Invertebrates of Siberia, a Potential Source of Animal Protein for Innovative Food and Feed Production: Biomass Nutrient Composition Change in the Earthworm Eisenia fetida (Savigny, 1826) and the House Cricket Acheta domesticus (Linnaeus, 1758)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Statistical Analysis
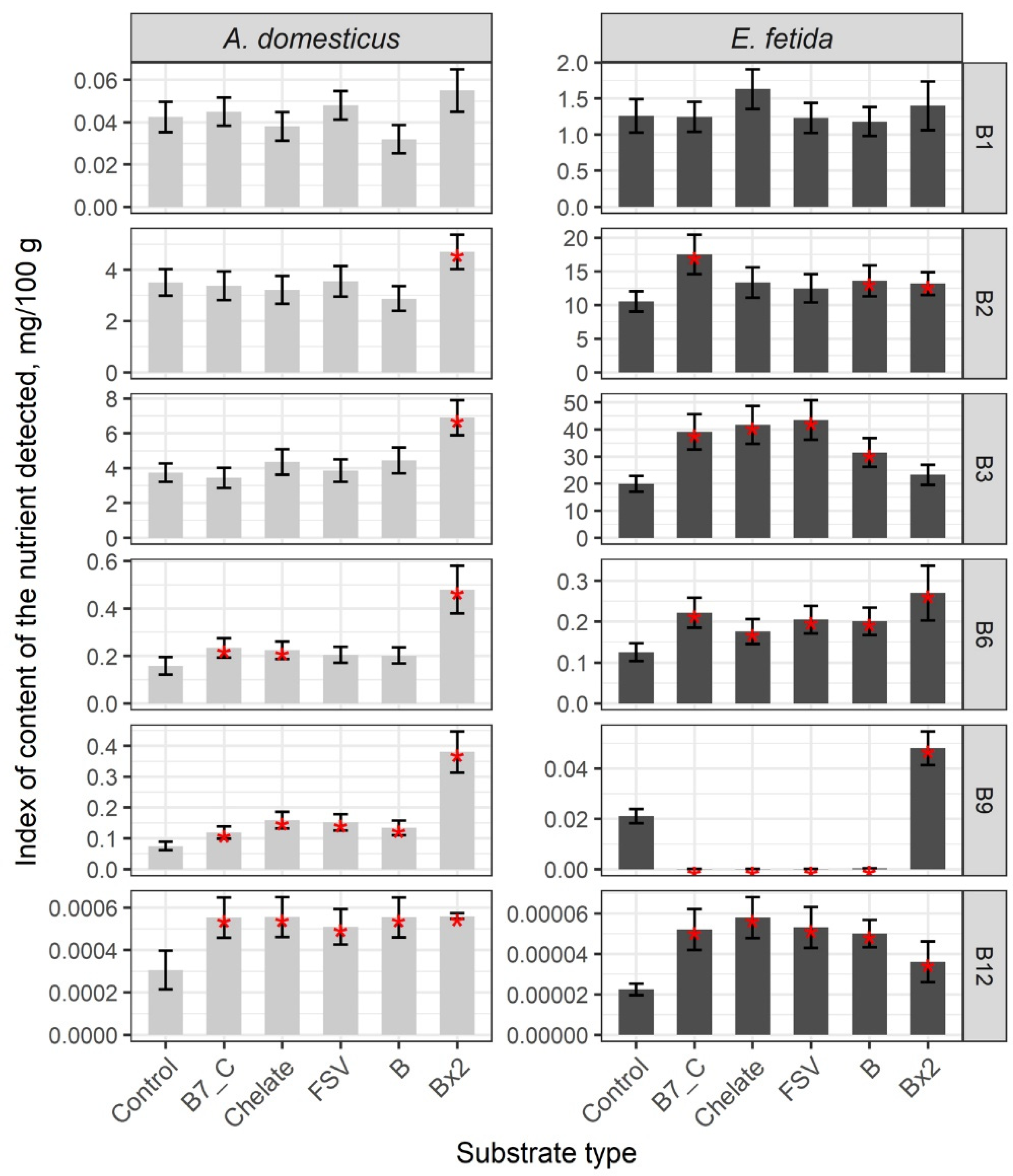
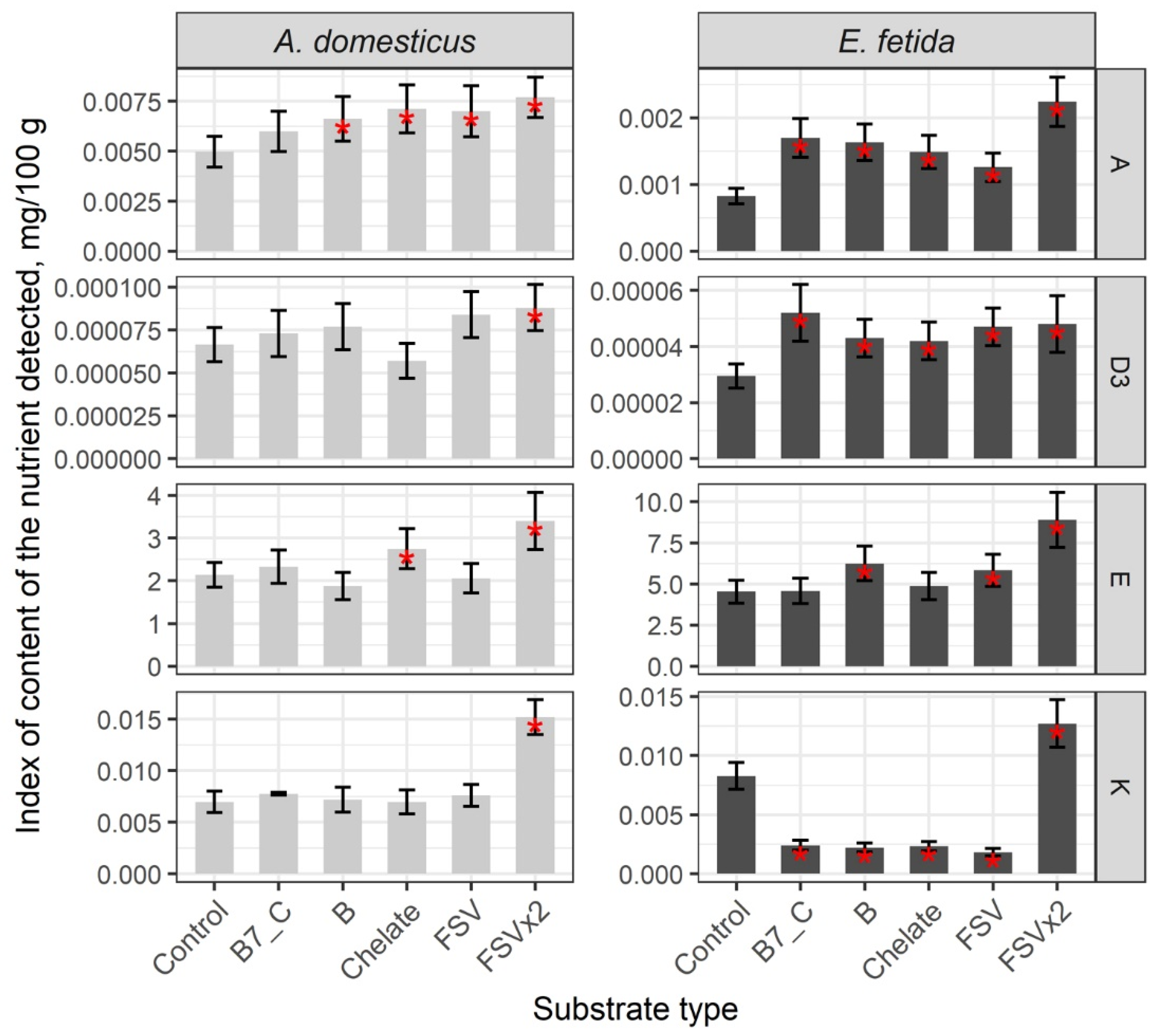
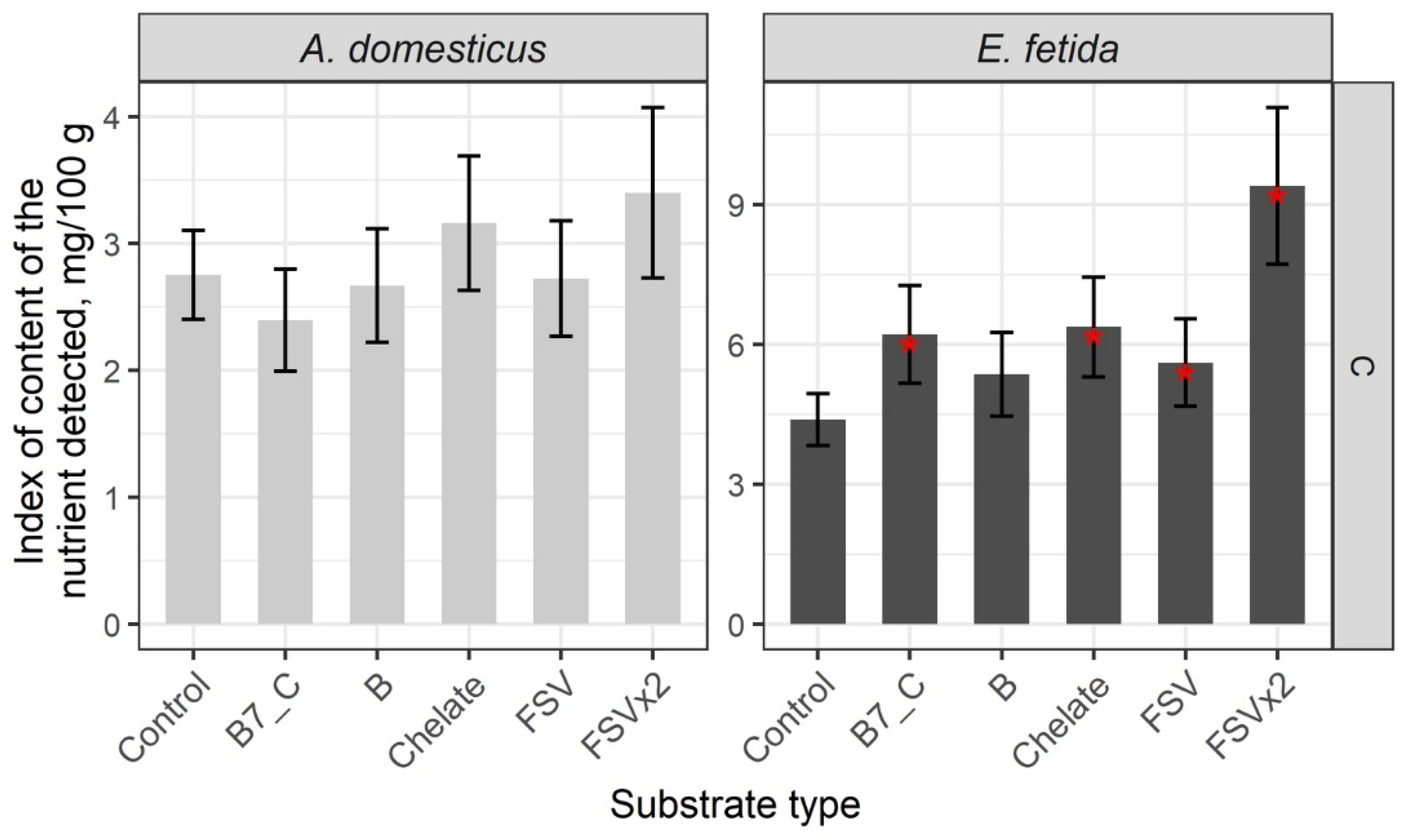
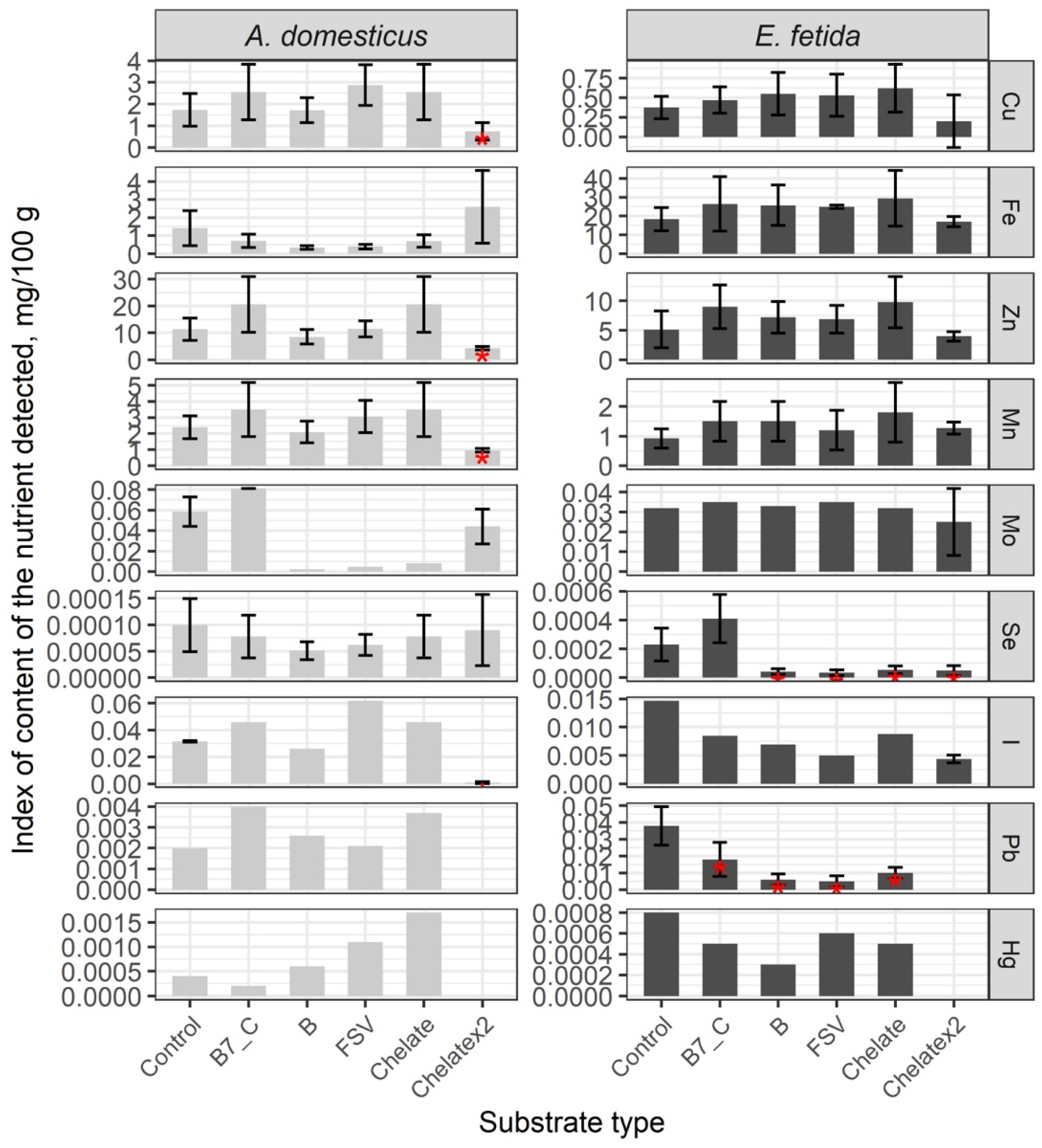
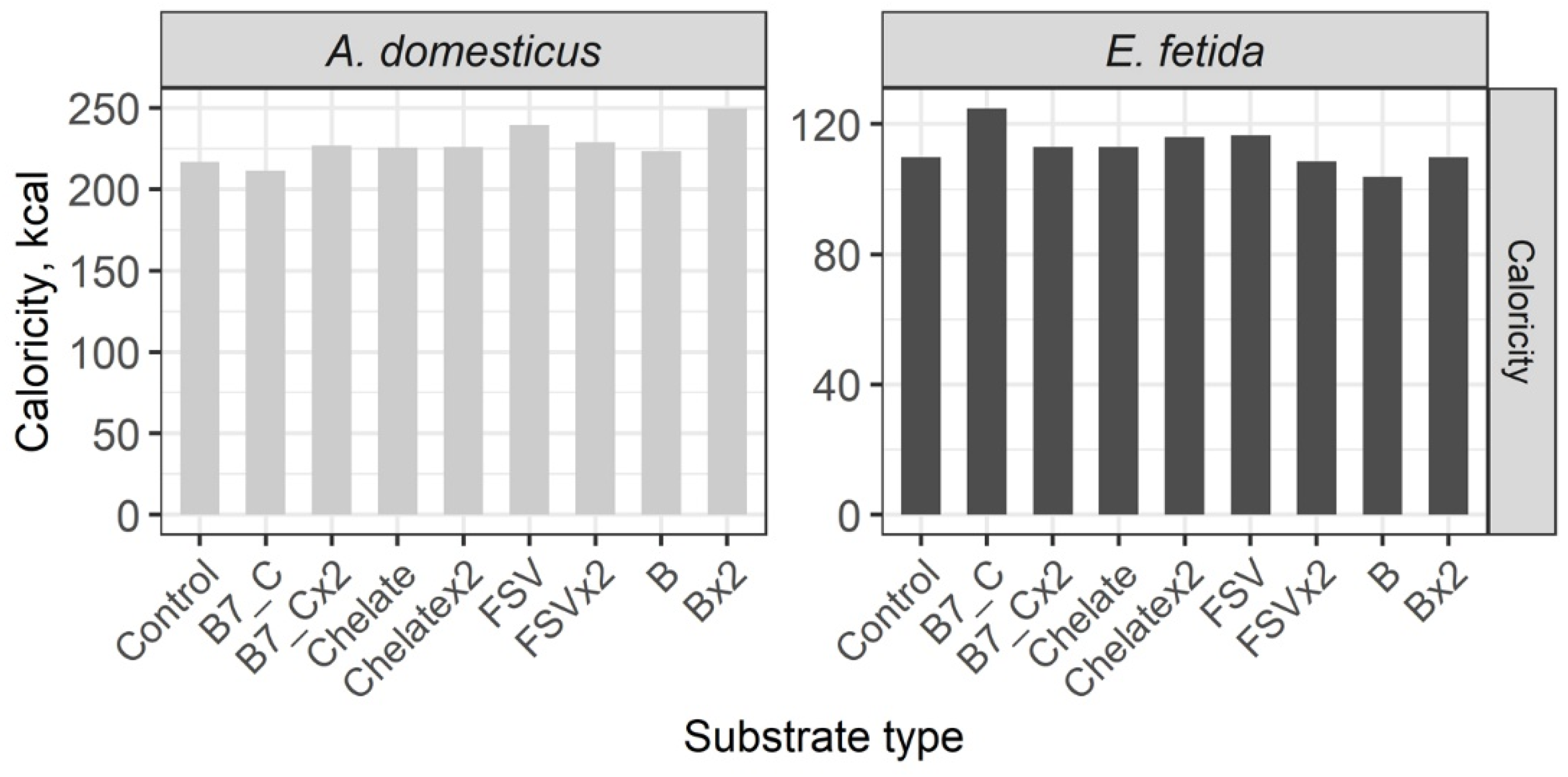
3. Results
Nutrient Composition of the Biomass of Model Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson-Sanders, S.E. Invertebrate models for biomedical research, testing, and education. ILAR J. 2011, 52, 126–152. [Google Scholar] [CrossRef]
- Scanes, C. Invertebrates and their use by humans. In Animals and Human Society; Academic Press: San Diego, CA, USA, 2018; pp. 181–193. [Google Scholar] [CrossRef]
- Pollo, S.; Vitale, A. Invertebrates and humans: Science, ethics, and policy. In The Welfare of Invertebrate Animal; Springer: Cham, Switzerland, 2019; pp. 7–22. [Google Scholar] [CrossRef]
- Rueda García, A.M.; Fracassi, P.; Scherf, B.D.; Hamon, M.; Iannotti, L. Unveiling the nutritional quality of terrestrial animal source foods by species and characteristics of livestock systems. Nutrients 2024, 16, 3346. [Google Scholar] [CrossRef]
- Iannotti, L.; Rueda García, A.M.; Palma, G.; Fontaine, F.; Scherf, B.; Neufeld, L.M.; Zimmerman, R.; Fracassi, P. Terrestrial animal source foods and health outcomes for those with special nutrient needs in the life course. Nutrients 2024, 16, 3231. [Google Scholar] [CrossRef]
- Drewnowski, A.; Fulgoni, V.L., III. Nutrient density: Principles and evaluation tools. Am. J. Clin. Nutr. 2014, 99, 1223–1228. [Google Scholar] [CrossRef]
- Van Raamsdonk, L.W.D.; Van der Fels-Klerx, H.J.; De Jong, J. New feed ingredients: The insect opportunity. Food Addit. Contam. Part A 2017, 34, 1384–1397. [Google Scholar] [CrossRef]
- Tang, C.; Yang, D.; Liao, H.; Sun, H.; Liu, C.; Wei, L.; Li, F. Edible insects as a food source: A review. Food Prod. Process. Nutr. 2019, 1, 8. [Google Scholar] [CrossRef]
- Tobolkova, B. Edible insects—The future of a healthy diet? Nov. Tech. Nutr. Food Sci. 2019, 4, 325–328. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; van der Poel, A.F.B. Effects of diet on the chemical composition of migratory locusts (Locusta migratoria). Zoo Biol. 2011, 30, 9–16. [Google Scholar] [CrossRef]
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; p. 190. Available online: https://www.fao.org/3/i3253e/i3253e.pdf (accessed on 1 April 2025).
- Zielińska, E.; Baraniak, B.; Karaś, M.; Rybczyńska, K.; Jakubczyk, A. Selected species of edible insects as a source of nutrient composition. Food Res. Int. 2015, 77, 460–466. [Google Scholar] [CrossRef]
- Derrien, C.; Boccuni, A. Current status of the insect producing industry in Europe. In Edible Insects in Sustainable Food Systems; Springer: Cham, Switzerland, 2018; pp. 471–479. [Google Scholar] [CrossRef]
- Gorbunova, N.A.; Zakharov, A.N. Edible insects as a source of alternative protein. A review. Theory Pract. Meat Process. 2021, 6, 23–32. [Google Scholar] [CrossRef]
- Tshernyshev, S.E.; Babkina, I.B.; Modyaeva, V.P.; Morozova, M.D.; Subbotina, E.Y.; Shcherbakov, M.V.; Simakova, A.V. Invertebrates of Siberia, a potential source of animal protein for innovative food production. 1. The keelback slugs (Gastropoda: Limacidae). Acta Biol. Sib. 2022, 8, 749–762. [Google Scholar] [CrossRef]
- Tshernyshev, S.E.; Baghirov, R.T.-O.; Modyaeva, V.P.; Morozova, M.D.; Skriptcova, K.E.; Subbotina, E.Y.; Shcherbakov, M.V.; Simakova, A.V. Invertebrates of Siberia, a potential source of animal protein for innovative human food production. 3. Principles of biomass nutrient composition design. Euroasian Entomol. J. 2023, 22, 246–255. [Google Scholar] [CrossRef]
- Tshernyshev, S.E.; Baghirov, R.T.-O.; Modyaeva, V.P.; Morozova, M.D.; Skriptcova, K.E.; Subbotina, E.Y.; Shcherbakov, M.V.; Simakova, A.V. Invertebrates of Siberia, a potential source of animal protein for innovative food production. 4. New method of protein food and feed products generation. Euroasian Entomol. J. 2023, 22, 285–290. [Google Scholar] [CrossRef]
- Olivadese, M.; Dindo, M.L. Edible insects: A historical and cultural perspective on entomophagy with a focus on western societies. Insects 2023, 14, 690. [Google Scholar] [CrossRef]
- Siaw, C.L.; Idrus, Z.; Yu, S.Y. Intermediate technology for fish craker (“keropok”) production. Int. J. Food Sci. Technol. 1985, 20, 17–21. [Google Scholar] [CrossRef]
- Widati, U.S. The Long Influence of Boiling on the Level of Development of Rabbit Skin Rambak Crackers After Frying; University of Brawijaya: Jawa Timur, Indonesia, 1988; p. 50. [Google Scholar]
- Kartiwa, U.M. Utilization of Fish Skin as Raw Material for Making Skin Crackers. Master’s Thesis, Agricultural Institute, Bogor, Indonesia, 2002; p. 42. [Google Scholar]
- Junianto; Haetami, K.; Maulina, I. Fish Skin Production and Utilization it as a Basic Ingredient in Making Crackers; Hibah Research Report Competing IV Year I; Faculty of Fisheries and Marine Sciences Hasanuddin University: Makassar, Indonesia, 2006. [Google Scholar]
- Lilir, F.B.; Palar, C.K.M.; Lontaan, N.N. Effect of time drying on the processing of cow skin crackers. Zootec 2021, 41, 214–222. [Google Scholar] [CrossRef]
- Susanti, S.; Hintono, A.; Mulyani, S.; Ardi, F.A.; Arifan, F. Comparison of chemical characteristics of various commercial animal skin crackers. Am. J. Anim. Vet. Sci. 2022, 17, 314–321. [Google Scholar] [CrossRef]
- Chairil, A.; Irhami; Umar, H.A.; Irmayanti. The effect of long skin soaking in the calcium solution on the quality of rambak crackers from buffalo skin. Theory Pract. Meat Process. 2023, 8, 12–18. [Google Scholar] [CrossRef]
- Mandal, F.B.; Dutta, M. The potential of entomophagy against malnutrition and ensuring food sustainability. Afr. J. Biol. Sci. 2022, 4, 15–22. [Google Scholar] [CrossRef]
- Premalatha, M.; Abbasi, T.; Abbasi, T.; Abbasi, S.A. Energy-efficient food production to reduce global warming and ecodegradation: The use of edible insects. Renew. Sustain. Energy Rev. 2011, 15, 4357–4360. [Google Scholar] [CrossRef]
- Belluco, S.; Losasso, C.; Maggioletti, M.; Alonzi, C.C.; Paoletti, M.G.; Ricci, A. Edible insects in a food safety and nutritional perspective: A critical review. Compr. Rev. Food Sci. Food Saf. 2013, 129, 296–313. [Google Scholar] [CrossRef]
- Hanboonsong, Y.; Jamjanya, T.; Durst, P.B. Six-Legged Livestock: Edible Insect Farming, Collection and Marketing in Thailand Bangkok; Food and Agriculture Organization of the United Nations Regional Office for Asia and the Pacific: Bangkok, Thailand, 2013; p. 58. Available online: https://www.fao.org/3/i3246e/i3246e00.htm (accessed on 1 April 2025).
- Mlcek, J.; Rop, O.; Borkovcova, M.; Bednarova, M. A comprehensive look at the possibilities of edible insects as food in Europe —A review. Pol. J. Food Nutr. Sci. 2014, 64, 147–157. [Google Scholar] [CrossRef]
- Assielou, B.; Due, E.A.; Koff, M.D.; Dabonne, S.; Kouame, P.L. Oryctes owariensis larvae as good alternative protein source: Nutritional and functional properties. Annu. Res. Rev. Biol. 2015, 8, 1–9. [Google Scholar] [CrossRef]
- Han, R.; Shin, J.T.; Kim, J.; Choi, Y.S.; Kim, Y.W. An overview of the South Korean edible insect food industry: Challenges and future pricing/promotion strategies. Entomol. Res. 2017, 47, 141–151. [Google Scholar] [CrossRef]
- Van Huis, A.; Oonincx, D.G.A.B. The environmental sustainability of insects as food and feed. A review. Agron. Sustain. Dev. 2017, 37, 43. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Potential and challenges of insects as an innovative source for food and feed production. Innov. Food Sci. Emerg. Technol. 2013, 17, 1–11. [Google Scholar] [CrossRef]
- Ayensu, J.; Annan, R.A.; Edusei, A.; Lutterodt, H. Beyond nutrients, health effects of entomophagy: A systematic review. Nutr. Food Sci. 2019, 49, 2–17. [Google Scholar] [CrossRef]
- Van Huis, A. Edible insects contributing to food security? Agric. Food Secur. 2015, 4, 20. [Google Scholar] [CrossRef]
- Murefu, T.R.; Macheka, L.; Musundire, R.; Manditsera, F.A. Safety of wild harvested and reared edible insects: A review. Food Control 2019, 101, 209–224. [Google Scholar] [CrossRef]
- EFSA Scientific Committee. Risk profile related to production and consumption of insects as food and feed. EFSA J. 2015, 13, 4257. [Google Scholar] [CrossRef]
- EFSA. Safety of frozen and freeze and dried formulations of the lesser mealworm (Alphitobius diaperinus larva) as a Novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2022, 20, 7325. [Google Scholar] [CrossRef]
- Lahteenmaki-Uutela, A.; Grmelova, N. European law on insects in food and feed. Eur. Food Feed Law Rev. 2016, 11, 2–8. Available online: https://www.jstor.org/stable/43958606 (accessed on 7 October 2024).
- Jansson, A.; Berggren, A. Insects as Food—Something for the Future? Future Agriculture, Swedish University of Agricultural Sciences (SLU): Uppsala, Sweden, 2015; p. 36. Available online: https://pub.epsilon.slu.se/12935/7/jansson_a_berggren_a_151230.pdf (accessed on 15 March 2025).
- Kim, T.-K.; Yong, H.I.; Kim, Y.-B.; Kim, H.-W.; Choi, Y.-S. Edible insects as a protein source: A review of public perception, processing technology, and research trends. Food Sci. Anim. Resour. 2019, 39, 521–540. [Google Scholar] [CrossRef]
- Hlongwane, Z.T.; Slotow, R.; Munyai, T.C. Nutritional composition of edible insects consumed in Africa: A systematic review. Nutrients 2020, 12, 2786. [Google Scholar] [CrossRef]
- Van Peer, M.; Frooninckx, L.; Coudron, C.; Berrens, S.; Álvarez, C.; Deruytter, D.; Verheyen, G.; Van Miert, S. Valorisation Potential of using organic side streams as feed for Tenebrio molitor, Acheta domesticus and Locusta migratoria. Insects 2021, 12, 796. [Google Scholar] [CrossRef] [PubMed]
- Magara, H.J.O.; Niassy, S.; Ayieko, M.A.; Mukundamago, M.; Egonyu, J.P.; Tanga, C.M.; Kimathi, E.K.; Ongere, J.O.; Fiaboe, K.K.M.; Hugel, S.; et al. Edible Crickets (Orthoptera) around the world: Distribution, nutritional value, and other benefits—A review. Front. Nutr. 2021, 7, 537915. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Jiang, H. Nutritive Evaluation of Earthworms as Human Food. In Future Foods; IntechOpen: London, UK, 2017; pp. 127–141. [Google Scholar] [CrossRef]
- EU Commission. Commission Implementing Regulation (EU) 2023/58 of 5 January 2023 Authorising the Placing on the Market of the Frozen, Paste, Dried and Powder Forms of Alphitobius diaperinus larvae (Lesser Mealworm) as a Novel Food and Amending Implementing Regulation (EU) 2017/2470. Available online: http://data.europa.eu/eli/reg_impl/2023/58/oj (accessed on 7 October 2024).
- Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 on the Hygiene of Foodstuffs. Available online: http://data.europa.eu/eli/reg/2004/853/oj (accessed on 7 October 2024).
- Tshernyshev, S.E.; Babkina, I.B.; Baghirov, R.T.-O.; Modyaeva, V.P.; Morozova, M.D.; Skriptcova, K.E.; Subbotina, E.Y.; Shcherbakov, M.V.; Simakova, A.V. Invertebrates of Siberia, a potential source of animal protein for innovative food and feed production. 2. Nutrient composition of the two new model species. Acta Biol. Sib. 2024, 10, 1337–1358. [Google Scholar] [CrossRef]
- Gunya, B.; Muchenje, V.; Masika, P.J. The effect of earthworm Eisenia foetida meal as a protein source on carcass characteristics and physico-chemical attributes of broilers. Pak. J. Nutr. 2019, 18, 657–664. [Google Scholar] [CrossRef]
- Isea-León, F.; Acosta-Balbás, V.; Rial-Betancoutd, L.B.; Medina-Gallardo, A.L.; Mélécony Célestin, B. Evaluation of the fatty acid composition of earthworm Eisenia andrei Meal as an alternative lipid source for fish feed. J. Food Nutr. Res. 2019, 7, 696–700. [Google Scholar] [CrossRef]
- Castro-Bedriñana, J.; Chirinos-Peinado, D.; Sosa-Blas, H. Digestibility, digestible and metabolizable energy of earthworm meal (Eisenia foetida) included in two levels in guinea pigs (Cavia porcellus). Adv. Sci. Technol. Eng. Syst. J. 2020, 5, 171–177. [Google Scholar] [CrossRef]
- Tshernyshev, S.E.; Babenko, A.S.; Babkina, I.B.; Baghirov, R.T.-O.; Modyaeva, V.P.; Morozova, M.D.; Skriptcova, K.E.; Subbotina, E.Y.; Shcherbakov, M.V.; Simakova, A.V. Invertebrates of Siberia, a potential source of animal protein for innovative food and feed production. 5. Changes of nutrient composition in worms and crickets after particular enrichment of feeding substrate. Euroasian Entomol. J. 2024, 23, 287–292. [Google Scholar] [CrossRef]
- Tshernyshev, S.E.; Babenko, A.S.; Babkina, I.B.; Baghirov, R.T.-O.; Modyaeva, V.P.; Morozova, M.D.; Skriptcova, K.E.; Subbotina, E.Y.; Shcherbakov, M.V.; Simakova, A.V. Invertebrates of Siberia as a promising source of animal protein for innovative feed and food production. 6. Potential of nutrient accumulation in biomass via feed enrichment. Acta Biol. Sib. 2024, 10, 1499–1518. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R. Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 7 October 2024).
- Myers, R. The ABCs of the Earthworm Business; Shields Publications: Eagle River, WI, USA, 1969; p. 64. [Google Scholar]
- Munroe, G. Manual of On-Farm Vermicomposting and Vermiculture; Publication of Organic Agriculture Centre of Canada: Truro, NS, Canada, 2007; p. 40. Available online: https://www.researchgate.net/publication/268254767_Manual_of_On-Farm_Vermicomposting_and_Vermiculture (accessed on 27 January 2025).
- Sinha, R.K. Vermiculture for Sustainable Agriculture. Sustainable Agriculture; Surabhee Publication: Jaipur, India, 2004; p. 370. [Google Scholar]
- Kajal, G.; Ramandeep, K.; Pooja, P.; Duhan, K. Earthworms and sustainable agriculture: A review. In Futuristic Trends in Agriculture Engineering & Food Sciences; IIP Series; Indian Institute of Professionals & Engineers: Uttar Pradesh, India, 2024; Volume 3, pp. 66–74. [Google Scholar]
- Fuah, A.M.; Siregar, H.C.H.; Endravati, Y.C.; Astuti, D.A.; Khotijah, L.; Sunyoto, W.A. Cricket Farming in Indonesia: Challenge and Opportunity; Lambert Academic Publishing: Riga, Latvia, 2016; p. 56. [Google Scholar]
- Fuah, A.M.; Siregar, H.C.H.; Endravati, Y.C. Cricket farming for animal protein as profitable business for small farmers in Indonesia. J. Agric. Sci. Technol. A 2015, 5, 295–304. [Google Scholar] [CrossRef]
- Fernandez-Cassi, X.; Söderqvist, K.; Bakeeva, A.; Vaga, M.; Dicksved, J.; Vagsholm, I.; Jansson, A.; Boqvist, S. Microbial communities and food safety aspects of crickets (Acheta domesticus) reared under controlled conditions. J. Insects Food Feed. 2020, 6, 429–440. [Google Scholar] [CrossRef]
- Yaemkonga, S.; Incharoen, T.; Rattanachak, N.; Jongjitvimol, T. Comparative analysis of diet effects on growth performance and nutrient composition in house cricket, Acheta domestica as an alternative protein source in Thailand. Cogent Food Agric. 2024, 10, 2339543. [Google Scholar] [CrossRef]
- Udomsil, N.; Imsoonthornruksa, S.; Gosalawit, C.; Ketudat-Cairns, M. Nutritional values and functional properties of house cricket (Acheta domesticus) and field cricket (Gryllus bimaculatus). Food Sci. Technol. Res. 2019, 25, 597–605. [Google Scholar] [CrossRef]
- Gunya, B.; Masika, P.J.; Hugo, A.; Muchenje, V. Nutrient composition and fatty acid profiles of oven-dried and freeze-dried earthworm Eisenia foetida. J. Food Nutr. Res. 2016, 4, 343–348. [Google Scholar] [CrossRef]
- Arshanitsa, N.M.; Onishchenko, L.S.; Voronin, V.N. Materials of pathological and epizootological studies on sartan disease in fish. In Biological Resources and Rational Use of Water Bodies in the Vologda Region; GosNIORH: Leningrad, Russia, 1989; Volume 293, pp. 113–130. [Google Scholar]
- Ludupova, E.Y.; Sergeeva, L.A.; Gyrgeshkinova, N.S.; Oloeva, E.V.; Badmaeva, V.Y.; Budasheeva, A.B. A case of occurrence of Haff disease (alimentary-toxic paroxysmal myoglobinuria) in the Republic of Buryatia in the villages of the Pribaikalsky district located near Lake Kotokel. In Bulletin of the East Siberian Scientific Center of the Siberian Branch of the Russian Academy of Medical Sciences; RIO NTRVH SORAMN: Irkutsk, Russia, 2009; Volume 67, pp. 92–94. [Google Scholar]
- Arshanitsa, N.M.; Onishchenko, L.S. Gaff disease. Issues Leg. Regul. Vet. Med. 2016, 2, 87–90. [Google Scholar]
- Savchenko, A.A.; Krasnolobova, E.P.; Veremeeva, S.A.; Glazunova, L.A. Pathomorphological changes in the detoxification organs of white mice during an outbreak of “Haff” disease. Bull. Bashkir State Agrar. Univ. 2023, 4, 109–114. [Google Scholar] [CrossRef]


| Type of Precursor | I Stage, Single Dose of Precursor | II Stage, Double Dose of Precursor | ||||
|---|---|---|---|---|---|---|
| Per 1 kg of Food Substrate, mg | Per Food Portion of Crickets, mg | Per Food Portion of earthworms, mg | Per 1 kg of Food Substrate, mg | Per Food Portion of Crickets, mg | Per Food Portion of Earthworms, mg | |
| C | 50 | 1.2 | 0.25 | 100 | 2.4 | 0.5 |
| B7 | 25 | 0.6 | 0.125 | 50 | 1.2 | 0.25 |
| Minerals (chelate) | 5 | 0.12 | 0.025 | 10 | 0.24 | 0.05 |
| B1 | 2 | 0.048 | 0.01 | 4 | 0.096 | 0.02 |
| B3 | 30 | 0.72 | 0.15 | 60 | 1.44 | 0.3 |
| B9 | 1 | 0.024 | 0.005 | 1 | 0.048 | 0.01 |
| A | 25 | 0.6 | 0.125 | 50 | 1.2 | 0.25 |
| D | 2.5 | 0.06 | 0.0125 | 5 | 0.12 | 0.025 |
| E | 20 | 0.48 | 0.1 | 40 | 0.96 | 0.2 |
| K | 2 | 0.048 | 0.01 | 4 | 0.096 | 0.02 |
| Substrate | B12 | B2 | B3 | B6 | B9 | A | D3 | E | K | C |
|---|---|---|---|---|---|---|---|---|---|---|
| A. domesticus | ||||||||||
| Control | 0.00031 | 3.51 | 3.75 | 0.16 | 0.08 | 0.0050 | 0.000067 | 2.14 | 0.0070 | 2.75 |
| B7_C | 0.00055 | – | – | 0.23 | 0.12 | – | – | – | – | – |
| Chelate | 0.00056 | – | – | 0.22 | 0.16 | 0.0071 | – | 2.75 | – | – |
| B | 0.00055 | – | – | – | 0.13 | 0.0066 | – | – | – | – |
| Bx2 | 0.00056 | 4.70 | 6.90 | 0.48 | 0.38 | – | – | – | – | – |
| FSV | 0.00051 | – | – | – | 0.15 | 0.0070 | – | – | – | – |
| FSVx2 | – | – | – | – | – | 0.0077 | 0.000088 | 3.40 | 0.0152 | – |
| E. fetida | ||||||||||
| Control | 0.00002 | 10.53 | 19.92 | 0.13 | 0.021 | 0.0008 | 0.000030 | 4.54 | 0.0083 | 4.39 |
| B7_C | 0.00005 | 17.52 | 39.14 | 0.22 | – | 0.0017 | 0.000052 | – | – | 6.21 |
| Chelate | 0.00006 | – | 41.72 | 0.18 | – | 0.0015 | 0.000042 | – | – | 6.38 |
| B | 0.00005 | 13.61 | 31.53 | 0.20 | – | 0.0016 | 0.000043 | 6.25 | – | – |
| Bx2 | 0.00004 | 13.20 | – | 0.27 | 0.048 | – | – | – | – | – |
| FSV | 0.00005 | – | 43.54 | 0.21 | – | 0.0013 | 0.000047 | 5.83 | – | 5.61 |
| FSVx2 | – | – | – | – | – | 0.0022 | 0.000048 | 8.90 | 0.0127 | 9.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tshernyshev, S.E.; Babenko, A.S.; Babkina, I.B.; Baghirov, R.T.-O.; Modyaeva, V.P.; Morozova, M.D.; Skribtcova, K.E.; Subbotina, E.Y.; Shcherbakov, M.V.; Simakova, A.V. Invertebrates of Siberia, a Potential Source of Animal Protein for Innovative Food and Feed Production: Biomass Nutrient Composition Change in the Earthworm Eisenia fetida (Savigny, 1826) and the House Cricket Acheta domesticus (Linnaeus, 1758). Insects 2025, 16, 632. https://doi.org/10.3390/insects16060632
Tshernyshev SE, Babenko AS, Babkina IB, Baghirov RT-O, Modyaeva VP, Morozova MD, Skribtcova KE, Subbotina EY, Shcherbakov MV, Simakova AV. Invertebrates of Siberia, a Potential Source of Animal Protein for Innovative Food and Feed Production: Biomass Nutrient Composition Change in the Earthworm Eisenia fetida (Savigny, 1826) and the House Cricket Acheta domesticus (Linnaeus, 1758). Insects. 2025; 16(6):632. https://doi.org/10.3390/insects16060632
Chicago/Turabian StyleTshernyshev, Sergei E., Andrei S. Babenko, Irina B. Babkina, Ruslan T.-O. Baghirov, Vera P. Modyaeva, Margarita D. Morozova, Ksenia E. Skribtcova, Elena Y. Subbotina, Mikhail V. Shcherbakov, and Anastasia V. Simakova. 2025. "Invertebrates of Siberia, a Potential Source of Animal Protein for Innovative Food and Feed Production: Biomass Nutrient Composition Change in the Earthworm Eisenia fetida (Savigny, 1826) and the House Cricket Acheta domesticus (Linnaeus, 1758)" Insects 16, no. 6: 632. https://doi.org/10.3390/insects16060632
APA StyleTshernyshev, S. E., Babenko, A. S., Babkina, I. B., Baghirov, R. T.-O., Modyaeva, V. P., Morozova, M. D., Skribtcova, K. E., Subbotina, E. Y., Shcherbakov, M. V., & Simakova, A. V. (2025). Invertebrates of Siberia, a Potential Source of Animal Protein for Innovative Food and Feed Production: Biomass Nutrient Composition Change in the Earthworm Eisenia fetida (Savigny, 1826) and the House Cricket Acheta domesticus (Linnaeus, 1758). Insects, 16(6), 632. https://doi.org/10.3390/insects16060632







