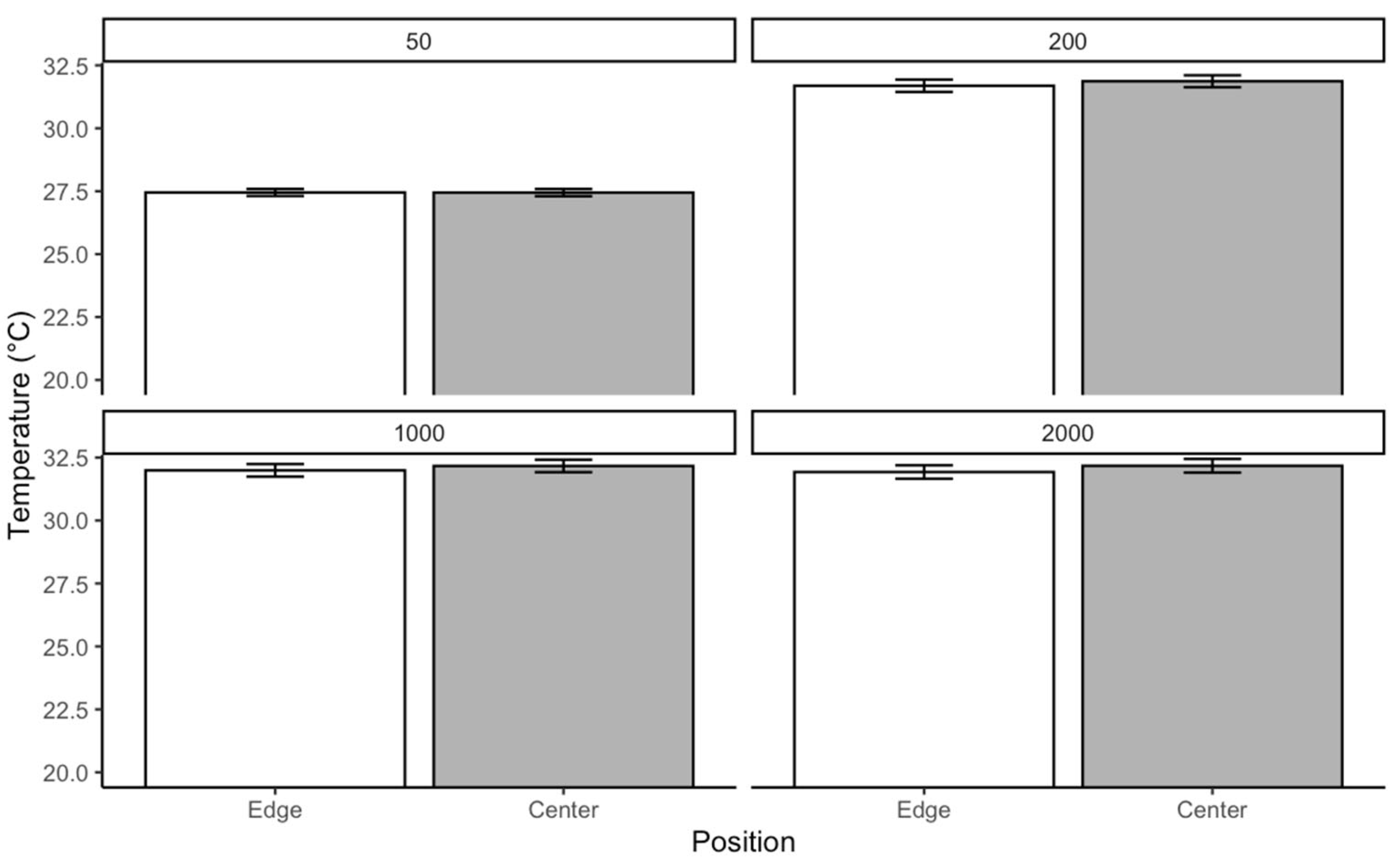
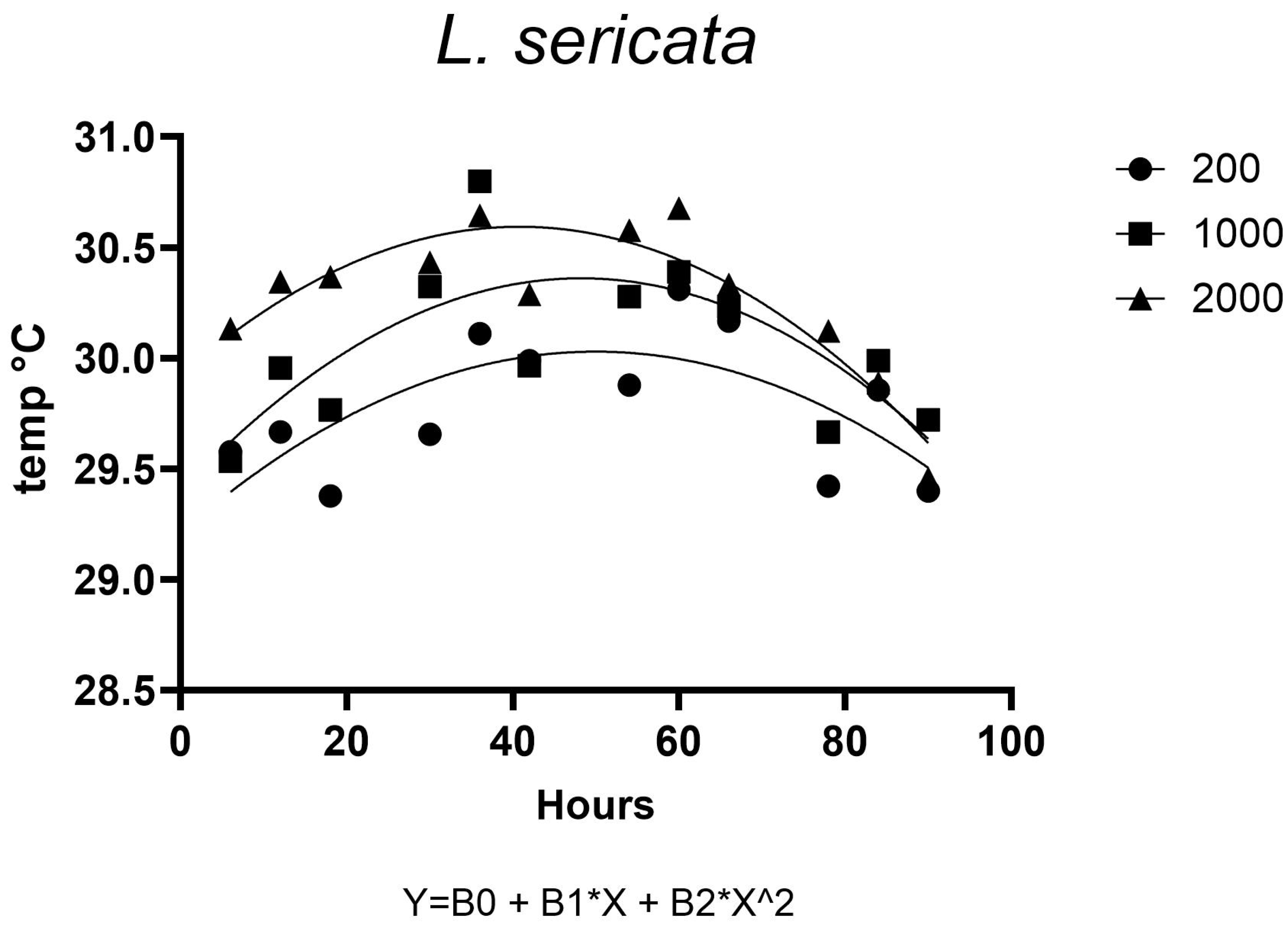
Temperature distribution within maggot masses follows characteristic spatial patterns, with maximum temperatures occurring at the center of medium-sized aggregations (200–2000 larvae). This thermal architecture reflects the balance between heat production from larval metabolism and insulation effects from surrounding biomass [
38]. However, as aggregation density increases beyond this threshold, enhanced surface area and convective cooling mechanisms serve to stabilize internal temperatures [
40].
Notably, at ambient temperatures of 30 °C, we observed limited additional heat accumulation in larger aggregations. This suggests that environmental conditions may constrain the thermoregulatory capacity of larval masses, potentially limiting their ability to maintain optimal developmental temperatures. These findings have important implications for forensic applications, as they demonstrate how microclimate conditions can substantially alter developmental timelines used in postmortem interval estimation.
The species-specific differences observed in thermal regulation align with known ecological adaptations of these forensically important flies; specifically, C. vicina, being more temperate in distribution, shows greater sensitivity to thermal extremes than the more cosmopolitan L. sericata.
Preconditioning has been widely recognized as a mechanism that enhances thermal tolerance in insects by inducing physiological changes that allow them to withstand extreme temperatures [
41,
42]. In this study, preconditioning significantly increased the lethal temperature of
L. sericata larvae, supporting previous findings that short-term exposure to sublethal heat stress can improve heat resistance through mechanisms such as heat shock protein (HSP) expression and cellular repair processes [
43]. The observed increase in thermal tolerance supports the idea that short-term acclimation plays a role in insect survival under fluctuating environmental conditions. Furthermore, Monzon et al. [
8] demonstrated that adult
L. sericata could survive at temperatures up to 43 °C, though oviposition success declined at higher temperatures. The present study extends this understanding by showing that larvae of
L. sericata can tolerate temperatures beyond those lethal to adults, particularly when preconditioned.
In
C. vicina, preconditioning also influenced thermal tolerance, though the effect appeared less pronounced. The increase in lethal temperature following short-term preconditioning suggests that heat exposure can induce some level of thermotolerance. However, the lack of a strong trend with extended preconditioning time indicates that additional exposure may not enhance heat resistance beyond a certain threshold. Similar findings have been reported in other insect species, where rapid thermal acclimation provides immediate but limited benefits [
41]. Given the natural exposure of blow fly larvae to fluctuating environmental temperatures, their ability to develop short-term heat resistance could be advantageous in thermally variable habitats.
In contrast, preconditioning did not appear to influence the lethal temperature of C. vomitoria larvae. Among the three species studied, C. vomitoria appears to be more heat tolerant up to 49 °C. The consistent thermal tolerance observed across treatments suggests that this species may possess an inherently higher capacity to withstand extreme temperatures. If C. vomitoria larvae have evolved to tolerate higher temperatures without preconditioning, this could indicate species-specific differences in heat adaptation strategies.
5. Conclusions
Forensic entomologists rely heavily on blow fly ecological succession patterns and temperature-dependent ADD models to estimate PMI in legal investigations. While these methods are well-established, a critical factor often overlooked is the impact of larval aggregation on development. Blow fly larvae naturally form dense masses, generating metabolic heat that can elevate internal temperatures by up to 20 °C above ambient conditions.
This study demonstrates that larval density significantly influences the developmental period of L. sericata and C. vicina. A critical density threshold was identified at approximately 1000 larvae, representing the optimal “maggot mass” density for studying aggregation effects. Competition becomes biologically significant at this density, leading to extended developmental times. Below this threshold, resource availability remains sufficient for optimal growth, while higher densities result in severe physiological stress and reduced survival.
A key finding is the non-uniform temperature distribution within larval masses, where heat generated by metabolic activity increases from the edges toward the center. Medium-sized masses (200–2000 larvae) exhibit the most pronounced thermal gradient, with the highest temperatures recorded at the core. However, this self-generated heat does not necessarily accelerate development, as the combined effects of crowding and thermal stress can instead delay growth. Our results indicate that using only internal maggot mass temperatures for forensic development models may lead to overestimating growth rates. The adverse effects of competition and waste accumulation can counteract the potential benefits of metabolic heat.
Additionally, species-specific responses indicate varying thermal tolerances: C. vicina shows significant developmental delays under high-density, high-temperature conditions, whereas L. sericata demonstrates notable metabolic plasticity, maintaining stable growth rates despite crowding. These differences suggest that climate change may disproportionately affect blow fly species, with temperate-adapted flies like C. vicina facing more significant challenges in warming environments.
These findings critically impact PMI estimation, as traditional models often assume that maggot mass temperatures accelerate development. Our study demonstrates that crowding effects can undermine the thermal benefits of metabolic heat in maggot masses. In addition, internal mass temperatures should not be used as the sole metric for developmental predictions. Instead, density-dependent competition must be incorporated into forensic models to avoid overestimating growth rates in casework.
Rising temperatures may impact the aggregation dynamics of blow flies in the context of climate change, potentially benefiting heat-tolerant species such as L. sericata while disadvantaging thermally sensitive species like C. vicina. An increase in the frequency of heatwaves could push larval masses beyond their thermoregulatory limits, leading to higher mortality rates or disrupted development. Additionally, decreased carrion availability might increase larval crowding, further delaying development and complicating forensic estimations.