Simple Summary
The aim is to understand the host preferences of Thrips hawaiiensis from a chemoecological perspective. The olfactory responses of female T. hawaiiensis to the floral volatiles of different plants were studied using electroantennography (EAG) and behavioral bioassays in different types of olfactometers. Then, the components of the volatile profile of the preferred host (Magnolia grandiflora flower) were further analyzed by gas chromatography–mass spectrometry. According to the EAG and behavioral bioassays, we found that T. hawaiiensis showed significant olfactory preference to β-elemen, bicyclogermacren, and (E)-α-bisabolene, which were three main components identified from the volatiles of M. grandiflora flowers. In short, our results indicated that semiochemical volatiles played important roles in searching the preferred host plant species of T. hawaiiensis. In addition, β-elemen showed the greatest potential to be used in the integrated management of T. hawaiiensis.
Abstract
Volatile cues are important in the host detection and discrimination of phytophagous insects, allowing them to find suitable hosts. Here, the electroantennography (EAG) and behavioral responses of female Thrips hawaiiensis to the floral volatiles of different plants, Magnolia grandiflora L. (Magnoliales: Magnoliaceae), Gerbera jamesonii Bolus (Asterales: Asteraceae), Lilium brownii Baker (Liliales: Liliaceae), and Rosa rugosa Thunb. (Rosales: Rosaceae), were studied. Y-tube olfactometer bioassays revealed that M. grandiflora was the preferred host for T. hawaiiensis. Fifty-two compounds were identified from the volatile profile of M. grandiflora by gas chromatography–mass spectrometry analysis, of which β-elemen (15.39%), bicyclogermacren (11.99%), and (E)-α-bisabolene (6.05%) showed the highest relative contents. The EAG bioassays revealed that the antennae of T. hawaiiensis could perceive these tested volatile compounds at different concentrations. In six-arm olfactometer bioassays, T. hawaiiensis showed significant positive responses to β-elemen and (E)-α-bisabolene at various concentrations, and 10 μg/μL was found to be the most attractive concentration for β-elemen, and 50 and 100 μg/μL for (E)-α-bisabolene. Based on the four-arm olfactometer bioassays, β-elemen was more attractive than (E)-α-bisabolene to T. hawaiiensis when compared at their optimal concentrations. Therefore, T. hawaiiensis could perceive and distinguish the floral volatiles from the preferred host plant (M. grandiflora). These findings assist in better understanding the mechanism of host preferences of T. hawaiiensis from a chemoecological perspective. In particular, β-elemen showed the greatest potential to be developed as a novel attractant for the monitoring and control of T. hawaiiensis.
1. Introduction
Thrips are members of the order Thysanoptera, which includes more than 6000 described extant species. They are opportunistic and ubiquitous insects with small bodies, and many species are preadapted to an invasive lifestyle. The body length of the adult ranges from less than 1 mm to only a few millimeters, so they can be carried by the wind over long distances. Thrips hawaiiensis (Morgan, 1913) (Thysanoptera: Thripidae) is a common flower-thrips species that is native to the Asia and Pacific regions [1]. As a result of increasing international trade, it is now distributed in Asia, Australia, America, Africa, and Europe [2,3,4,5] and has become an important agricultural and horticultural pest worldwide.
Thrips hawaiiensis has a wide host range among crop plants, including various ornamental plants, fruits, and vegetables [6,7,8,9]. However, T. hawaiiensis shows clear host preferences in the field, with different population sizes and associated degrees of damage among different host plant species, e.g., Gardenia jasminoides (Gentianales: Rubiaceae), Hydrangea macrophylla L. (Saxifragales: Saxifragaceae), and 19 other plants [8,9]. Particularly, when T. hawaiiensis, Thrips flavidulus Bagnall (Thysanoptera: Thripidae), Frankliniella occidentalis Pergande (Thysanoptera: Thripidae), and Thrips coloratus Schmutz (Thysanoptera: Thripidae) coexisted on M. grandiflora flowers, T. hawaiiensis showed a significantly higher number than the other three thrips species, indicating the dominant position of this thrips on this host plant [10]. In addition, T. hawaiiensis prefers to feed and live in flowers, rather than in other plant structures [1,6,11]. Therefore, this thrip pest not only shows host preferences, but also preferences for different parts of the host plant.
There is increasing evidence that olfactory cues are important for host-searching by flower-inhabiting thrips, including T. hawaiiensis, and that thrips use plant volatiles to locate more suitable host plants, as it has been reported that F. occidentalis were attracted to the volatiles of Rosa rugosa (Rosales: Rosaceae) flower and T. hawaiiensis were attracted to the volatiles of G. jasminoides flower [12,13,14]. These findings would be useful in building a push–pull system for thrips pest control, thereby reducing the need for chemical sprays [15,16].
According to our previous studies, Magnolia grandiflora L., Gerbera jamesonii Bolus, Lilium brownii Baker, and R. rugosa Thunb. are four host plants of T. hawaiiensis, and the population fitness of T. hawaiiensis differs among these hosts, indicating this pest could cause different degrees of damage to different plant species [9,10,14]. In the present study, the olfactory responses of T. hawaiiensis to these different plant flowers above were studied using electroantennography (EAG) and behavioral bioassays in Y-tube, six-arm, and four-arm olfactometers. Our study provides new information for further exploring the mechanism of host selection and damage in T. hawaiiensis, based on the chemoecology of its interaction with potential host plants. The findings of this study also provide new information that will be useful for ecological regulation or control of thrips pests. In particular, these results could provide candidate compounds for the development of new attractants/repellents for the integrated management of T. hawaiiensis.
2. Materials and Methods
2.1. Insects and Host Plants
Thrips hawaiiensis adults were collected from different weeds, as well as vegetable and flowering plant species, in the Nanming district (106°77′78.24″ E, 26°55′73.56″ N), Guiyang area, Guizhou Province, China. After being taken back to the laboratory, these thrips were reared on green bean pods of Phaseolus vulgaris L. (Fabales: Fabaceae) in plastic containers (20 cm × 14 cm × 9 cm) with snap-on lids and used to establish laboratory colonies [17]. The thrips colonies were reared for more than five generations before being used for bioassays and were kept in a climate-controlled room (RTOP-400Y, Tuopu Yunnong Technology Co., Ltd., Hangzhou, China) at 25 °C ± 1 °C, 70% ± 5% relative humidity under a 14 h light–10 h dark photoperiod.
Different flower plant species, M. grandiflora, G. jamesonii, L. brownii, and R. rugosa, were grown in greenhouses in the nursery of Guiyang University, Guizhou Province, China [8]. Greenhouses were kept free of pests by insect-proof netting, and no insecticides were applied to these plants. Plant flowers at anthesis were collected for olfactory tests and gas chromatography–mass spectrometry (GC-MS) analysis.
2.2. Y-Tube Olfactometer Bioassays
The olfactory responses of T. hawaiiensis to the volatiles of different plant flowers were tested in a Y-tube olfactometer by the method of Colazza et al. [18] and Cao et al. [17]. Here, we made two types of comparisons: (1) plant flowers (each 20.0 g) versus clean air (CA); and (2) all the possible pairings of these four plant flowers (20.0 g each). Because thrips females are more sensitive to volatiles from host plants [14], only T. hawaiiensis females were used in this study. For each comparison, 60 T. hawaiiensis females (2–3 days old) were tested individually, and flower material was replaced with an equal quantity after testing 10 individuals. Before each test treatment, T. hawaiiensis adults were initially starved for 6 h. Airflow was set at 200 mL/min. All bioassays were done between 09:00 and 17:00 at room temperature (25 °C ± 2 °C).
2.3. GC-MS Analysis
According to the Y-tube olfactometer bioassays, as T. hawaiiensis were most attracted to the mixture of volatile organic compounds (VOCs) from M. grandiflora flowers, the components of M. grandiflora floral VOCs were further analyzed. The collected VOCs were analyzed by solid phase microextraction–gas chromatography–mass spectrometry (SPME–GC–MS) (HP6890/5975C, Agilent Technologies, Santa Clara, CA, USA), as detailed in Báez et al. [19] and Cao et al. [13]. The chemical identities of the main peaks in the chromatograms were determined by comparing the mass spectra of compounds with those in databases (NIST 2017 and WILEY 275) [20].
2.4. Behavioral Responses of T. hawaiiensis to Main M. grandiflora VOCs
β-Elemen, bicyclogermacren, and (E)-α-bisabolene were the most abundant compounds that were identified from the VOCs profile of M. grandiflora (the most attractive plant species). Therefore, the behavioral responses of T. hawaiiensis to these compounds at different concentrations (0.1, 1, 10, 50, and 100 μg/μL) were further tested in different types of olfactometers.
2.5. Odor Stimuli
Solutions of β-elemen (MedChemExpress, Monmouth Junction, NJ, USA), bicyclogermacren (Sigma-Aldrich, St. Louis, MO, USA), and (E)-α-bisabolene (Sigma-Aldrich) in mineral oil (Sigma-Aldrich) were prepared at different concentrations for EAG and olfactometer bioassays and were stored at −20 °C until use.
2.6. Electroantennography Tests
In brief, a data acquisition collector, a stimulus flow controller, an AC/DC amplifier, a single-ended probe, and a micromanipulator were the five main components of the EAG system (SYNTECH, IDAC-2, Kirchzarten, Germany). The antennal sensitivity of T. hawaiiensis females (2–3 days old) to increasing concentrations of the three test compounds was evaluated by EAG using a technique described elsewhere [21,22,23]. To achieve better contact with the electrodes, the antennae of T. hawaiiensis females were excised at the groove between antennal segments 6 and 7 (away from the head). Then, the recording electrode was placed in contact with the last antennal segment of the thrips, and the neutral electrode was inserted into the base of the head. Mineral oil (10 μL) was used as a control; each compound was separately diluted in mineral oil to obtain different concentrations; and 10 μL of each compound at each concentration (0.001, 0.01, 0.1, 1, and 10 μg/μL) was used as the stimulus. Each compound at each dose was adsorbed onto a filter paper strip inserted in a Pasteur pipette, which was used as an odor cartridge. The measurements were conducted as described by Cao et al. [23].
2.7. Six-Arm Olfactometer Bioassays
The behavioral responses of T. hawaiiensis females to different doses of each of the three compounds [β-elemen, bicyclogermacren, and (E)-α-bisabolene] were also evaluated in a six-arm olfactometer using the method of Turlings et al. [24] with necessary modifications. The six-arm olfactometer consisted of a central chamber (120 mm internal diameter) with six arms (60 mm length and 15 mm internal diameter), and details were described in our previous study [25]. Solutions of these three compounds (0.1, 1, 10, 50, and 100 μg/μL, respectively) were also prepared with mineral oil, which were adsorbed onto a filter paper disk (1.0 cm diameter) and were used as stimuli (10 μL of each compound solution). Mineral oil (10 μL) was used as the control. In the six-arm olfactometer, each odor source was driven to thrips by the airflow at a flow rate of 200 mL min−1. Unmated female T. hawaiiensis adults (2–3 days old) were starved for 6 h and were then introduced into the olfactometer in groups of 200 individuals. The numbers of T. hawaiiensis that entered the arms of the olfactometer were counted and considered to have made a choice for an odor source, which should be accomplished within 30 min. Bioassays were repeated five times and were conducted between 09:00 and 17:00 at room temperature (25 °C ± 2 °C).
2.8. Four-Arm Olfactometer Bioassays
As tested in the six-arm olfactometer, bicyclogermacren was not attractive to T. hawaiiensis at any of the tested concentrations. The most attractive concentration of β-elemen was 10 μg/μL, and those of (E)-α-bisabolene were 50 and 100 μg/μL. Hence, the attractiveness of β-elemen and (E)-α-bisabolene at their optimal concentrations was compared in further four-arm bioassays [23,26], which only differed in the number of olfactometer arms as compared with the six-arm olfactometer bioassay described in the preceding section. The airflow was also maintained at 200 mL min−1. Female T. hawaiiensis adults were introduced in groups of 180 individuals, with five replicates.
2.9. Statistical Analyses
The null hypothesis that T. hawaiiensis adults showed no preference for either Y-tube arm (a response equal to 50:50) was analyzed using a chi-square goodness-of-fit test (in all cases, df = 1). The numbers of thrips showing preferences for odors in the 6-arm and 4-arm olfactometers were subjected to analysis of variance (ANOVA), followed by Tukey’s honestly significant difference (HSD) test (p < 0.05) for separation of means. All statistical analyses were performed using SPSS 18.0 for Windows (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Y-Tube Olfactometer Bioassays
When provided with different floral volatiles versus clean air (CA), female T. hawaiiensis exhibited significant preferences for M. grandiflora (χ2 = 40.16, p < 0.001), G. jamesonii (χ2 = 34.57, p < 0.001), L. brownii (χ2 = 32.67, p < 0.001), and R. rugosa (χ2 = 28.70, p < 0.001) over CA (Figure 1). When presented with pairs of floral volatiles of these four plant species, T. hawaiiensis females significantly preferred M. grandiflora to G. jamesonii (χ2 = 6.00, p = 0.014), M. grandiflora to L. brownii (χ2 = 15.87, p < 0.001), M. grandiflora to R. rugosa: χ2 = 12.52, p < 0.001), G. jamesonii to L. brownii (χ2 = 5.45, p = 0.02), G. iamesonii to R. rugosa (χ2 = 5.26, p = 0.022), and L. brownii to R. rugosa (χ2 = 4.74, p = 0.029) (Figure 1).
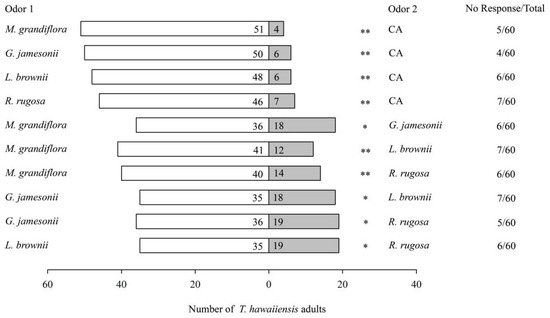
Figure 1.
Olfactory responses of female T. hawaiiensis to different plant flowers. CA: clean air. Asterisks indicate highly significant (** p < 0.01) and significant (* p < 0.05) differences in selectivity of T. hawaiiensis between two odors by χ2 test.
3.2. Analysis of M. grandiflora Flower Volatiles
Fifty-two components were identified in the floral VOCs profile of M. grandiflora (Table 1). β-Elemen showed the highest relative content (15.39%), followed by bicyclogermacren (11.99%), and (E)-α-bisabolene (6.05%). No other component showed a relative content exceeding 5% of the M. grandiflora flower volatiles, except for germacrene D (5.13%). Notably, β-elemen and bicyclogermacren are isomers of each other. There were 25 isomers of spathulenol in total, together accounting for 64.71% of the total VOCs profile. In addition, four components were not identified, with relative contents of 2.25%, 2.13%, 1.86%, and 1.05%, respectively.

Table 1.
Components of M. grandiflora flower volatiles.
3.3. Electroantennogram (EAG) Tests
The EAG responses of female T. hawaiiensis to increasing doses of β-elemen, bicyclogermacren, and (E)-α-bisabolene are shown in Figure 2A. In the dose range tested (starting from the 0.01 μg dose), all three compounds elicited typical sigmoid-shaped dose–responses in T. hawaiiensis. As determined by ANOVA, the thrips showed significantly different responses to the three compounds at the 1 μg dose (F = 96.69; df = 4, 20; p < 0.001), 10 μg (F = 33.64; df = 4, 20; p < 0.001), and 100 μg (F = 66.83; df = 4, 20; p < 0.001) (Figure 2B). Comparing the compounds at the 1 μg dose, the mean EAG values for bicyclogermacren and (E)-α-bisabolene were not significantly different from each other, but both were significantly higher than that for β-elemen. Comparing the compounds at the 10 μg dose, the mean EAG values were not significantly different between β-elemen and (E)-α-bisabolene, nor between bicyclogermacren and (E)-α-bisabolene, but were significantly higher for β-elemen than for bicyclogermacren. Comparing the compounds at the 100 μg dose, the mean EAG values were not significantly different between β-elemen and (E)-α-bisabolene, nor between β-elemen and bicyclogermacren, but were significantly higher for (E)-α-bisabolene than for bicyclogermacren.
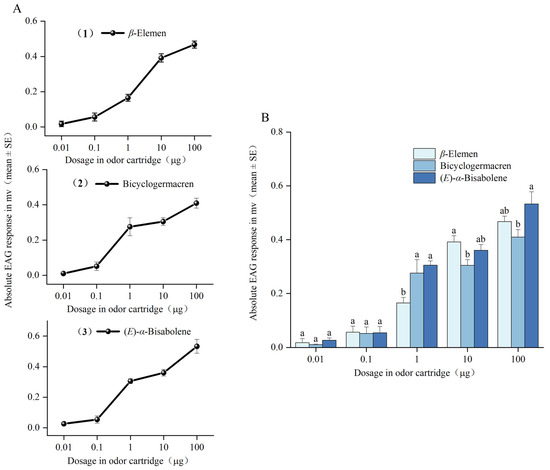
Figure 2.
Electroantennography responses of female T. hawaiiensis. (A) EAG dose–response curves and (B) EAG responses of T. hawaiiensis to different doses of β-elemen, bicyclogermacren, and (E)-α-bisabolene. Mean values are shown. Different letters indicate significant differences among different compounds at the same dose (one-way analysis of variance followed by Tukey’s HSD test, p < 0.05).
3.4. Six-Arm Olfactometer Bioassays
Six-arm bioassays showed that each concentration (0.1, 1, 10, 50, and 100 μg/μL) of β-elemen (F = 351.06; df = 5, 24; p < 0.001) and (E)-α-bisabolene (F = 176.04; df = 5, 24; p < 0.001) were significantly more attractive to T. hawaiiensis, compared with the control of mineral oil (Figure 3). The most attractive concentration of β-elemen was 10 μg/μL. For (E)-α-bisabolene, the most attractive concentrations were 50 and 100 μg/μL, but the degree of attractiveness did not differ between these two concentrations. However, bicyclogermacren at any concentration was not more attractive than mineral oil to T. hawaiiensis (F = 1.68; df = 5, 24; p = 0.18).
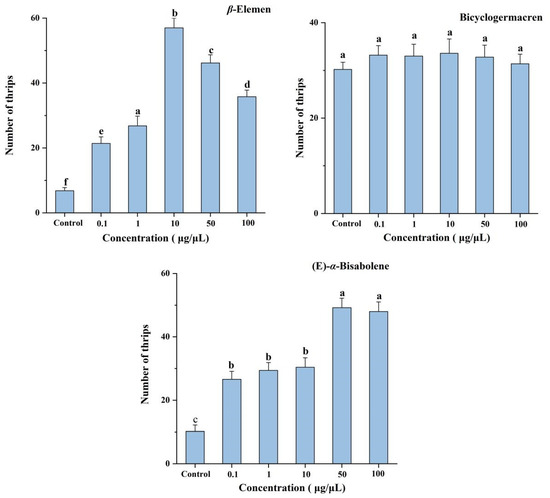
Figure 3.
Olfactory responses of female T. hawaiiensis to β-elemen, bicyclogermacren, and (E)-α-bisabolene at different concentrations in a six-arm olfactometer. Control was mineral oil. Data are means ± SE. Different letters above bars indicate significant differences (one-way analysis of variance followed by Tukey’s HSD test, p < 0.05).
3.5. Four-Arm Olfactometer Bioassays
As mentioned above, the most attractive concentration of β-elemen was 10 μg/μL, and the most attractive concentrations of (E)-α-bisabolene were 50 and 100 μg/μL. Compared with the control (mineral oil), although both β-elemen and (E)-α-bisabolene at their respective optimal concentrations were significantly more attractive to T. hawaiiensis (F = 284.03; df = 3, 16; p < 0.001) (Figure 4), T. hawaiiensis significantly preferred β-elemen to (E)-α-bisabolene, but showed no significant difference in the olfactory preference between 50 and 100 μg/μL of (E)-α-bisabolene.
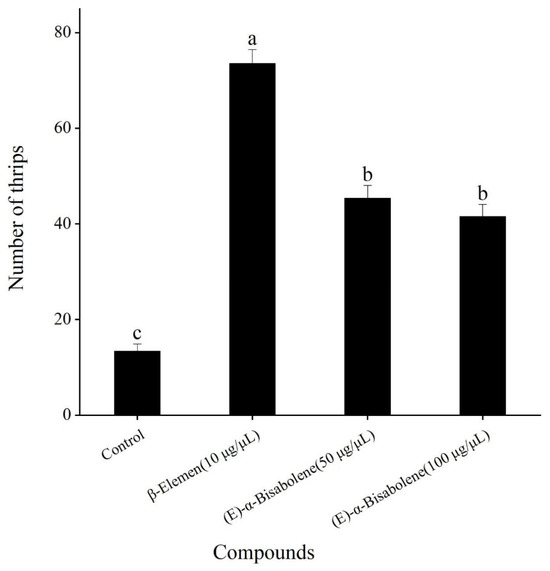
Figure 4.
Olfactory responses of female T. hawaiiensis to β-elemen and (E)-α-bisabolene at their most attractive concentrations in a four-arm olfactometer. Control was mineral oil. Data are means ± standard errors (SE). Different lowercase letters on bars indicate significant differences (one-way analysis of variance followed by Tukey’s HSD test, p < 0.05).
4. Discussion
Magnolia grandiflora is recognized as a preferred host plant for T. hawaiiensis, and large numbers of this thrips species attack this species in the field [10]. Here, Y-tube olfactometer bioassays showed that T. hawaiiensis had clear olfactory preferences among the floral volatiles of four plant species, ranked as follows: M. grandiflora > G. jamesonii > L. brownii > R. rugosa. This rank order is consistent with the host plant fitness levels for T. hawaiiensis, as determined in our previous studies [9,10]. Similar results were reported for Frankliniella occidentalis (Thysanoptera: Thripidae), whose olfactory preferences were also closely related to their fitness levels among different host plants [13]. These results confirmed that volatile cues play a vital role in guiding insects as they search for suitable food, oviposition sites, or nutrient sources [27,28]. Our results may also partly explain why T. hawaiiensis populations reach different sizes and cause different degrees of damage to these four flowering plants.
The SPME–GC–MS analysis detected 52 compounds in the floral VOCs profile of M. grandiflora, among which β-elemen, bicyclogermacren, and (E)-α-bisabolene were the most abundant. These three main compounds were not detected in the floral VOCs profiles of G. jamesonii, L. brownii, or R. rugosa [13,14], which may explain why T. hawaiiensis prefers the floral VOCs of M. grandiflora. The EAG analyses revealed that these three main tested compounds were perceived by the peripheral olfactory system of T. hawaiiensis at a wide range of concentrations, and then their biological activity was further investigated in six- and four-arm olfactometers. The six-arm olfactometer bioassays indicated that T. hawaiiensis was significantly attracted to β-elemen and (E)-α-bisabolene at a range of concentrations, but not bicyclogermacren at any concentration. In addition, the most attractive concentrations were 10 μg/μL for β-elemen and 50 and 100 μg/μL for (E)-α-bisabolene. Furthermore, in the four-arm olfactometer bioassays, T. hawaiiensis preferred β-elemen to (E)-α-bisabolene when the two compounds were compared at their most attractive concentrations. Therefore, β-elemen has the greatest potential to be developed as a lure for T. hawaiiensis. Probably because of the differences in the cultivation conditions or geographical environment, the abundant compounds from the VOCs profile of M. grandiflora flowers differed in other research, while β-elemen was also one of the main compounds [19]. In addition, β-elemen is attractive to Araecerus fasciculatus (Coleoptera: Anthribidae) [29]. Thus, β-elemen has potential uses in the management of both field pests and insect pests of stored products. However, these results were obtained exclusively under laboratory conditions. Further investigation into field trapping tests is warranted to ensure the practical applicability of pest monitoring and control strategies.
Previous studies have reported that methyl anthranilate, linalool, (E-3,E-7)-4,8,12-trimethyltrideca-1,3,7,11-tetraene, (Z)-3-hexenyl tiglate, o-anisidine, and other volatile compounds are also attractive to T. hawaiiensis [14,30,31]. Therefore, the synergistic attractiveness of these volatile compounds as well as β-elemen to T. hawaiiensis should be further studied, with a range of tests at appropriate ratios and concentrations [32,33]. This would be useful to develop more efficient attractants for the control or integrated management of T. hawaiiensis. In addition, to our knowledge, only a small number of volatile compounds out of all of those emitted by host plants are involved in host detection by phytophagous insects [34,35,36]. Here, only the attractiveness of the most abundant components of the floral VOCs to thrips was assessed. The biological activity of minute amounts of other components (among the floral VOCs of M. grandiflora) for attracting T. hawaiiensis should be further investigated.
Although floral VOCs of M. grandiflora were able to elicit significant EAG and behavioral responses in female T. hawaiiensis, it is still unknown how T. hawaiiensis perceives these volatile stimuli by the olfactory neurons inside the antennal sensilla. Further studies should explore the molecular and cellular olfaction mechanism of host plant recognition in thrips [37,38,39]. This will help us to better understand the interaction between host plants and thrips at the chemoecological level and provide new information to elucidate the mechanism of the host preferences of thrips.
Our results suggest that, amongst the volatiles tested in this study, β-elemen showed the greatest potential to be developed as a new lure for T. hawaiiensis. Including this compound in attractants may make them more efficient and effective for the monitoring and control of this pest, especially when combined with sex pheromones and thrips parasitoid attraction [30,40].
Author Contributions
Conceptualization, Y.C. and Y.G.; methodology, T.Z. and Y.Y.; software, F.M.; validation, all authors; formal analysis, T.Z., F.J. and Y.Y.; investigation, T.Z., Y.Y., F.J., R.Y. and L.H.; resources, T.Z. and Y.Y.; data curation, T.Z., Y.Y., L.H., F.M. and X.Z.; writing—original draft preparation, T.Z. and Y.Y.; writing—review and editing, T.Z., Y.Y. and Y.C.; visualization, R.Y.; supervision, Y.G.; project administration, Y.C. and Y.G.; funding acquisition, Y.C. and T.Z. All authors have read and agreed to the published version of the manuscript.
Funding
We thank the Training Project for High-Level Innovative Talent in Guizhou Province (GCC [2023]074), Key Project of the Natural Science Foundation of Guizhou Province (ZK [2022]001), Guizhou Key Laboratory of Agricultural Biosecurity (Qiankehe ZSYS [2025]024), Program for Natural Science Research in Guizhou Education Department (QJJ [2023]024), and Doctoral Foundation Project of Guizhou University of Traditional Chinese Medicine (043230030) for financial support.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We express our sincere thanks to David A. J. Teulon (New Zealand Institute for Plant & Food Research, Ltd., Christchurch, New Zealand) for useful suggestions and comments on an earlier draft of this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mound, L.A. Thysanoptera: Diversity and interactions. Annu. Rev. Entomol. 2005, 50, 247–269. [Google Scholar] [CrossRef] [PubMed]
- Atakan, E.; Ölçülü, M.; Pehlivan, S.; Satar, S. A new thrips species recorded in Turkey: Thrips hawaiiensis (Morgan, 1913) (Thysanoptera: Thripidae). Türk. Entomol. Bült. 2015, 2, 77–84. [Google Scholar] [CrossRef]
- Goldarazena, A. First record of Thrips hawaiiensis (Morgan, 1913) (Thysanoptera: Thripidae), an Asian pest thrips in Spain. Bull. OEPP 2011, 41, 170–173. [Google Scholar] [CrossRef]
- Reynau, P.; Balmès, V.; Pizzol, J. Thrips hawaiiensis (Morgan, 1913) (Thysanoptera: Thripidae), an Asian pest thrips now established in Europe. Bull. OEPP 2008, 1, 155–160. [Google Scholar] [CrossRef]
- Marullo, A.; De Grazia, A. Thrips hawaiiensis a pest thrips from Asia newly introduced into Italy. Bull. Insectol. 2017, 70, 27–30. [Google Scholar]
- Murai, T. Development and reproductive capacity of Thrips hawaiiensis (Thysanoptera: Thripidae) and its potential as a major pest. Bull. Entomol. Res. 2001, 91, 193–198. [Google Scholar] [CrossRef]
- Aliakbarpour, H.; Salmah, M.R.C. Seasonal abundance and spatial distribution of larval and adult thrips (Thysanoptera) on weed host plants in mango orchards in Penang, Malaysia. Appl. Entomol. Zool. 2011, 2, 185–194. [Google Scholar] [CrossRef]
- Cao, Y.; Zhi, J.R.; Zhang, R.Z.; Li, C.; Liu, Y.; Lv, Z.Y.; Gao, Y.L. Different population performances of Frankliniella occidentalis and Thrips hawaiiensis on flowers of two horticultural plants. J. Pest Sci. 2018, 1, 79–91. [Google Scholar] [CrossRef]
- Cao, Y.; Reitz, S.R.; Germinara, G.S.; Wang, C.; Wang, L.J.; Yang, S.Y.; Gao, Y.L.; Zhang, W.Q.; Li, C. Host preference of Thrips hawaiiensis for different ornamental plants. J. Pest Sci. 2022, 95, 761–770. [Google Scholar] [CrossRef]
- Cao, Y.; Qi, G.L.; Jiang, F.Y.; Meng, Y.L.; Wang, C.; Gu, Z.Y.; Gao, Y.L.; Reitz, S.R.; Li, C. Population performance and detoxifying and protective enzyme activities of four thrips species feeding on flowers of Magnolia grandiflora (Ranunculales: Magnolia). Pest Manag. Sci. 2023, 79, 3239–3249. [Google Scholar] [CrossRef]
- Fu, B.L.; Qiu, H.Y.; Li, Q.; Tang, L.D.; Zeng, D.Q.; Liu, K.; Gao, Y.L. Flower injection of imidacloprid and spirotetramat: A novel tool for the management of banana thrips Thrips hawaiiensis. J. Pest Sci. 2020, 93, 1073–1084. [Google Scholar] [CrossRef]
- Avellaneda, J.; Díaz, M.; Coy-Barrera, E.; Rodríguez, D.; Osorio, C. Rose volatile compounds allow the design of new control strategies for the western fower thrips (Frankliniella occidentalis). J. Pest Sci. 2021, 94, 129–142. [Google Scholar] [CrossRef]
- Cao, Y.; Li, C.; Yang, H.; Li, J.; Li, S.; Wang, Y.W.; Gao, Y.L. Laboratory and field investigation on the orientation of Frankliniella occidentalis (Thysanoptera: Thripidae) to more suitable host plants driven by volatiles and component analysis of volatiles. Pest Manag. Sci. 2019, 75, 598–606. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, J.; Germinara, G.S.; Wang, L.J.; Yang, H.; Gao, Y.L.; Li, C. Behavioral Responses of Thrips hawaiiensis (Thysanoptera: Thripidae) to Volatile Compounds Identified from Gardenia jasminoides Ellis (Gentianales: Rubiaceae). Insects 2020, 11, 408. [Google Scholar] [CrossRef] [PubMed]
- Teulon, D.A.J.; Hollister, B.; Butler, R.C.; Cameron, E.A. Colour and odour responses of flying western flower thrips: Wind tunnel and greenhouse experiments. Entomol. Exp. Appl. 1999, 93, 9–19. [Google Scholar] [CrossRef]
- Van Tol, R.W.H.M.; James, D.E.; de Kogel, W.J.; Teulon, D.A.J. Plant odours with potential for a push-pull strategy to control the onion thrips, Thrips tabaci. Entomol. Exp. Appl. 2007, 122, 69–76. [Google Scholar] [CrossRef]
- Cao, Y.; Zhi, J.R.; Cong, C.L.; Margolies, D.C. Olfactory cues used in host selection by Frankliniella occidentalis (Thysanoptera: Thripidae) in relation to host suitability. J. Insect Behav. 2014, 27, 41–56. [Google Scholar] [CrossRef]
- Colazza, S.; Rosi, M.C.; Clemente, A. Response of egg parasitoid Telenomus busseolae to sex pheromone of Sesamia nonagrioides. J. Chem. Ecol. 1997, 23, 2437–2444. [Google Scholar] [CrossRef]
- Báez, D.; Pino, J.A.; Pino, D. Volatiles from Magnolia grandiflora Flowers: Comparative Analysis by Simultaneous Distillation-Extraction and Solid Phase Microextraction. Nat. Prod. Commun. 2012, 7, 237–238. [Google Scholar] [CrossRef]
- NIST 17, Mass Spectral Library (NIST/EPA/NIH); National Institute of Standards and Technology: Gaithersburg, MD, USA, 2017.
- Abdullah, Z.S.; Ficken, K.J.; Greenfield, B.P.J.; Butt, T.M. Innate responses to putative ancestral hosts: Is the attraction of western flower thrips to pine pollen a result of relict olfactory receptors? J. Chem. Ecol. 2014, 40, 534–540. [Google Scholar] [CrossRef]
- Tian, H.J.; Chen, Y.X.; Chen, Y.; Chen, X.Q.; Lin, S.; Zhang, J.; Yang, G.; Wei, H. A mixture of p-anisaldehyde and ethyl nicotinate elicits positive antennal and behavioral responses in Frankliniella occidentalis. Entomol. Exp. Appl. 2022, 170, 603–611. [Google Scholar] [CrossRef]
- Cao, Y.; Pistillo, O.M.; Lou, Y.B.; D’isita, I.; Maggi, F.; Hu, Q.Q.; Germinara, G.S.; Li, C. Electrophysiological and behavioural responses of Stegobium paniceum to volatile compounds from Chinese medicinal plant materials. Pest Manag. Sci. 2022, 78, 3697–3703. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Davison, A.C.; Tamo, C.A. Six-arm olfactometer permitting simultaneous observation of insect attraction and odour trapping. Physiol. Entomol. 2010, 29, 45–55. [Google Scholar] [CrossRef]
- Cao, Y.; Benelli, G.; Germinara, G.S.; Maggi, F.; Zhang, Y.; Luo, S.L.; Yang, H.; Li, C. Innate positive chemotaxis to paeonal from highly attractive Chinese medicinal herbs in the cigarette beetle, Lasioderma serricorne. Sci. Rep. 2019, 9, 6995. [Google Scholar] [CrossRef]
- Liu, X.F.; Chen, H.H.; Li, J.K.; Zhang, R.; Chen, L. Volatiles released by Chinese liquorice roots mediate host location behaviour by neonate Porphyrophora sophorae (Hemiptera: Margarodidae). Pest Manag. Sci. 2016, 72, 1959–1964. [Google Scholar] [CrossRef] [PubMed]
- Bruce, T.J.A.; Wadhams, L.J.; Woodcock, C.M. Insect host location: A volatile situation. Trends Plant Sci. 2005, 10, 269–274. [Google Scholar] [CrossRef]
- Knolhoff, L.M.; Heckel, D.G. Behavioral assays for studies of host plant choice and adaptation in herbivorous insects. Annu. Rev. Entomol. 2014, 59, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Xie, S.H.; Jian, L.Y.; Agrafoti, P.; Wu, K.X.; Athanassiou, C.G.; Cao, Y. Behavioral responses of Araecerus fasciculatus (Coleoptera: Anthribidae) to volatiles of selected stored Chinese medicinal plant products. J. Econ. Entomol. 2024, 117, 2669–2677. [Google Scholar] [CrossRef]
- Murai, T.; Imai, T.; Maekawa, M. Methyl Anthranilate as an Attractant for Two Thrips Species and the Thrips Parasitoid Ceranisus menes. J. Chem. Ecol. 2000, 26, 2557–2565. [Google Scholar] [CrossRef]
- Imai, T.; Maekawa, M.; Murai, T. Attractiveness of methyl anthranilate and its related compounds to the flower thrips, Thrips hawaiiensis (Morgan), T. coloratus Schmutz, T. flavus Schrank and Megalurothrips distalis (Karny) (Thysanoptera: Thripidae). Appl. Entomol. Zool. 2001, 36, 475–478. [Google Scholar] [CrossRef]
- Najar-Rodriguez, A.J.; Galizia, C.G.; Stierle, J.; Dorn, S. Behavioral and neurophysiological responses of an insect to changing ratios of constituents in host plant-derived volatile mixtures. J. Exp. Biol. 2010, 213, 3388–3397. [Google Scholar] [CrossRef]
- Cha, D.H.; Linn, C.E.; Teal, P.E.A.; Zhang, A.; Roelofs, W.L.; Loeb, G.M.; Frederic, M.P. Eavesdropping on plant volatiles by a specialist moth: Significance of ratio and concentration. PLoS ONE 2011, 6, e17033. [Google Scholar] [CrossRef]
- Rojas, C.J. Electrophysiological and behavioural responses of the cabbage moth to plant volatiles. J. Chem. Ecol. 1999, 25, 1867–1883. [Google Scholar] [CrossRef]
- Tasin, M.; Backamanì, A.C.; Bengtsson, M.; Ioriatti, C.; Witzgall, P. Essential host plant cues in the grapevine moth. Naturwissenschaften 2006, 93, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Dickens, J.C. Orientation of Colorado potato beetle to natural and synthetic blends of volatiles emitted by potato plants. Agric. For. Entomol. 2000, 2, 167–172. [Google Scholar] [CrossRef]
- Guo, X.J.; Yu, Q.Q.; Chen, D.F.; Wei, J.L.; Yang, P.C.; Yu, J.; Wang, X.H.; Kang, L. 4-Vinylanisole is an aggregation pheromone in locusts. Nature 2020, 584, 584–588. [Google Scholar] [CrossRef]
- Wang, B.; Dong, W.Y.; Li, H.M.; D’Onofriox, C.; Bai, P.H.; Chen, R.P.; Yang, L.L.; Wu, J.A.; Wang, X.Q.; Wang, B.; et al. Molecular basis of (E)-β-farnesene-mediated aphid location in the predator Eupeodes corollae. Curr. Biol. 2022, 32, 951–962.e7. [Google Scholar] [CrossRef]
- Zhang, L.W.; Sun, H.W.; Grosse-Wilde, E.; Zhang, L.; Hansson, B.S.; Dweck, H.K.M. Cross-generation pheromonal communication drives Drosophila oviposition site choice. Curr. Biol. 2023, 33, 2095–2103. [Google Scholar] [CrossRef]
- Kirk, W.D.J.; de Kogel, W.J.; Koschier, E.H.; Teulon, D.A.J. Semiochemicals for Thrips and Their Use in Pest Management. Annu. Rev. Entomol. 2021, 66, 101–119. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
