Electrophysiological and Behavioral Responses of Thrips hawaiiensis (Thysanoptera: Thripidae) to the Floral Volatiles of the Horticultural Plant Magnolia grandiflora (Magnoliales: Magnoliaceae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Host Plants
2.2. Y-Tube Olfactometer Bioassays
2.3. GC-MS Analysis
2.4. Behavioral Responses of T. hawaiiensis to Main M. grandiflora VOCs
2.5. Odor Stimuli
2.6. Electroantennography Tests
2.7. Six-Arm Olfactometer Bioassays
2.8. Four-Arm Olfactometer Bioassays
2.9. Statistical Analyses
3. Results
3.1. Y-Tube Olfactometer Bioassays
3.2. Analysis of M. grandiflora Flower Volatiles
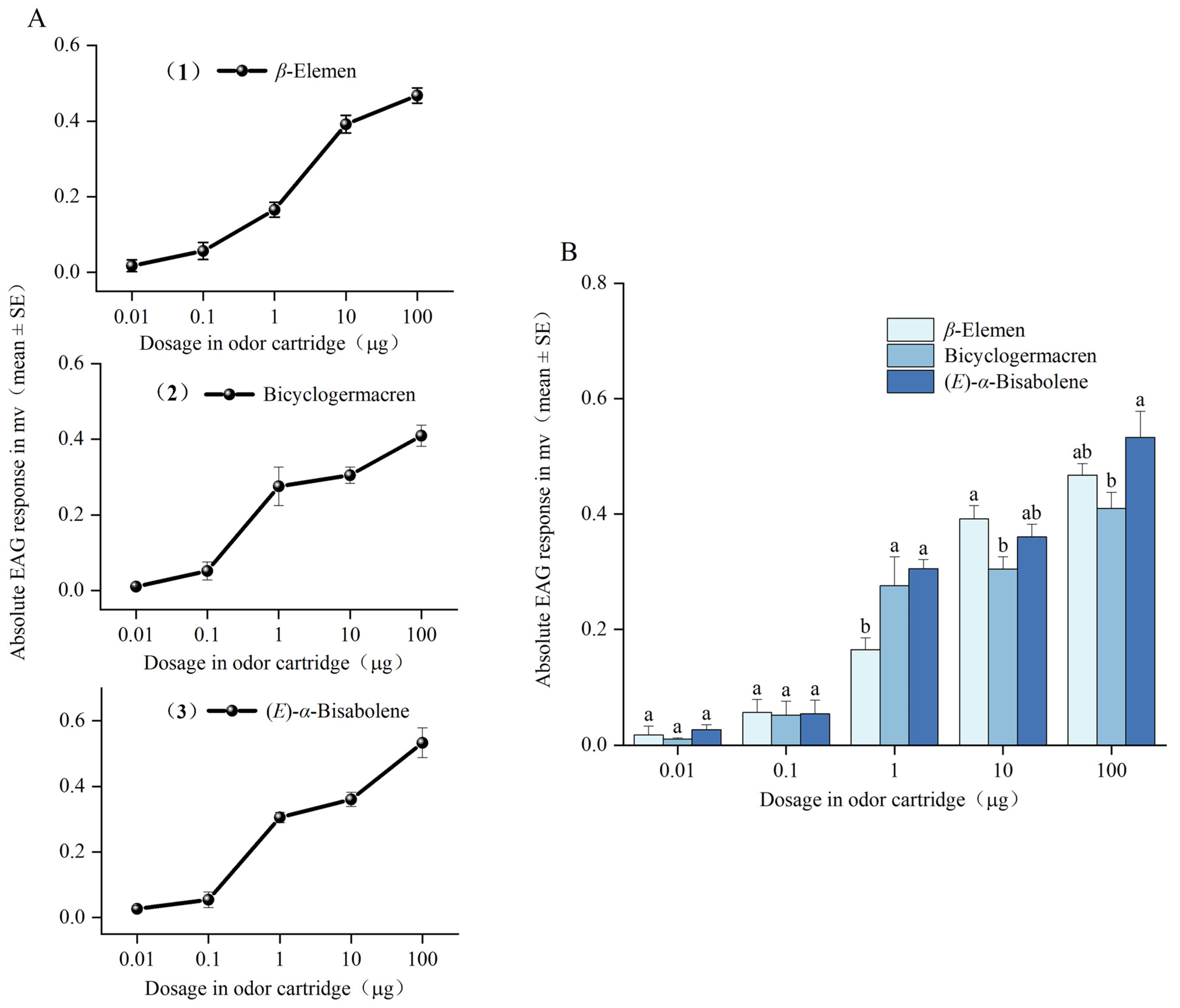
3.3. Electroantennogram (EAG) Tests
3.4. Six-Arm Olfactometer Bioassays
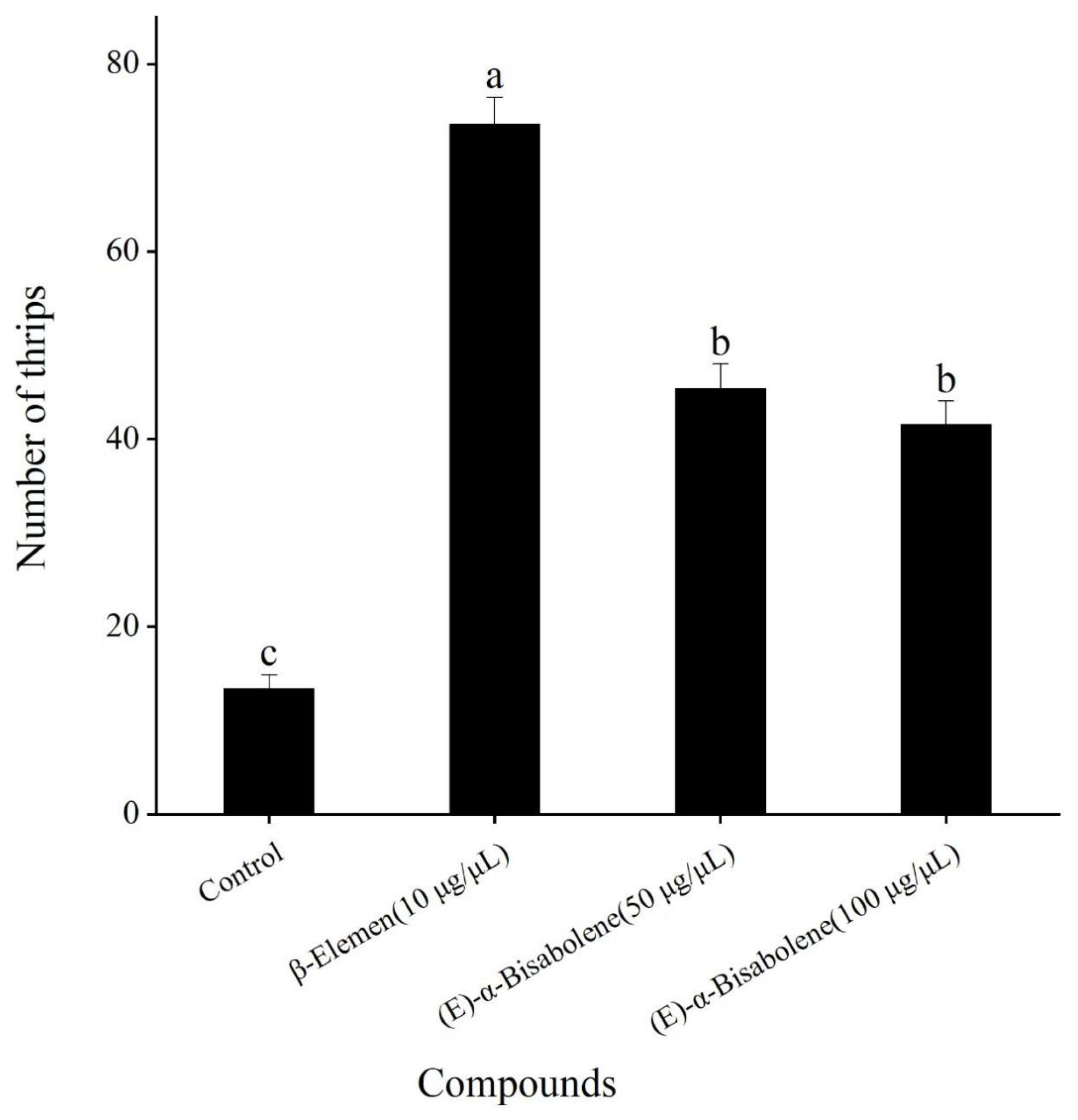
3.5. Four-Arm Olfactometer Bioassays
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mound, L.A. Thysanoptera: Diversity and interactions. Annu. Rev. Entomol. 2005, 50, 247–269. [Google Scholar] [CrossRef] [PubMed]
- Atakan, E.; Ölçülü, M.; Pehlivan, S.; Satar, S. A new thrips species recorded in Turkey: Thrips hawaiiensis (Morgan, 1913) (Thysanoptera: Thripidae). Türk. Entomol. Bült. 2015, 2, 77–84. [Google Scholar] [CrossRef]
- Goldarazena, A. First record of Thrips hawaiiensis (Morgan, 1913) (Thysanoptera: Thripidae), an Asian pest thrips in Spain. Bull. OEPP 2011, 41, 170–173. [Google Scholar] [CrossRef]
- Reynau, P.; Balmès, V.; Pizzol, J. Thrips hawaiiensis (Morgan, 1913) (Thysanoptera: Thripidae), an Asian pest thrips now established in Europe. Bull. OEPP 2008, 1, 155–160. [Google Scholar] [CrossRef]
- Marullo, A.; De Grazia, A. Thrips hawaiiensis a pest thrips from Asia newly introduced into Italy. Bull. Insectol. 2017, 70, 27–30. [Google Scholar]
- Murai, T. Development and reproductive capacity of Thrips hawaiiensis (Thysanoptera: Thripidae) and its potential as a major pest. Bull. Entomol. Res. 2001, 91, 193–198. [Google Scholar] [CrossRef]
- Aliakbarpour, H.; Salmah, M.R.C. Seasonal abundance and spatial distribution of larval and adult thrips (Thysanoptera) on weed host plants in mango orchards in Penang, Malaysia. Appl. Entomol. Zool. 2011, 2, 185–194. [Google Scholar] [CrossRef]
- Cao, Y.; Zhi, J.R.; Zhang, R.Z.; Li, C.; Liu, Y.; Lv, Z.Y.; Gao, Y.L. Different population performances of Frankliniella occidentalis and Thrips hawaiiensis on flowers of two horticultural plants. J. Pest Sci. 2018, 1, 79–91. [Google Scholar] [CrossRef]
- Cao, Y.; Reitz, S.R.; Germinara, G.S.; Wang, C.; Wang, L.J.; Yang, S.Y.; Gao, Y.L.; Zhang, W.Q.; Li, C. Host preference of Thrips hawaiiensis for different ornamental plants. J. Pest Sci. 2022, 95, 761–770. [Google Scholar] [CrossRef]
- Cao, Y.; Qi, G.L.; Jiang, F.Y.; Meng, Y.L.; Wang, C.; Gu, Z.Y.; Gao, Y.L.; Reitz, S.R.; Li, C. Population performance and detoxifying and protective enzyme activities of four thrips species feeding on flowers of Magnolia grandiflora (Ranunculales: Magnolia). Pest Manag. Sci. 2023, 79, 3239–3249. [Google Scholar] [CrossRef]
- Fu, B.L.; Qiu, H.Y.; Li, Q.; Tang, L.D.; Zeng, D.Q.; Liu, K.; Gao, Y.L. Flower injection of imidacloprid and spirotetramat: A novel tool for the management of banana thrips Thrips hawaiiensis. J. Pest Sci. 2020, 93, 1073–1084. [Google Scholar] [CrossRef]
- Avellaneda, J.; Díaz, M.; Coy-Barrera, E.; Rodríguez, D.; Osorio, C. Rose volatile compounds allow the design of new control strategies for the western fower thrips (Frankliniella occidentalis). J. Pest Sci. 2021, 94, 129–142. [Google Scholar] [CrossRef]
- Cao, Y.; Li, C.; Yang, H.; Li, J.; Li, S.; Wang, Y.W.; Gao, Y.L. Laboratory and field investigation on the orientation of Frankliniella occidentalis (Thysanoptera: Thripidae) to more suitable host plants driven by volatiles and component analysis of volatiles. Pest Manag. Sci. 2019, 75, 598–606. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, J.; Germinara, G.S.; Wang, L.J.; Yang, H.; Gao, Y.L.; Li, C. Behavioral Responses of Thrips hawaiiensis (Thysanoptera: Thripidae) to Volatile Compounds Identified from Gardenia jasminoides Ellis (Gentianales: Rubiaceae). Insects 2020, 11, 408. [Google Scholar] [CrossRef] [PubMed]
- Teulon, D.A.J.; Hollister, B.; Butler, R.C.; Cameron, E.A. Colour and odour responses of flying western flower thrips: Wind tunnel and greenhouse experiments. Entomol. Exp. Appl. 1999, 93, 9–19. [Google Scholar] [CrossRef]
- Van Tol, R.W.H.M.; James, D.E.; de Kogel, W.J.; Teulon, D.A.J. Plant odours with potential for a push-pull strategy to control the onion thrips, Thrips tabaci. Entomol. Exp. Appl. 2007, 122, 69–76. [Google Scholar] [CrossRef]
- Cao, Y.; Zhi, J.R.; Cong, C.L.; Margolies, D.C. Olfactory cues used in host selection by Frankliniella occidentalis (Thysanoptera: Thripidae) in relation to host suitability. J. Insect Behav. 2014, 27, 41–56. [Google Scholar] [CrossRef]
- Colazza, S.; Rosi, M.C.; Clemente, A. Response of egg parasitoid Telenomus busseolae to sex pheromone of Sesamia nonagrioides. J. Chem. Ecol. 1997, 23, 2437–2444. [Google Scholar] [CrossRef]
- Báez, D.; Pino, J.A.; Pino, D. Volatiles from Magnolia grandiflora Flowers: Comparative Analysis by Simultaneous Distillation-Extraction and Solid Phase Microextraction. Nat. Prod. Commun. 2012, 7, 237–238. [Google Scholar] [CrossRef]
- NIST 17, Mass Spectral Library (NIST/EPA/NIH); National Institute of Standards and Technology: Gaithersburg, MD, USA, 2017.
- Abdullah, Z.S.; Ficken, K.J.; Greenfield, B.P.J.; Butt, T.M. Innate responses to putative ancestral hosts: Is the attraction of western flower thrips to pine pollen a result of relict olfactory receptors? J. Chem. Ecol. 2014, 40, 534–540. [Google Scholar] [CrossRef]
- Tian, H.J.; Chen, Y.X.; Chen, Y.; Chen, X.Q.; Lin, S.; Zhang, J.; Yang, G.; Wei, H. A mixture of p-anisaldehyde and ethyl nicotinate elicits positive antennal and behavioral responses in Frankliniella occidentalis. Entomol. Exp. Appl. 2022, 170, 603–611. [Google Scholar] [CrossRef]
- Cao, Y.; Pistillo, O.M.; Lou, Y.B.; D’isita, I.; Maggi, F.; Hu, Q.Q.; Germinara, G.S.; Li, C. Electrophysiological and behavioural responses of Stegobium paniceum to volatile compounds from Chinese medicinal plant materials. Pest Manag. Sci. 2022, 78, 3697–3703. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Davison, A.C.; Tamo, C.A. Six-arm olfactometer permitting simultaneous observation of insect attraction and odour trapping. Physiol. Entomol. 2010, 29, 45–55. [Google Scholar] [CrossRef]
- Cao, Y.; Benelli, G.; Germinara, G.S.; Maggi, F.; Zhang, Y.; Luo, S.L.; Yang, H.; Li, C. Innate positive chemotaxis to paeonal from highly attractive Chinese medicinal herbs in the cigarette beetle, Lasioderma serricorne. Sci. Rep. 2019, 9, 6995. [Google Scholar] [CrossRef]
- Liu, X.F.; Chen, H.H.; Li, J.K.; Zhang, R.; Chen, L. Volatiles released by Chinese liquorice roots mediate host location behaviour by neonate Porphyrophora sophorae (Hemiptera: Margarodidae). Pest Manag. Sci. 2016, 72, 1959–1964. [Google Scholar] [CrossRef] [PubMed]
- Bruce, T.J.A.; Wadhams, L.J.; Woodcock, C.M. Insect host location: A volatile situation. Trends Plant Sci. 2005, 10, 269–274. [Google Scholar] [CrossRef]
- Knolhoff, L.M.; Heckel, D.G. Behavioral assays for studies of host plant choice and adaptation in herbivorous insects. Annu. Rev. Entomol. 2014, 59, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Xie, S.H.; Jian, L.Y.; Agrafoti, P.; Wu, K.X.; Athanassiou, C.G.; Cao, Y. Behavioral responses of Araecerus fasciculatus (Coleoptera: Anthribidae) to volatiles of selected stored Chinese medicinal plant products. J. Econ. Entomol. 2024, 117, 2669–2677. [Google Scholar] [CrossRef]
- Murai, T.; Imai, T.; Maekawa, M. Methyl Anthranilate as an Attractant for Two Thrips Species and the Thrips Parasitoid Ceranisus menes. J. Chem. Ecol. 2000, 26, 2557–2565. [Google Scholar] [CrossRef]
- Imai, T.; Maekawa, M.; Murai, T. Attractiveness of methyl anthranilate and its related compounds to the flower thrips, Thrips hawaiiensis (Morgan), T. coloratus Schmutz, T. flavus Schrank and Megalurothrips distalis (Karny) (Thysanoptera: Thripidae). Appl. Entomol. Zool. 2001, 36, 475–478. [Google Scholar] [CrossRef]
- Najar-Rodriguez, A.J.; Galizia, C.G.; Stierle, J.; Dorn, S. Behavioral and neurophysiological responses of an insect to changing ratios of constituents in host plant-derived volatile mixtures. J. Exp. Biol. 2010, 213, 3388–3397. [Google Scholar] [CrossRef]
- Cha, D.H.; Linn, C.E.; Teal, P.E.A.; Zhang, A.; Roelofs, W.L.; Loeb, G.M.; Frederic, M.P. Eavesdropping on plant volatiles by a specialist moth: Significance of ratio and concentration. PLoS ONE 2011, 6, e17033. [Google Scholar] [CrossRef]
- Rojas, C.J. Electrophysiological and behavioural responses of the cabbage moth to plant volatiles. J. Chem. Ecol. 1999, 25, 1867–1883. [Google Scholar] [CrossRef]
- Tasin, M.; Backamanì, A.C.; Bengtsson, M.; Ioriatti, C.; Witzgall, P. Essential host plant cues in the grapevine moth. Naturwissenschaften 2006, 93, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Dickens, J.C. Orientation of Colorado potato beetle to natural and synthetic blends of volatiles emitted by potato plants. Agric. For. Entomol. 2000, 2, 167–172. [Google Scholar] [CrossRef]
- Guo, X.J.; Yu, Q.Q.; Chen, D.F.; Wei, J.L.; Yang, P.C.; Yu, J.; Wang, X.H.; Kang, L. 4-Vinylanisole is an aggregation pheromone in locusts. Nature 2020, 584, 584–588. [Google Scholar] [CrossRef]
- Wang, B.; Dong, W.Y.; Li, H.M.; D’Onofriox, C.; Bai, P.H.; Chen, R.P.; Yang, L.L.; Wu, J.A.; Wang, X.Q.; Wang, B.; et al. Molecular basis of (E)-β-farnesene-mediated aphid location in the predator Eupeodes corollae. Curr. Biol. 2022, 32, 951–962.e7. [Google Scholar] [CrossRef]
- Zhang, L.W.; Sun, H.W.; Grosse-Wilde, E.; Zhang, L.; Hansson, B.S.; Dweck, H.K.M. Cross-generation pheromonal communication drives Drosophila oviposition site choice. Curr. Biol. 2023, 33, 2095–2103. [Google Scholar] [CrossRef]
- Kirk, W.D.J.; de Kogel, W.J.; Koschier, E.H.; Teulon, D.A.J. Semiochemicals for Thrips and Their Use in Pest Management. Annu. Rev. Entomol. 2021, 66, 101–119. [Google Scholar] [CrossRef]


| Number | Compound | RI | Molecular Formula | Molecular Weight | Relative Peak Area (%) |
|---|---|---|---|---|---|
| 1 | α-Pinene | 937 | C10H16 | 136 | 1.17 |
| 2 | Camphene | 952 | C10H16 | 136 | 0.11 |
| 3 | Butanoic acid, 2-methyl-, 1-methylpropyl ester | 971 | C9H18O2 | 158 | 0.23 |
| 4 | β-Pinene | 979 | C10H16 | 136 | 4.22 |
| 5 | Myrcene | 991 | C10H16 | 136 | 1.02 |
| 6 | Limonene | 1031 | C10H16 | 136 | 0.77 |
| 7 | 1,8-Cineole | 1032 | C10H18O | 154 | 0.28 |
| 8 | Terpinolene | 1088 | C10H16 | 136 | 0.13 |
| 9 | Linalool | 1099 | C10H18O | 154 | 0.23 |
| 10 | Pinocarvone | 1164 | C10H14O | 150 | 0.69 |
| 11 | α-Terpineol | 1189 | C10H18O | 154 | 0.15 |
| 12 | Myrtenol | 1213 | C10H16O | 152 | 0.17 |
| 13 | Citronellol | 1228 | C10H20O | 156 | 0.26 |
| 14 | Geraniol | 1255 | C10H18O | 154 | 0.17 |
| 15 | cis-Myrtanol | 1261 | C10H18O | 154 | 0.17 |
| 16 | trans-Pinocarvyl acetate | 1297 | C12H18O2 | 194 | 0.41 |
| 17 | α-Cubebene | 1351 | C15H24 | 204 | 0.22 |
| 18 | α-Ylangene | 1372 | C15H24 | 204 | 0.19 |
| 19 | α-Copaene | 1376 | C15H24 | 204 | 0.28 |
| 20 | β-Cubebene | 1390 | C15H24 | 204 | 0.34 |
| 21 | β-Elemene | 1391 | C15H24 | 204 | 15.39 |
| 22 | Isocaryophyllene | 1406 | C15H24 | 204 | 0.31 |
| 23 | α-Gurjunene | 1409 | C15H24 | 204 | 0.17 |
| 24 | (E)-Caryophyllene | 1419 | C15H24 | 204 | 2.88 |
| 25 | β-Copaene | 1432 | C15H24 | 204 | 0.36 |
| 26 | γ-Elemene | 1433 | C15H24 | 204 | 1.62 |
| 27 | Aromandendrene | 1440 | C15H24 | 204 | 1.06 |
| 28 | Selina-5,11-diene | 1447 | C15H24 | 204 | 0.36 |
| 29 | α-Humulene | 1454 | C15H24 | 204 | 1.01 |
| 30 | Valerena-4,7(11)-diene | 1460 | C15H24 | 204 | 2.78 |
| 31 | cis-Muurola-4(15),5-diene | 1463 | C15H24 | 204 | 0.69 |
| 32 | γ-Gurjunene | 1473 | C15H24 | 204 | 1.09 |
| 33 | γ-Muurolene | 1477 | C15H24 | 204 | 3.01 |
| 34 | Germacrene D | 1481 | C15H24 | 204 | 5.13 |
| 35 | β-Selinene | 1486 | C15H24 | 204 | 0.61 |
| 36 | Bicyclogermacren | 1495 | C15H24 | 204 | 11.99 |
| 37 | δ-Guaiene | 1505 | C15H24 | 204 | 4.47 |
| 38 | (E)-α-Bisabolene | 1512 | C15H24 | 204 | 6.04 |
| 39 | γ-Cadinene | 1513 | C15H24 | 204 | 0.68 |
| 40 | δ-Cadinene | 1524 | C15H24 | 204 | 2.43 |
| 41 | Germacrene B | 1557 | C15H24 | 204 | 0.39 |
| 42 | (E)-Nerolidol | 1564 | C15H24 | 204 | 1.21 |
| 43 | Spathulenol | 1576 | C15H24O | 220 | 0.78 |
| 44 | Caryophyllene oxide | 1581 | C15H24O | 220 | 0.21 |
| 45 | Globulol | 1591 | C15H26O | 222 | 0.19 |
| 46 | Isospathulenol | 1638 | C15H24O | 220 | 0.37 |
| 47 | α-Cadinol | 1640 | C15H26O | 222 | 0.31 |
| 48 | n-Nonadecane | 1900 | C19H40 | 268 | 0.37 |
| 49 | n-Heneicosane | 2100 | C21H44 | 296 | 0.46 |
| 50 | n-Tricosane | 2300 | C23H48 | 324 | 0.18 |
| 51 | Cyclohexane,2-ethenyl-1,1-dimethyl-3-methylene- | 2368 | C11H18 | 150 | 3.77 |
| 52 | Longiverbenone | 2453 | C15H22O | 218 | 2.51 |
| 53 | unidentified | 2.25 | |||
| 54 | unidentified | 2.13 | |||
| 55 | unidentified | 1.86 | |||
| 56 | unidentified | 1.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Yang, Y.; Maggi, F.; Jiang, F.; Yuan, R.; Huang, L.; Zhang, X.; Cao, Y.; Gao, Y. Electrophysiological and Behavioral Responses of Thrips hawaiiensis (Thysanoptera: Thripidae) to the Floral Volatiles of the Horticultural Plant Magnolia grandiflora (Magnoliales: Magnoliaceae). Insects 2025, 16, 633. https://doi.org/10.3390/insects16060633
Zhang T, Yang Y, Maggi F, Jiang F, Yuan R, Huang L, Zhang X, Cao Y, Gao Y. Electrophysiological and Behavioral Responses of Thrips hawaiiensis (Thysanoptera: Thripidae) to the Floral Volatiles of the Horticultural Plant Magnolia grandiflora (Magnoliales: Magnoliaceae). Insects. 2025; 16(6):633. https://doi.org/10.3390/insects16060633
Chicago/Turabian StyleZhang, Tao, Yuping Yang, Filippo Maggi, Feiyu Jiang, Rongrong Yuan, Lujie Huang, Xueyan Zhang, Yu Cao, and Yulin Gao. 2025. "Electrophysiological and Behavioral Responses of Thrips hawaiiensis (Thysanoptera: Thripidae) to the Floral Volatiles of the Horticultural Plant Magnolia grandiflora (Magnoliales: Magnoliaceae)" Insects 16, no. 6: 633. https://doi.org/10.3390/insects16060633
APA StyleZhang, T., Yang, Y., Maggi, F., Jiang, F., Yuan, R., Huang, L., Zhang, X., Cao, Y., & Gao, Y. (2025). Electrophysiological and Behavioral Responses of Thrips hawaiiensis (Thysanoptera: Thripidae) to the Floral Volatiles of the Horticultural Plant Magnolia grandiflora (Magnoliales: Magnoliaceae). Insects, 16(6), 633. https://doi.org/10.3390/insects16060633








