Predicting Suitable Habitat for Glipa (Coleoptera: Mordellidae: Mordellinae) Under Current and Future Climates Using MaxEnt Modeling
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
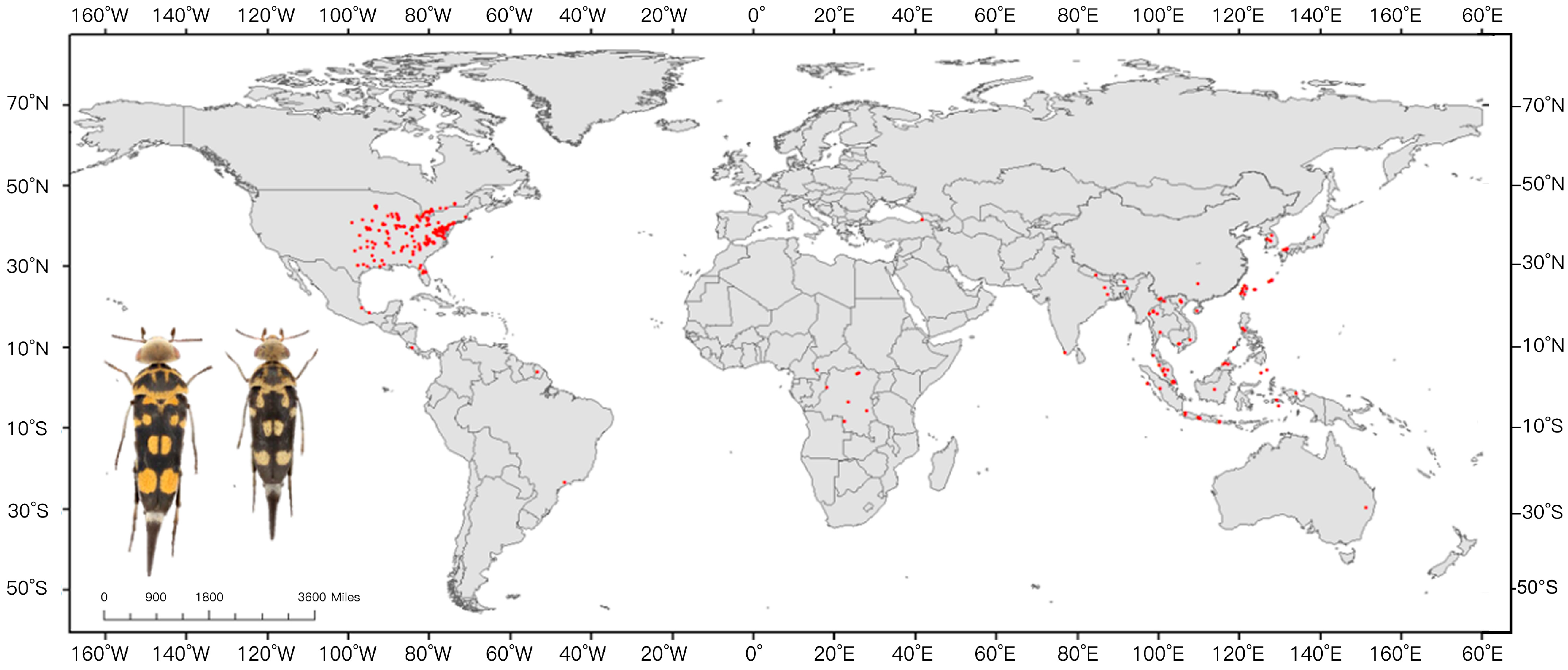
2.1. Geographic Coordinate Acquisition for Glipa
2.2. Selection of Environmental Variables
2.3. Model Optimization and Setup
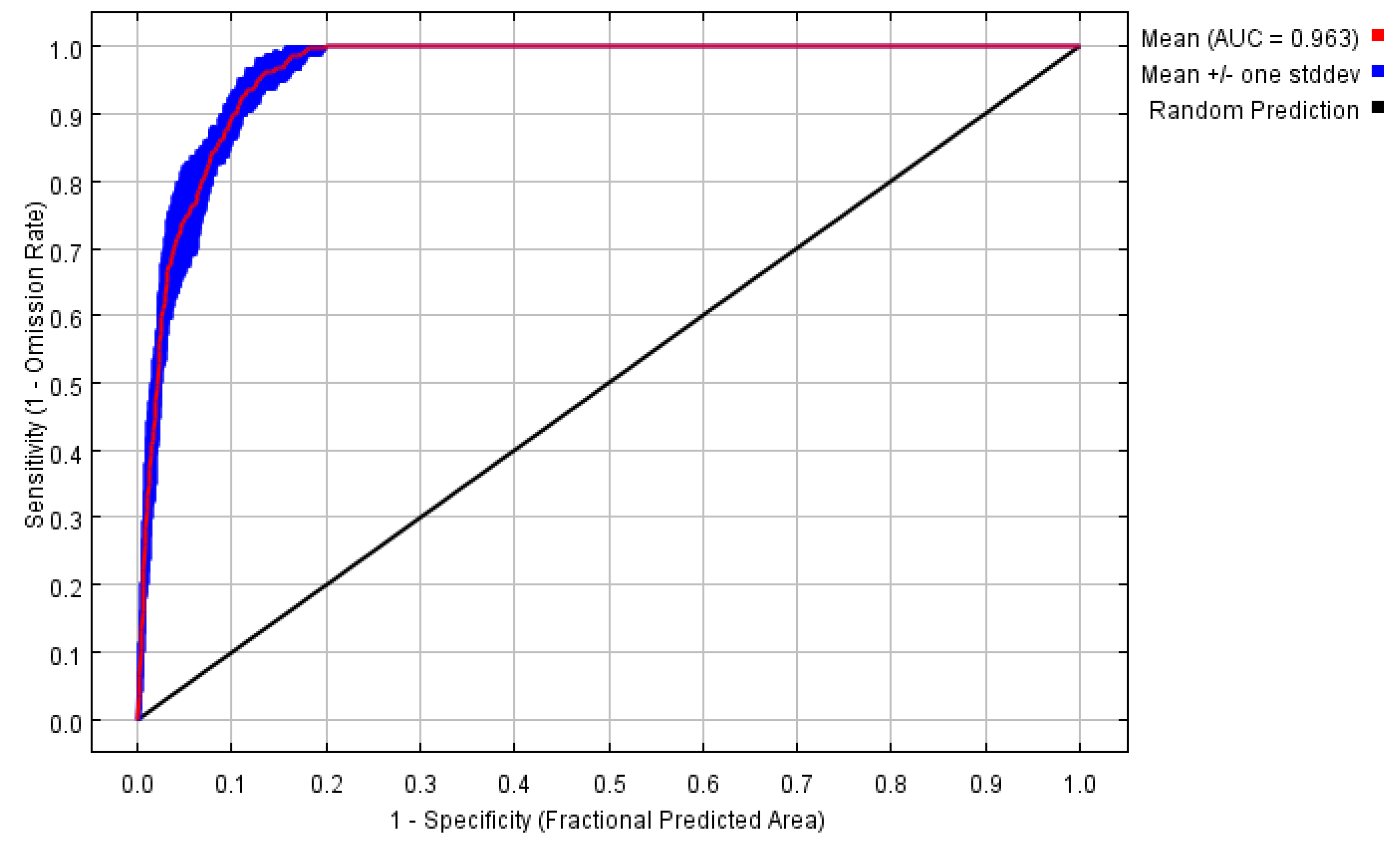
2.4. Model Accuracy
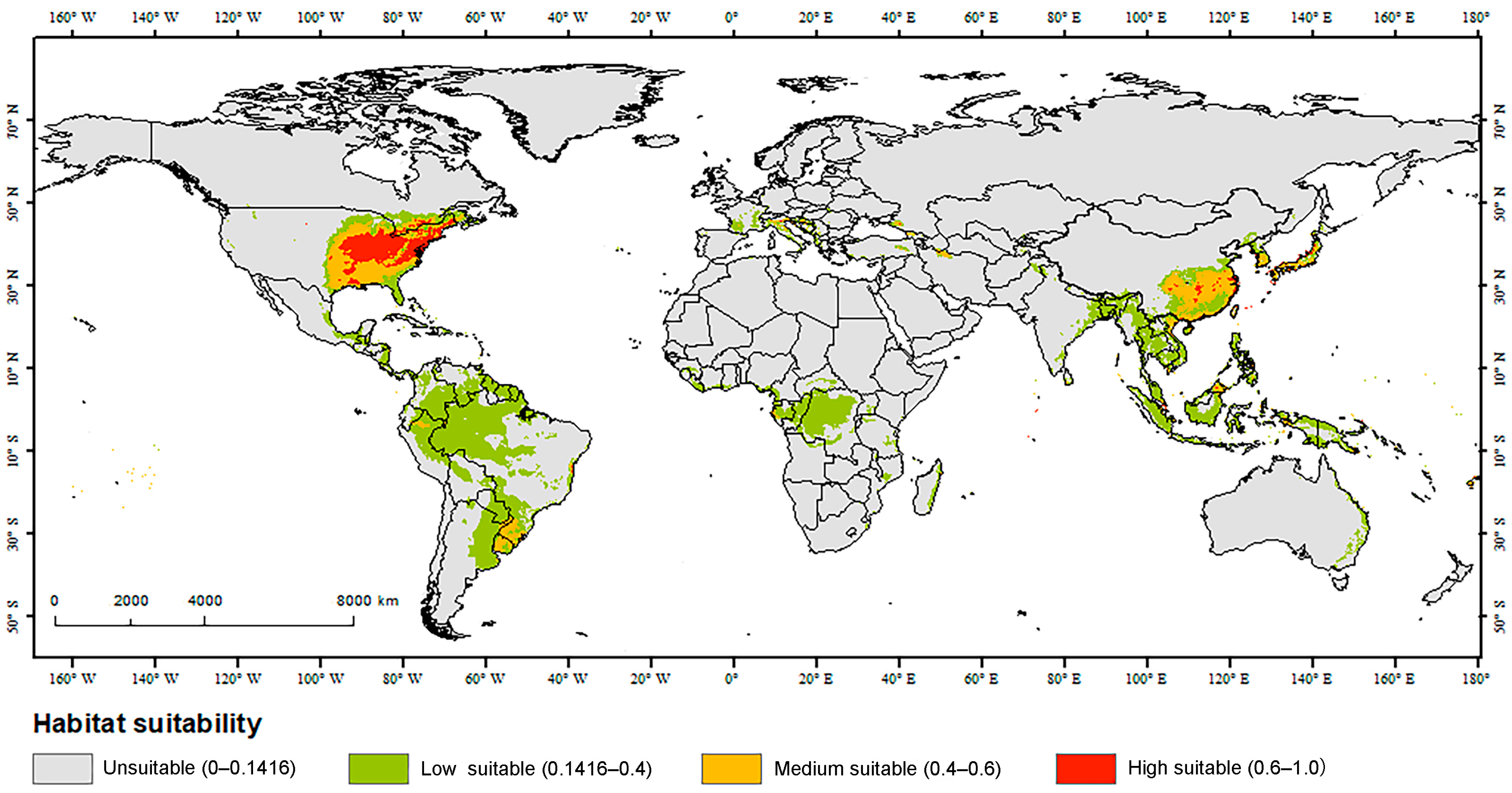
2.5. Analysis of Suitable Habitats and Their Variation
3. Results
4. Discussion
4.1. Analysis of Environmental Variables
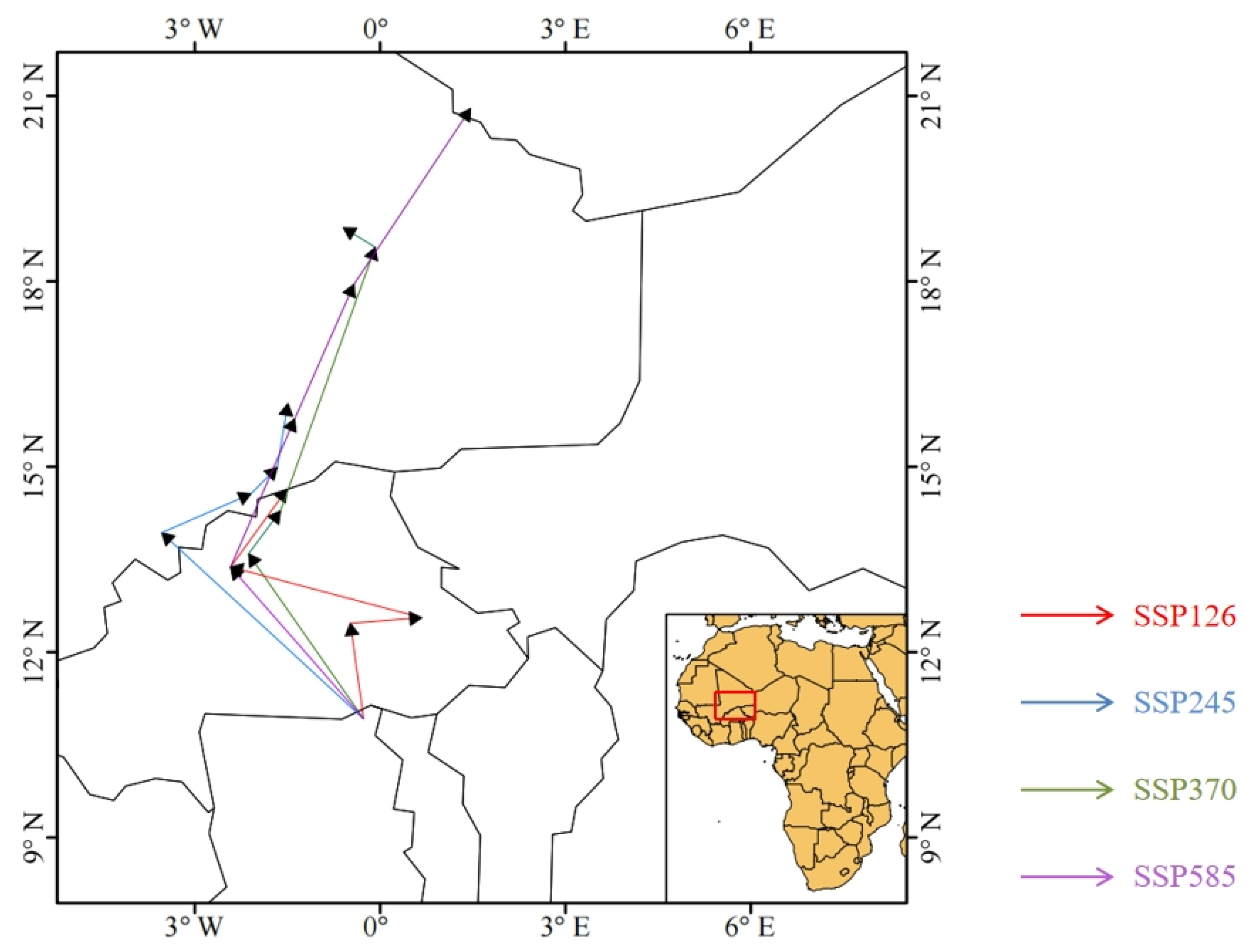
4.2. Changes in the Potential Suitable Habitat of Glipa Under Future Climate Scenarios
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cohen, J.M.; Lajeunesse, M.J.; Rohr, J.R. A global synthesis of animal phenological responses to climate change. Nat. Clim. Change 2018, 8, 224–228. [Google Scholar] [CrossRef]
- Kirchengast, G.; Pichler, M. A traceable global warming record and clarity for the 1.5 °C and well-below-2 °C goals. Commun. Earth Environ. 2025, 6, 402. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef]
- Kearns, C.A.; Inouye, D.W.; Waser, N.M. Endangered mutualisms: The conservation of plant-pollinator interactions. Annu. Rev. Ecol. Evol. Syst. 1998, 29, 83–112. [Google Scholar] [CrossRef]
- Ollerton, J.; Winfree, R.; Tarrant, S. How many flowering plants are pollinated by animals? Oikos 2011, 120, 321–326. [Google Scholar] [CrossRef]
- Ghisbain, G.; Thiery, W.; Massonnet, F.; Erazo, D.; Rasmont, P.; Michez, D.; Dellicour, S. Projected decline in European bumblebee populations in the twenty-first century. Nature 2024, 628, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Ollerton, J. Pollinator diversity: Distribution, ecological function, and conservation. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 353–376. [Google Scholar] [CrossRef]
- Klein, A.M.; Vaissiere, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef]
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T.; Aizen, M.A.; Biesmeijer, J.C.; Breeze, T.D.; Vanbergen, A.J. Safeguarding pollinators and their values to human well-being. Nature 2016, 540, 220–229. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Kharouba, H.M.; Ehrlen, J.; Gelman, A.; Bolmgren, K.; Allen, J.M.; Travers, S.E.; Wolkovich, E.M. Global shifts in the phenological synchrony of species interactions over recent decades. Proc. Natl. Acad. Sci. 2018, 115, 5211–5216. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xue, H.; Yang, X. Advances in systematics of Chinese Mordellidae (Coleoptera). Environ. Entomol. 2015, 37, 865–870. [Google Scholar]
- Bao, T.; Wang, B.; Li, J.; Dilcher, D. Pollination of Cretaceous flowers. Proc. Natl. Acad. Sci. USA 2019, 116, 24707–24711. [Google Scholar] [CrossRef]
- Fan, X. Revision of the genus Glipa of China (Coleoptera: Mordellidae). Mem. Beijing Nat. Hist. Mus. 1993, 53, 45–68. [Google Scholar]
- Liu, Y.; Erwin, T.L.; Yang, X. Mordellidae (Coleoptera) research: A review based on the zoological record from 1864 through 2013. Insects 2018, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Takakuwa, M. Discovery of a peculiar new species of the genus Glipa (Coleoptera, ordellidae) from western Honshu of Japan. Elytra 2007, 35, 545–549. [Google Scholar]
- Kim, J.; Park, S.; Jung, S. A new record of Glipa (Macroglipa) oshimana Nomura (Coleoptera: Mordellidae: Mordellinae: Mordellini) from Korea, with first male description. J. Asia-Pac. Biodivers. 2021, 14, 439–443. [Google Scholar] [CrossRef]
- Ruzzier, E. A new species of Glipa LeConte, 1859 subgenus Stenoglipa Franciscolo, 1952 (Coleoptera: Mordellidae: Mordellini) from the Philippines. Zootaxa 2018, 4410, 397. [Google Scholar] [CrossRef]
- Lu, W.; Jackman, J.A.; Johnson, P.W. Male genitalia and phylogenetic relationships in North American Mordellidae (Coleoptera). Ann. Entomol. Soc. Am. 1997, 90, 742–767. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. Environmental niche equivalency versus conservatism: Quantitative approaches to niche evolution. Evolution 2008, 62, 2868–2883. [Google Scholar] [CrossRef] [PubMed]
- Sillero, N. What does ecological modelling model? A proposed classification of ecological niche models based on their underlying methods. Ecol. Model. 2011, 222, 1343–1346. [Google Scholar] [CrossRef]
- Guisan, A.; Zimmermann, N.E. Predictive habitat distribution models in ecology. Ecol. Model. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudı, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Stockwell, D.P. The GARP modelling system problems and solutions to automated. Int. J. Geogr. Inf. Sci. 1999, 13, 143–158. [Google Scholar] [CrossRef]
- Hirzel, A.H.; Hausser, J.; Chessel, D.; Perrin, N. Ecological-niche factor analysis: How to compute habitat-suitability maps without absence data? Ecology 2002, 83, 2027–2036. [Google Scholar] [CrossRef]
- Austin, M. Species distribution models and ecological theory: A critical assessment and some possible new approaches. Ecol. Model. 2007, 200, 1–19. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Guo, F.; Gao, G.; Sun, Q.; Guo, L.; Yang, Y. Predicting high-risk zones for pine wood nematodes invasion: Integrating climate suitability, host availability, and vector dominance. Sci. Total Environ. 2025, 969, 178902. [Google Scholar] [CrossRef]
- Kramer-Schadt, S.; Niedballa, J.; Pilgrim, J.D.; Schröder, B.; Lindenborn, J.; Reinfelder, V.; Wilting, A. The importance of correcting for sampling bias in MaxEnt species distribution models. Divers. Distrib. 2013, 19, 1366–1379. [Google Scholar] [CrossRef]
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Townsend Peterson, A. Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeogr. 2006, 34, 102–117. [Google Scholar] [CrossRef]
- Warren, D.L.; Matzke, N.J.; Cardillo, M.; Baumgartner, J.B.; Beaumont, L.J.; Turelli, M.; Dinnage, R. ENMTools 1.0: An R package for comparative ecological biogeography. Ecography 2021, 44, 504–511. [Google Scholar] [CrossRef]
- Hosni, E.M.; Al-Khalaf, A.A.; Nasser, M.G.; Abou-Shaara, H.F.; Radwan, M.H. Modeling the potential global distribution of honeybee pest, Galleria Mellonella under changing climate. Insects 2022, 13, 484. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Lee, D.S.; Kwon, T.S.; Athar, M.; Park, Y.S. Predicting the global distribution of Solenopsis geminata (Hymenoptera: Formicidae) under climate change using the maxent model. Insects 2021, 12, 229. [Google Scholar] [CrossRef]
- Lee, W.H.; Song, J.W.; Yoon, S.H.; Jung, J.M. Spatial evaluation of machine learning-based species distribution models for prediction of invasive ant species distribution. Appl. Sci. 2022, 12, 10260. [Google Scholar] [CrossRef]
- Eyring, V.; Bony, S.; Meehl, G.A.; Senior, C.A.; Stevens, B.; Stouffer, R.J.; Taylor, K.E. Overview of the Coupled Model Intercomparison Project Phase 6 (CMIP6) experimental design and organization. Geosci. Model Dev. 2016, 9, 1937–1958. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Bao, R.; Li, X.; Zheng, J. Feature tuning improves maxent predictions of the potential distribution of Pedicularis longiflora Rudolph and its variant. PeerJ 2022, 10, e13337. [Google Scholar] [CrossRef]
- Radosavljevic, A.; Anderson, R.P.; Araújo, M. Making better maxent models of species distributions: Complexity, overfitting and evaluation. J. Biogeogr. 2014, 41, 629–643. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A. Insights into the area under the receiver operating characteristic curve (AUC) as a discrimination measure in species distribution modelling. Glob. Ecol. Biogeogr. 2012, 21, 498–507. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Liu, C.; White, M.; Newell, G.; Pearson, R. Selecting thresholds for the prediction of species occurrence with presence-only data. J. Biogeogr. 2013, 40, 778–789. [Google Scholar] [CrossRef]
- Zhao, J.Q.; Gao, T.; Du, J.J.; Shi, J. Future trends in Obolodiplosis robiniae distribution across Eurasian continent under global climate change. Insects 2023, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.L.; Bennett, J.R.; French, C.M. Sdmtoolbox 2.0: The next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ 2017, 5, e4095. [Google Scholar] [CrossRef]
- Haoxiang, Z.; Xiaoqing, X.; Nianwan, Y.; Yongjun, Z.; Hui, L.; Fanghao, W.; Wanxue, L. Insights from the biogeographic approach for biocontrol of invasive alien pests: Estimating the ecological niche overlap of three egg parasitoids against Spodoptera frugiperda in China. Sci. Total Environ. 2023, 862, 160785. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, C.A.; Tewksbury, J.J.; Huey, R.B.; Sheldon, K.S.; Ghalambor, C.K.; Haak, D.C.; Martin, P.R. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl. Acad. Sci. India Sect. B 2008, 105, 6668–6672. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.W.; Lee, S.G.; Kim, Y.H. New Record of Silvanolomus inermis (Reitter, 1876) and New Distributional Record of Glipa (Macroglipa) oshimana Nomura, 1966 from Korea (Coleoptera: Silvanidae, Mordellidae). Entomol. Res. Bull. 2021, 37, 50–52. [Google Scholar]
- Huberty, A.F.; Denno, R.F. Plant water stress and its consequences for herbivorous insects: A new synthesis. Ecology 2004, 85, 1383–1398. [Google Scholar] [CrossRef]
- Jamieson, M.A.; Trowbridge, A.M.; Raffa, K.F.; Lindroth, R.L. Consequences of climate warming and altered precipitation patterns for plant-insect and multitrophic interactions. Plant Physiol. 2012, 160, 1719–1727. [Google Scholar] [CrossRef]
- Menzel, A.; Sparks, T.H.; Estrella, N.; Roy, D.B. Altered geographic and temporal variability in phenology in response to climate change. Glob. Ecol. Biogeogr. 2006, 15, 498–504. [Google Scholar] [CrossRef]
- Freimuth, J.; Bossdorf, O.; Scheepens, J.F.; Willems, F.M. Climate warming changes synchrony of plants and pollinators. Proc. Biol. Sci. 2022, 289, 20212142. [Google Scholar] [CrossRef]
- Bale, J.S. Insects at low temperature: A predictable relationship? Funct. Ecol. 1991, 5, 291–298. [Google Scholar] [CrossRef]
- Henri Huchon, G.D. La bioécologie de la processionnaire du pin: Dispersion potentielle, dispersion actuelle. Rev. Fr. 1970, 22, 220–234. [Google Scholar] [CrossRef]
- Arnaldo, P.S.; Torres, L.M. Spatial distribution and sampling of Thaumetopoea pityocampa (Den. & schiff.)(Lep. thaumetopoeidea) populations on Pinus pinaster ait. in montesinho, N. Portugal. Forest Ecol. Manag. 2005, 210, 1–7. [Google Scholar]
- Harvey, J.A.; Heinen, R.; Gols, R.; Thakur, M.P. Climate change-mediated temperature extremes and insects: From outbreaks to breakdowns. Glob. Ecol. Biogeogr. 2020, 26, 6685–6701. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Song, J.; Zhao, H.; Li, M.; Han, W. Predicting the distribution of the invasive species Leptocybe invasa: Combining MaxEnt and geodetector models. Insects 2021, 12, 92. [Google Scholar] [CrossRef]
- Memmott, J.; Craze, P.G.; Waser, N.M.; Price, M.V. Global warming and the disruption of plant-pollinator interactions. Ecol. Lett. 2007, 10, 710–717. [Google Scholar] [CrossRef]
- Burkle LA, M.J.; Knight, T.M. Plant-pollinator interactions over 120 years: Loss of species, co-occurrence, and function. Science 2013, 339, 1611–1615. [Google Scholar] [CrossRef]
- Biesmeijer, J.C.; Roberts, S.P.; Reemer, M.; Ohlemuller, R.; Edwards, M.; Peeters, T.; Kunin, W.E. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 2006, 313, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Hegland, S.J.; Nielsen, A.; Lazaro, A.; Bjerknes, A.L.; Totland, O. How does climate warming affect plant-pollinator interactions? Ecol. Lett. 2009, 12, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Ghisbain, G.; Gerard, M.; Wood, T.J.; Hines, H.M.; Michez, D. Expanding insect pollinators in the Anthropocene. Biol. Rev. Camb. Philos. Soc. 2021, 96, 2755–2770. [Google Scholar] [CrossRef]
- Ma, C.S.; Ma, G.; Pincebourde, S. Survive a warming climate: Insect responses to extreme high temperatures. Annu. Rev. Entomol. 2021, 66, 163–184. [Google Scholar] [CrossRef]
- Wu, C.H.; Holloway, J.D.; Hill, J.K.; Thomas, C.D.; Chen, I.C.; Ho, C.K. Reduced body sizes in climate-impacted Borneo moth assemblages are primarily explained by range shifts. Nat. Commun. 2019, 10, 4612. [Google Scholar] [CrossRef]
- Jepsen, J.U.; Hagen, S.B.; Ims, R.A.; Yoccoz, N.G. Climate change and outbreaks of the geometrids Operophtera brumata and Epirrita autumnata in subarctic birch forest: Evidence of a recent outbreak range expansion. J. Anim. Ecol. 2008, 77, 257–264. [Google Scholar] [CrossRef]
- Cudmore, T.J.; Björklund, N.; Carroll, A.L.; Staffan Lindgren, B. Climate change and range expansion of an aggressive bark beetle: Evidence of higher beetle reproduction in naïve host tree populations. J. Appl. Ecol. 2010, 47, 1036–1043. [Google Scholar] [CrossRef]
- Wang, H.Y.; Shen, S.F.; Chen, Y.S.; Kiang, Y.K.; Heino, M. Life histories determine divergent population trends for fishes under climate warming. Nat. Commun. 2020, 11, 4088. [Google Scholar] [CrossRef]
- Lago, P.K.; Mann, M.O. Survey of Coleoptera associated with flowers of wild carrot in northern Mississippi. Coleopt. Bull. 1987, 41, 1–8. [Google Scholar]
- Gerard, M.; Vanderplanck, M.; Wood, T.; Michez, D. Global warming and plant-pollinator mismatches. Emerg. Top. Life Sci. 2020, 4, 77–86. [Google Scholar]
- Northfield, T.D.; Ives, A.R. Coevolution and the effects of climate change on interacting species. PLoS Biol. 2013, 11, e1001685. [Google Scholar] [CrossRef] [PubMed]
- Merow, C.; Smith, M.J.; Silander, J.A., Jr. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, X.; Ouyang, X.; Ding, X.; Wang, Y.; Liu, W.; Liu, Y. Predicting Suitable Habitat for Glipa (Coleoptera: Mordellidae: Mordellinae) Under Current and Future Climates Using MaxEnt Modeling. Insects 2025, 16, 642. https://doi.org/10.3390/insects16060642
Su X, Ouyang X, Ding X, Wang Y, Liu W, Liu Y. Predicting Suitable Habitat for Glipa (Coleoptera: Mordellidae: Mordellinae) Under Current and Future Climates Using MaxEnt Modeling. Insects. 2025; 16(6):642. https://doi.org/10.3390/insects16060642
Chicago/Turabian StyleSu, Xie, Xianheng Ouyang, Xiaoqun Ding, Yang Wang, Wangang Liu, and Yang Liu. 2025. "Predicting Suitable Habitat for Glipa (Coleoptera: Mordellidae: Mordellinae) Under Current and Future Climates Using MaxEnt Modeling" Insects 16, no. 6: 642. https://doi.org/10.3390/insects16060642
APA StyleSu, X., Ouyang, X., Ding, X., Wang, Y., Liu, W., & Liu, Y. (2025). Predicting Suitable Habitat for Glipa (Coleoptera: Mordellidae: Mordellinae) Under Current and Future Climates Using MaxEnt Modeling. Insects, 16(6), 642. https://doi.org/10.3390/insects16060642




