Bee Hotels as a Tool for Post-Fire Recovery of Cavity-Nesting Native Bees
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Bee Hotels
2.3. Surveys of Bees Foraging on Flowers
2.4. Data Analysis
3. Results
3.1. Bee Hotel Colonisation
3.2. Bee Hotel Material
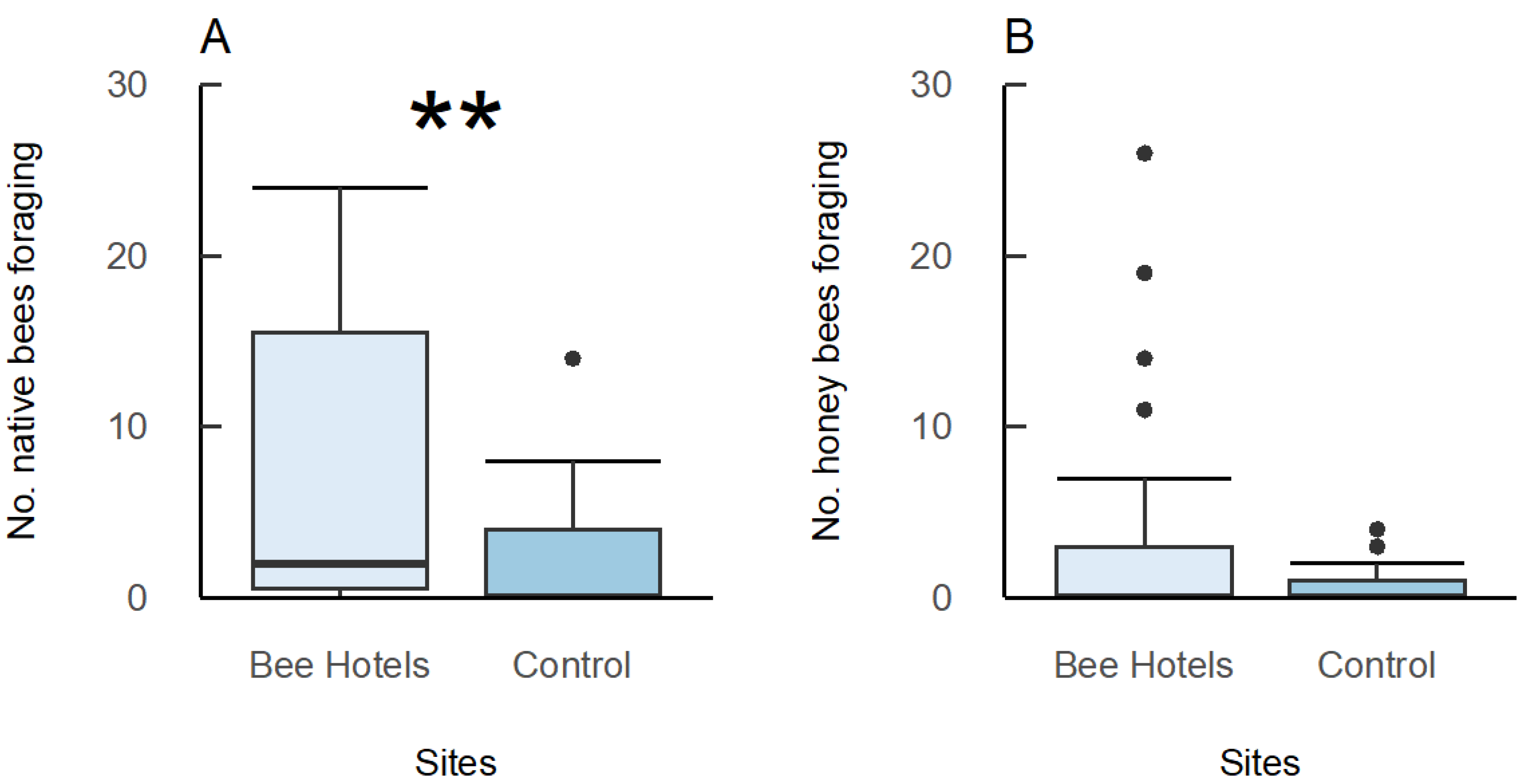
3.3. Foraging Bee Surveys
3.4. Taxonomic Composition of Bee Hotel Communities
3.5. Foraging Resources
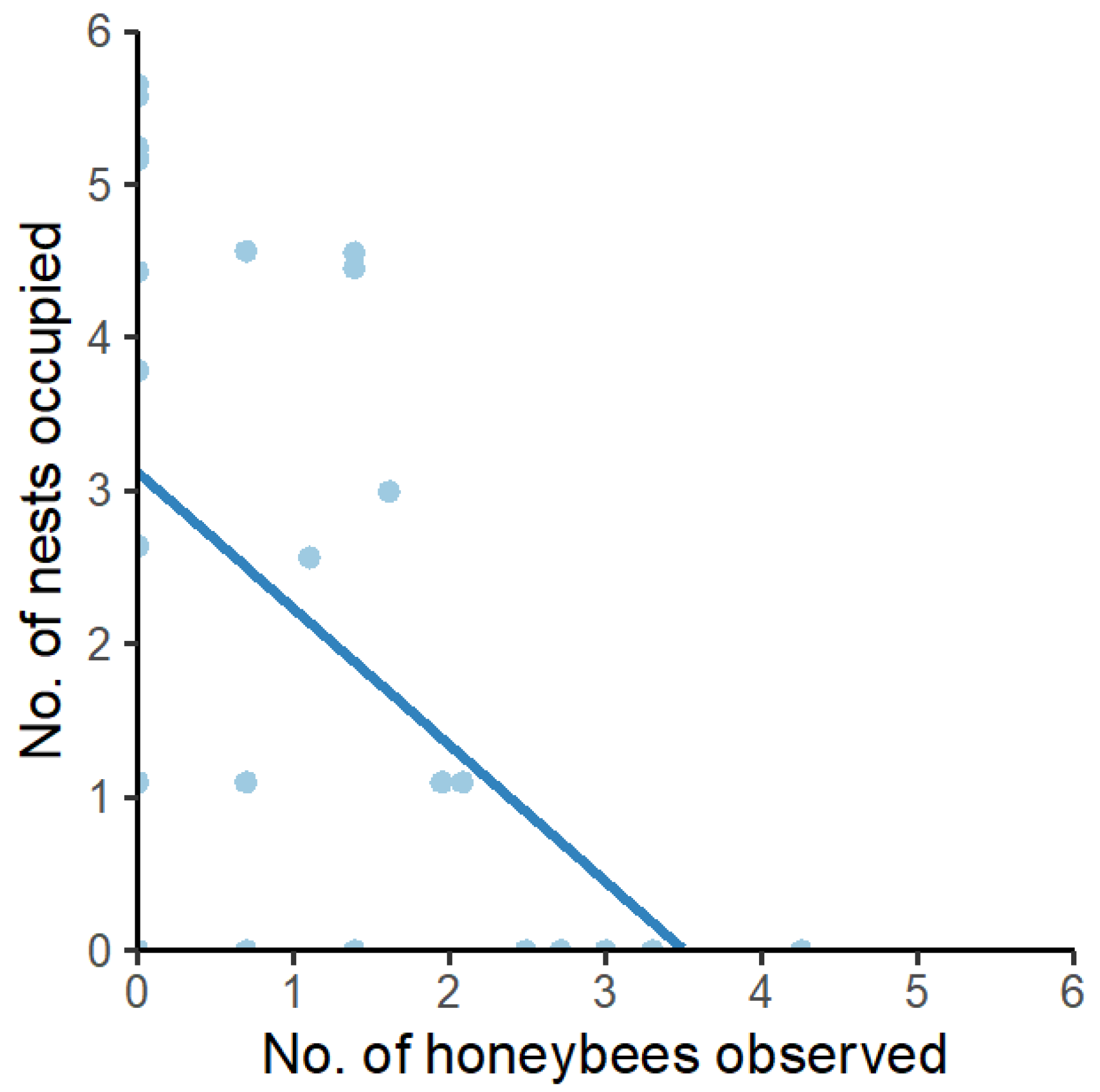
3.6. Honeybee Competition
4. Discussion
Future Research Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, M.W.; Smith, A.J.; Betts, R.; Canadell, J.G.; Prentice, I.C.; Le Quéré, C. Climate Change Increases the Risk of Wildfires: January 2020. ScienceBrief 2020. Available online: https://ueaeprints.uea.ac.uk/id/eprint/77982/ (accessed on 30 May 2024).
- McKenzie, D.; Gedalof, Z.e.; Peterson, D.L.; Mote, P. Climatic change, wildfire, and conservation. Conserv. Biol. 2004, 18, 890–902. [Google Scholar] [CrossRef]
- Brown, M.J.; Dicks, L.V.; Paxton, R.J.; Baldock, K.C.; Barron, A.B.; Chauzat, M.-P.; Freitas, B.M.; Goulson, D.; Jepsen, S.; Kremen, C. A horizon scan of future threats and opportunities for pollinators and pollination. PeerJ 2016, 4, e2249. [Google Scholar] [CrossRef]
- Carbone, L.M.; Tavella, J.; Pausas, J.G.; Aguilar, R. A global synthesis of fire effects on pollinators. Glob. Ecol. Biogeogr. 2019, 28, 1487–1498. [Google Scholar] [CrossRef]
- Dickman, C.; McDonald, T. Some personal reflections on the present and future of Australia’s fauna in an increasingly fire-prone continent. Ecol. Manag. Restor. 2020, 21, 86–96. [Google Scholar] [CrossRef]
- Boer, M.M.; Resco de Dios, V.; Bradstock, R.A. Unprecedented burn area of Australian mega forest fires. Nat. Clim. Change 2020, 10, 171–172. [Google Scholar] [CrossRef]
- Collins, L.; Bradstock, R.A.; Clarke, H.; Clarke, M.F.; Nolan, R.H.; Penman, T.D. The 2019/2020 mega-fires exposed Australian ecosystems to an unprecedented extent of high-severity fire. Environ. Res. Lett. 2021, 16, 044029. [Google Scholar] [CrossRef]
- Rumpuff, L.; Legge, S.; van Leeuwen, S.; Wintle, B.; Woinarski, J. Australia’s Megafires: Biodiversity Impacts and Lessons from 2019–2020; CSIRO Publishing: Clayton South, VIC, Australia, 2023. [Google Scholar]
- Carbone, L.M.; Tavella, J.; Marquez, V.; Ashworth, L.; Pausas, J.G.; Aguilar, R. Fire effects on pollination and plant reproduction: A quantitative review. Ann. Bot. 2025, 135, 43–56. [Google Scholar] [CrossRef]
- Mola, J.M.; Williams, N.M. Fire-induced change in floral abundance, density, and phenology benefits bumble bee foragers. Ecosphere 2018, 9, e02056. [Google Scholar] [CrossRef]
- Smith, J.P.; Heard, T.A.; Beekman, M.; Gloag, R. Flight range of the Australian stingless bee Tetragonula carbonaria (Hymenoptera: Apidae). Austral Entomol. 2017, 56, 50–53. [Google Scholar] [CrossRef]
- Zurbuchen, A.; Landert, L.; Klaiber, J.; Müller, A.; Hein, S.; Dorn, S. Maximum foraging ranges in solitary bees: Only few individuals have the capability to cover long foraging distances. Biol. Conserv. 2010, 143, 669–676. [Google Scholar] [CrossRef]
- Ulyshen, M.D.; Wilson, A.C.; Ohlson, G.C.; Pokswinksi, S.M.; Hiers, J.K. Frequent prescribed fires favour ground-nesting bees in southeastern U.S. forests. Insect Conserv. Divers. 2021, 14, 527–534. [Google Scholar] [CrossRef]
- Brokaw, J.; Portman, Z.M.; Bruninga-Socolar, B.; Cariveau, D.P. Prescribed fire increases the number of ground-nesting bee nests in tallgrass prairie remnants. Insect Conserv. Divers. 2023, 16, 355–367. [Google Scholar] [CrossRef]
- Grau-Andrés, R.; Moreira, B.; Pausas, J.G. Global plant responses to intensified fire regimes. Glob. Ecol. Biogeogr. 2024, 33, e13858. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Blanchard, W.; McBurney, L.; Blair, D.; Banks, S.; Likens, G.E.; Franklin, J.F.; Laurance, W.F.; Stein, J.A.; Gibbons, P. Interacting factors driving a major loss of large trees with cavities in a forest ecosystem. PLoS ONE 2012, 7, e41864. [Google Scholar] [CrossRef]
- Etchells, H.; O’Donnell, A.J.; McCaw, W.L.; Grierson, P.F. Fire severity impacts on tree mortality and post-fire recruitment in tall eucalypt forests of southwest Australia. For. Ecol. Manag. 2020, 459, 117850. [Google Scholar] [CrossRef]
- Fettig, C.J.; Runyon, J.B.; Homicz, C.S.; James, P.M.; Ulyshen, M.D. Fire and insect interactions in North American forests. Curr. For. Rep. 2022, 8, 301–316. [Google Scholar] [CrossRef]
- Lazarina, M.; Sgardelis, S.P.; Tscheulin, T.; Kallimanis, A.S.; Devalez, J.; Petanidou, T. Bee response to fire regimes in Mediterranean pine forests: The role of nesting preference, trophic specialization, and body size. Basic Appl. Ecol. 2016, 17, 308–320. [Google Scholar] [CrossRef]
- Potts, S.G.; Vulliamy, B.; Roberts, S.; O’Toole, C.; Dafni, A.; Ne’eman, G.; Willmer, P. Role of nesting resources in organising diverse bee communities in a Mediterranean landscape. Ecol. Entomol. 2005, 30, 78–85. [Google Scholar] [CrossRef]
- Williams, N.M.; Crone, E.E.; T’ai, H.R.; Minckley, R.L.; Packer, L.; Potts, S.G. Ecological and life-history traits predict bee species responses to environmental disturbances. Biol. Conserv. 2010, 143, 2280–2291. [Google Scholar] [CrossRef]
- Hogendoorn, K.; Glatz, R.V.; Leijs, R. Conservation management of the green carpenter bee Xylocopa aerata (Hymenoptera: Apidae) through provision of artificial nesting substrate. Austral Entomol. 2021, 60, 82–88. [Google Scholar] [CrossRef]
- Michener, C.D. The Bees of the World, 2nd ed.; Johns Hopkins: Baltimore, MD, USA, 2007; p. 992. [Google Scholar]
- MacIvor, J.S. Cavity-nest boxes for solitary bees: A century of design and research. Apidologie 2016, 48, 311–327. [Google Scholar] [CrossRef]
- Campbell, J.W.; Smithers, C.; Irvin, A.; Kimmel, C.B.; Stanley-Stahr, C.; Daniels, J.C.; Ellis, J.D. Trap nesting wasps and bees in agriculture: A comparison of sown wildflower and fallow plots in Florida. Insects 2017, 8, 107. [Google Scholar] [CrossRef]
- Murphy, M. Interactive Effects of Habitat Loss and Climate Change on Insect Species Networks. Ph.D. Thesis, University of Western Australia, Perth, Australia, 2015. [Google Scholar]
- Wilson, R.S.; Leonhardt, S.D.; Burwell, C.J.; Fuller, C.; Smith, T.J.; Kaluza, B.F.; Wallace, H.M. Landscape simplification modifies trap-nesting bee and wasp communities in the subtropics. Insects 2020, 11, 853. [Google Scholar] [CrossRef]
- Prendergast, K. Intra and interspecific interactions involving Meroglossa rubricata at bee blocks in urbanised south-western Australia. West. Aust. Nat. 2017, 31, 82–87. [Google Scholar]
- Prendergast, K.A. Establishment of the East Australian masked bee Hylaeus nubilosus (Smith) (Hymenoptera: Colletidae: Hylaeinae) in Western Australia. Aust. Entomol. 2017, 44, 213–218. [Google Scholar] [CrossRef]
- Prendergast, K.; Menz, M.H.; Bateman, B.; Dixon, K. The relative performance of sampling methods for native bees: An empirical test and review of the literature. Ecosphere 2020, 11, e03076. [Google Scholar] [CrossRef]
- Dainese, M.; Riedinger, V.; Holzschuh, A.; Kleijn, D.; Scheper, J.; Steffan-Dewenter, I. Managing trap-nesting bees as crop pollinators: Spatiotemporal effects of floral resources and antagonists. J. Appl. Ecol. 2018, 55, 195–204. [Google Scholar] [CrossRef]
- Silberbauer, L. Founding patterns of Exoneura bicolor Smith in Cobboboonee State Forest, southwestern Victoria. Aust. Zool. 1992, 28, 67–73. [Google Scholar] [CrossRef]
- Enright, N.J.; Fontaine, J.B.; Bowman, D.M.; Bradstock, R.A.; Williams, R.J. Interval squeeze: Altered fire regimes and demographic responses interact to threaten woody species persistence as climate changes. Front. Ecol. Environ. 2015, 13, 265–272. [Google Scholar] [CrossRef]
- Fairman, T.A.; Nitschke, C.R.; Bennett, L.T. Too much, too soon? A review of the effects of increasing wildfire frequency on tree mortality and regeneration in temperate eucalypt forests. Int. J. Wildland Fire 2016, 25, 831–848. [Google Scholar] [CrossRef]
- Gosper, C.R.; Miller, B.P.; Gallagher, R.V.; Kinloch, J.; van Dongen, R.; Adams, E.; Barrett, S.; Cochrane, A.; Comer, S.; McCaw, L.; et al. Mapping risk to plant populations from short fire intervals via relationships between maturation period and environmental productivity. Plant Ecol. 2022, 223, 769–787. [Google Scholar] [CrossRef]
- Krebs, C.J. Ecology: The Experimental Analysis of Distribution and Abundance; Pearson Education: London, UK, 2009. [Google Scholar]
- Prendergast, K.S.; Dixon, K.W.; Bateman, P.W. Interactions between the introduced European honey bee and native bees in urban areas varies by year, habitat type and native bee guild. Biol. J. Linn. Soc. 2021, 133, 725–743. [Google Scholar] [CrossRef]
- Prendergast, K.S.; Dixon, K.W.; Bateman, P.W. The evidence for and against competition between the European honeybee and Australian native bees. Pac. Conserv. Biol. 2022, 29, 89–109. [Google Scholar] [CrossRef]
- Prendergast, K.S.; Ollerton, J. Impacts of the introduced European honeybee on Australian bee-flower network properties in urban bushland remnants and residential gardens. Austral Ecol. 2022, 47, 35–53. [Google Scholar] [CrossRef]
- Brundrett, M.C. One biodiversity hotspot to rule them all: Southwestern Australia—An extraordinary evolutionary centre for plant functional and taxonomic diversity. J. R. Soc. West. Aust. 2021, 104, 91–122. [Google Scholar] [CrossRef]
- Thorn, S.; Wills, A.; McCaw, L. Biodiversity monitoring for the jarrah (Eucalyptus marginata) forest in south-west Western Australia: An extension to ten-year findings of Forestcheck. For. Ecol. Manag. 2024, 567, 122082. [Google Scholar] [CrossRef]
- Anderson, N.S.; Fontaine, J.B.; Lewandrowski, W.; Walden, L.; Ruthrof, K.X. Drought and Wildfire Legacies Highlight Vulnerability of a Mediterranean Climate-Type Forest. Austral Ecol. 2025, 50, e70011. [Google Scholar] [CrossRef]
- Dell, B.; Havel, J.J.; Malajczuk, N. The Jarrah Forest: A Complex Mediterranean Ecosystem; Springer: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Maher, D.; McCaw, L.; Yates, C.J. Vulnerability of Forests in South-West Western Australia to Timber Harvesting Under the Influence of Climate Change: Expert Panel Report; Western Australian Department of Environment and Conservation: South Perth, Australia, 2009. [Google Scholar]
- Burrows, N.; Harper, R. A Report on Silvicultural Guidelines for the 2024–2033 Forest Management Plan to the Western Australian Department of Biodiversity, Conservation and Attractions; Western Australian Department of Biodiversity, Conservation and Attractions: Joondalup, Australia, 2022. [Google Scholar]
- Burrows, N.; Ward, B.; Robinson, A. Jarrah forest fire history from stem analysis and anthropological evidence. Aust. For. 1995, 58, 7–16. [Google Scholar] [CrossRef]
- Burrows, N.; McCaw, L. Prescribed burning in southwestern Australian forests. Front. Ecol. Environ. 2013, 11, e25–e34. [Google Scholar] [CrossRef]
- Bradshaw, S.; Dixon, K.; Lambers, H.; Cross, A.; Bailey, J.; Hopper, S. Understanding the long-term impact of prescribed burning in mediterranean-climate biodiversity hotspots, with a focus on south-western Australia. Int. J. Wildland Fire 2018, 27, 643–657. [Google Scholar] [CrossRef]
- Chandler, R.E.; Bates, B.C.; Charles, S.P. Rainfall trends in southwest Western Australia. In Statistical Methods for Trend Detection and Analysis in the Environmental Sciences; Wiley: Hoboken, NJ, USA, 2011; pp. 283–306. [Google Scholar]
- Burrows, N.; Ward, B.; Robinson, A. Fire regimes and tree growth in low rainfall jarrah forest of south-west Australia. Environ. Manag. 2010, 45, 1332–1343. [Google Scholar] [CrossRef]
- Gallagher, R.V.; Allen, S.; Mackenzie, B.D.; Yates, C.J.; Gosper, C.R.; Keith, D.A.; Merow, C.; White, M.D.; Wenk, E.; Maitner, B.S. High fire frequency and the impact of the 2019–2020 megafires on Australian plant diversity. Divers. Distrib. 2021, 27, 1166–1179. [Google Scholar] [CrossRef]
- Gathmann, A.; Tscharntke, T. Foraging ranges of solitary bees. J. Anim. Ecol. 2002, 71, 757–764. [Google Scholar] [CrossRef]
- Prendergast, K. Are bees in the ‘burbs checking in at bee hotels? In Proceedings of the Murdoch University Annual Research Symposium (MARS) 2019, Murdoch, Australia, 3 June 2019; Murdoch University: Murdoch, Australia, 2019. [Google Scholar]
- Prendergast, K.S. Checking in at bee hotels: Trap-nesting occupancy and fitness of cavity-nesting bees in an urbanised biodiversity hotspot. Urban Ecosyst. 2023, 26, 1381–1395. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.M.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. The Comprehensive R Archive Network (CRAN), R Package Version 0.3. 2.0; 2020. Available online: https://cran.r-project.org/web/packages/DHARMa/vignettes/DHARMa.html (accessed on 30 May 2024).
- Harrison, X.A. Using observation-level random effects to model overdispersion in count data in ecology and evolution. PeerJ 2014, 2, e616. [Google Scholar] [CrossRef]
- Bolker, B.M.; Brooks, M.E.; Clark, C.J.; Geange, S.W.; Poulsen, J.R.; Stevens, M.H.H.; White, J.-S.S. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol. Evol. 2009, 24, 127–135. [Google Scholar] [CrossRef]
- Barton, K.; Barton, M.K. Package ‘mumin’. Version 2015, 1, 439. [Google Scholar]
- Hothorn, L.A.; Ritz, C.; Schaarschmidt, F. Examples of Tukey’s Trend Test in General Parametric Models; R: CRAN Packages; 2020. Available online: https://cran.r-project.org/web/packages/tukeytrend/vignettes/ExamplesTukeytrendtest.pdf (accessed on 30 May 2024).
- Prendergast, K. Plant-Pollinator Network Interaction Matrices and Flowering Plant Species Composition in Urban Bushland Remnants and Residential Gardens in the Southwest Western Australian Biodiversity Hotspot; Curtin University: Perth, Australia, 2020. [Google Scholar] [CrossRef]
- Prendergast, K. Species of Native Bees in the Urbanised Region of the Southwest Western Australian Biodiversity Hotspot; Curtin University: Perth, Australia, 2020. [Google Scholar] [CrossRef]
- Gilpin, A.M.; Brettell, L.E.; Cook, J.M.; Power, S.A. The use of trap-nests to support crop pollinators in agricultural areas. Ecol. Res. 2022, 37, 768–779. [Google Scholar] [CrossRef]
- Houston, T.F. A Guide to the Native Bees of Australia; CSIRO Publishing: Clayton, Australia, 2018; p. 280. [Google Scholar]
- Dorey, J.B.; Rebola, C.M.; Davies, O.K.; Prendergast, K.S.; Parslow, B.A.; Hogendoorn, K.; Leijs, R.; Hearn, L.R.; Leitch, E.J.; O’Reilly, R.L.; et al. Continental risk assessment for understudied taxa post-catastrophic wildfire indicates severe impacts on the Australian bee fauna. Glob. Change Biol. 2021, 27, 6551–6567. [Google Scholar] [CrossRef] [PubMed]
- Cane, J.H. Pollinating bees crucial to farming wildflower seed for US habitat restoration. In Bee Pollination in Agricultural Ecosystems; Oxford University Press (OUP): Oxford, UK, 2008; pp. 48–64. [Google Scholar]
- Cane, J.H.; Love, B. Floral guilds of bees in sagebrush steppe: Comparing bee usage of wildflowers available for postfire restoration. Nat. Areas J. 2016, 36, 377–391. [Google Scholar] [CrossRef]
- Pyke, G.H.; Prendergast, K.S.; Ren, Z.-X. Pollination crisis Down-Under: Has Australasia dodged the bullet? Ecol. Evol. 2023, 13, e10639. [Google Scholar] [CrossRef]
- Graham, J.R.; Campbell, J.W.; Ellis, J.D. Uninvited Guests: Identifying Parasites and Other Nest Associates of Solitary Bees and Wasps Using Artificial Nest Sites in North Central Florida. Southeast. Nat. 2023, 22, 192–206. [Google Scholar] [CrossRef]
- Prendergast, K.S. Native flora receive more visits than exotics from bees, especially native bees, in an urbanised biodiversity hotspot. Pac. Conserv. Biol. 2023, 30, PC22033. [Google Scholar] [CrossRef]
- Bell, D.T.; Koch, J.M. Post-fire succession in the northern jarrah forest of Western Australia. Aust. J. Ecol. 1980, 5, 9–14. [Google Scholar] [CrossRef]
- Wajon, E. Response of native vegetation in the Great Southern region of Western Australia to fire. Australas. Plant Conserv. J. Aust. Netw. Plant Conserv. 2020, 29, 8–11. [Google Scholar] [CrossRef]
- Prendergast, K.S.; Willers, N. Corymbia calophylla (Marri) (K. D. Hill & L. A. S. Johnson) (Myrtaceae) is a major resource for native bees in the southwest western Australian biodiversity hotspot. Pac. Conserv. Biol. 2024, 30, PC24054. [Google Scholar] [CrossRef]
- Prendergast, K.; Murphy, M.; Kevan, P.; Ren, Z.-X.; Milne, L. Introduced honey bees have the potential to reduce fitness of cavity-nesting native bees in terms of a male bias sex ratio, brood mortality and reduced reproduction. Front. Bee Sci. 2025, 3, 1508958. [Google Scholar] [CrossRef]


| Model | ∆ R2 | LRT | Tukey’s Post-Hoc | ||||
|---|---|---|---|---|---|---|---|
| Marginal | Conditional | χ2 | df | p | Levels (Direction) | p | |
| N native bees foraging~Treatment | 0.2013 | 0.9954 | 9.683 | 1 | 0.0018 | Bee hotels > Control | 0.0023 |
| N honeybees foraging~Treatment | 0.0567 | 0.9614 | 1.396 | 1 | 0.2337 | Bee hotels > Control | 0.2000 |
| Group | Taxon | Bamboo | Wooden | Total | % Nests |
|---|---|---|---|---|---|
| Native bees | Megachilidae | 521 | 285 | 806 | 88.67 |
| Hylaeinae | 9 | 13 | 22 | 2.42 | |
| Exoneura | 2 | 2 | 4 | 0.44 | |
| Wasps | Eumeninae | 8 | 4 | 12 | 1.32 |
| Ants | Formicidae | 28 | 11 | 39 | 4.29 |
| Spiders | Aranae | 13 | 13 | 26 | 2.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prendergast, K.S.; Wilson, R.S. Bee Hotels as a Tool for Post-Fire Recovery of Cavity-Nesting Native Bees. Insects 2025, 16, 659. https://doi.org/10.3390/insects16070659
Prendergast KS, Wilson RS. Bee Hotels as a Tool for Post-Fire Recovery of Cavity-Nesting Native Bees. Insects. 2025; 16(7):659. https://doi.org/10.3390/insects16070659
Chicago/Turabian StylePrendergast, Kit Stasia, and Rachele S. Wilson. 2025. "Bee Hotels as a Tool for Post-Fire Recovery of Cavity-Nesting Native Bees" Insects 16, no. 7: 659. https://doi.org/10.3390/insects16070659
APA StylePrendergast, K. S., & Wilson, R. S. (2025). Bee Hotels as a Tool for Post-Fire Recovery of Cavity-Nesting Native Bees. Insects, 16(7), 659. https://doi.org/10.3390/insects16070659







